Cooperation between Lactococcus lactis NRRL B-50571 and NRRL B-50572 for Aroma Formation in Fermented Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Sample Preparation
2.3. Microbial Growth Monitoring and pH Determination
2.4. Proteolytic Activity in Fermented Milk
2.5. Determination of Free Amino Acids
2.6. Solid-Phase Microextraction Gas Chromatography Mass Spectrometry (SPME-GC-MS) Analysis
2.7. Aroma Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Microbial Growth and Acidifying Activity
3.2. Milk Proteolysis and Free Amino Acid Determination
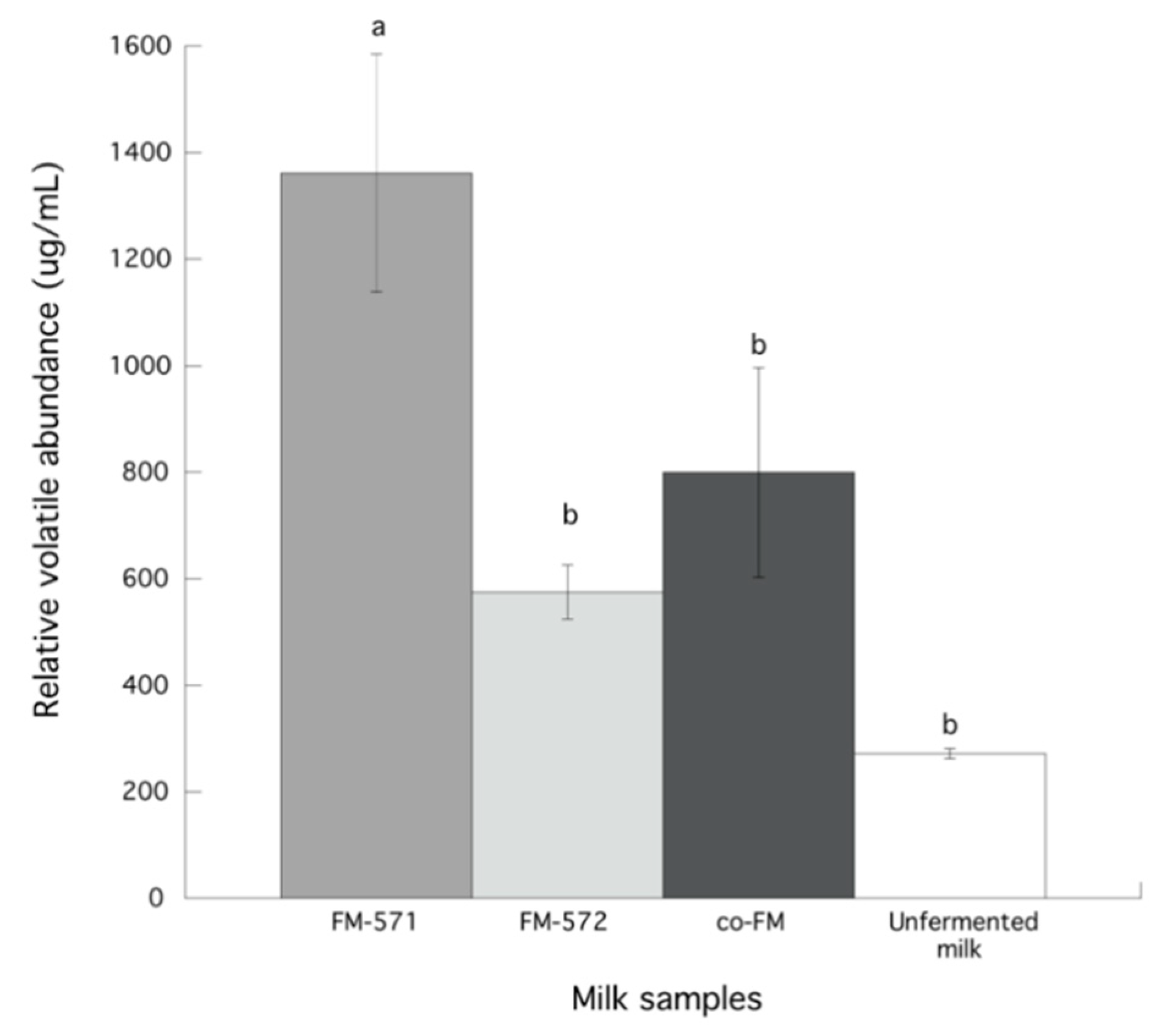
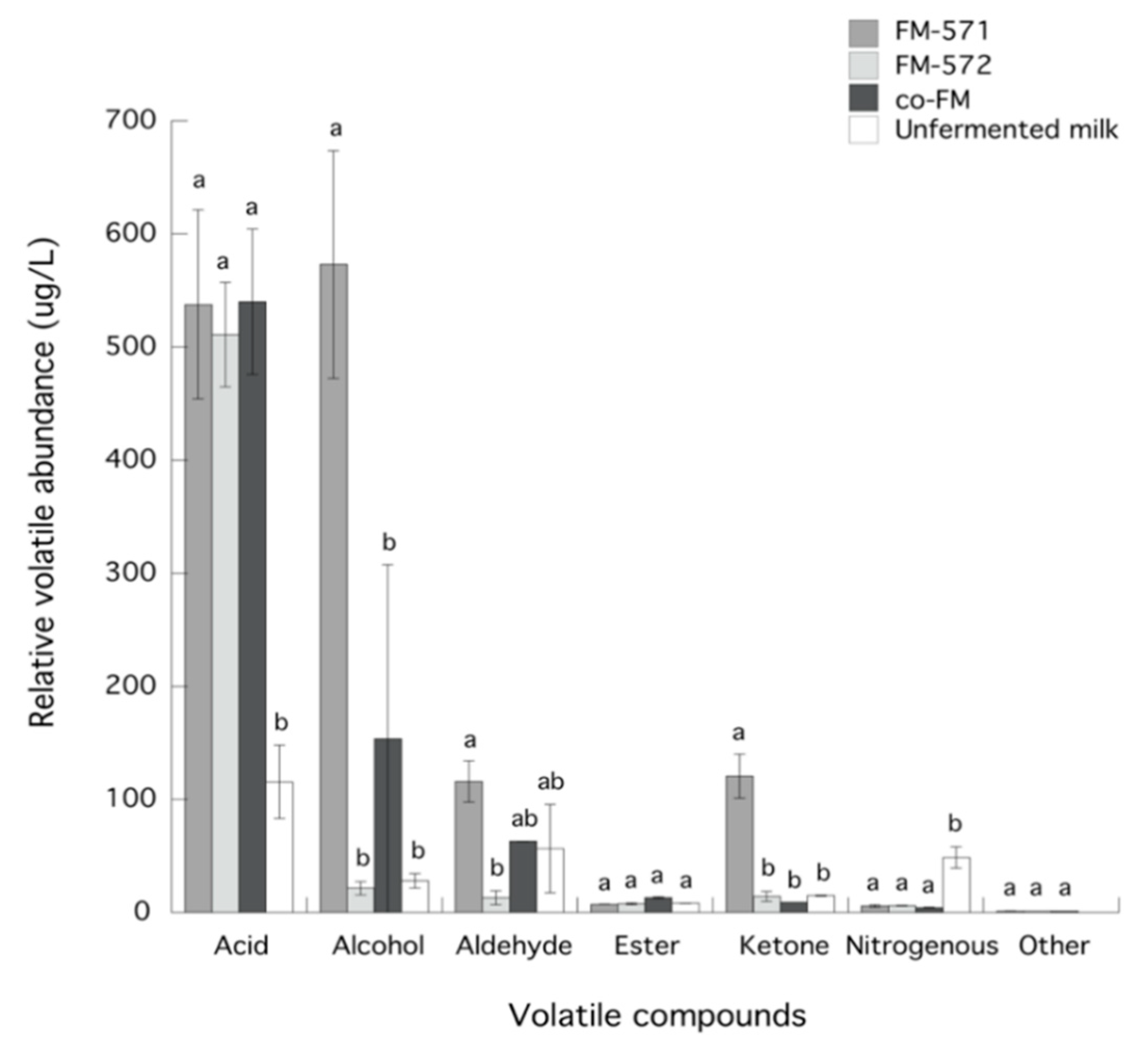
3.3. Identification and Chemical Groups of Volatile Compounds
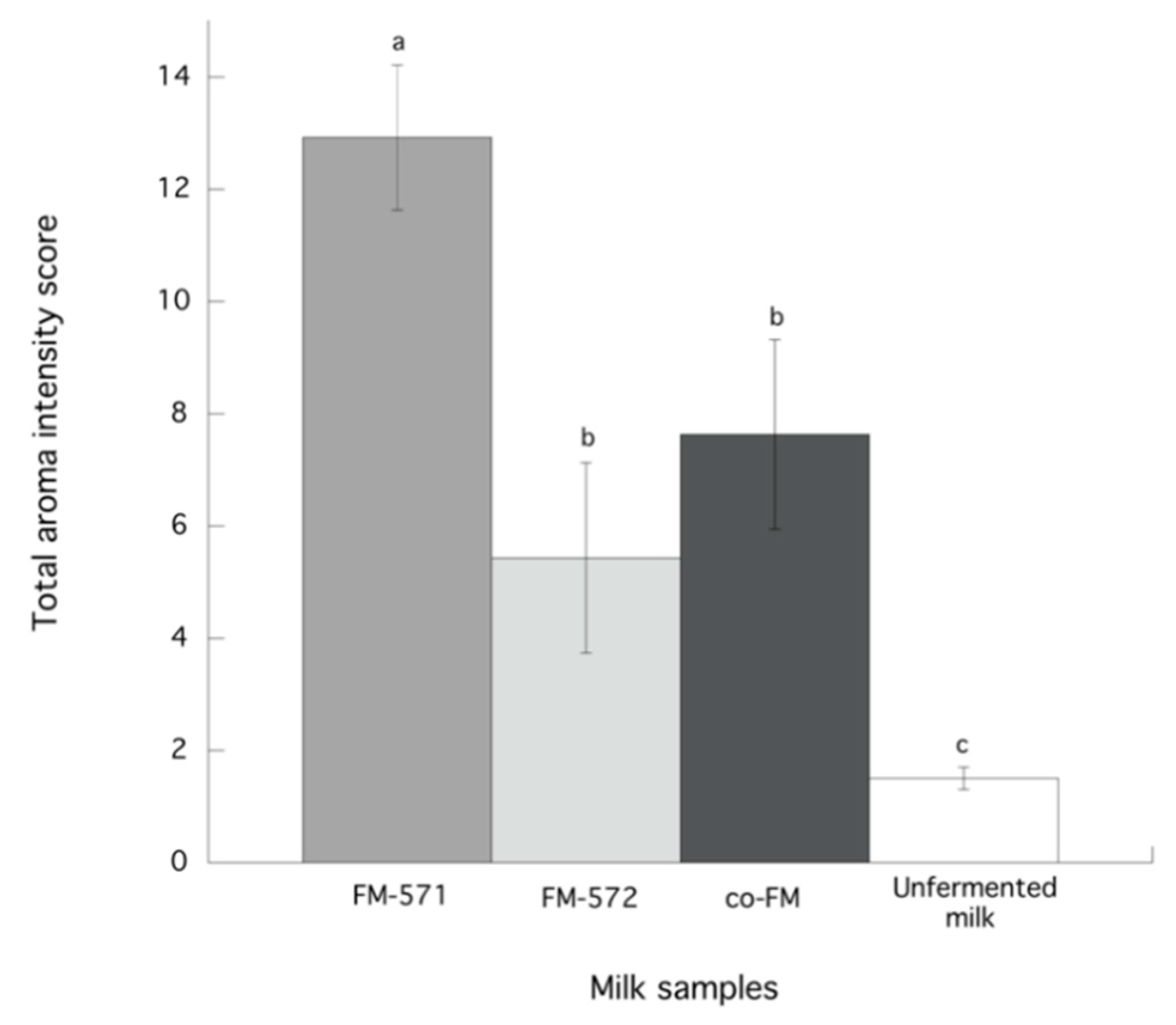
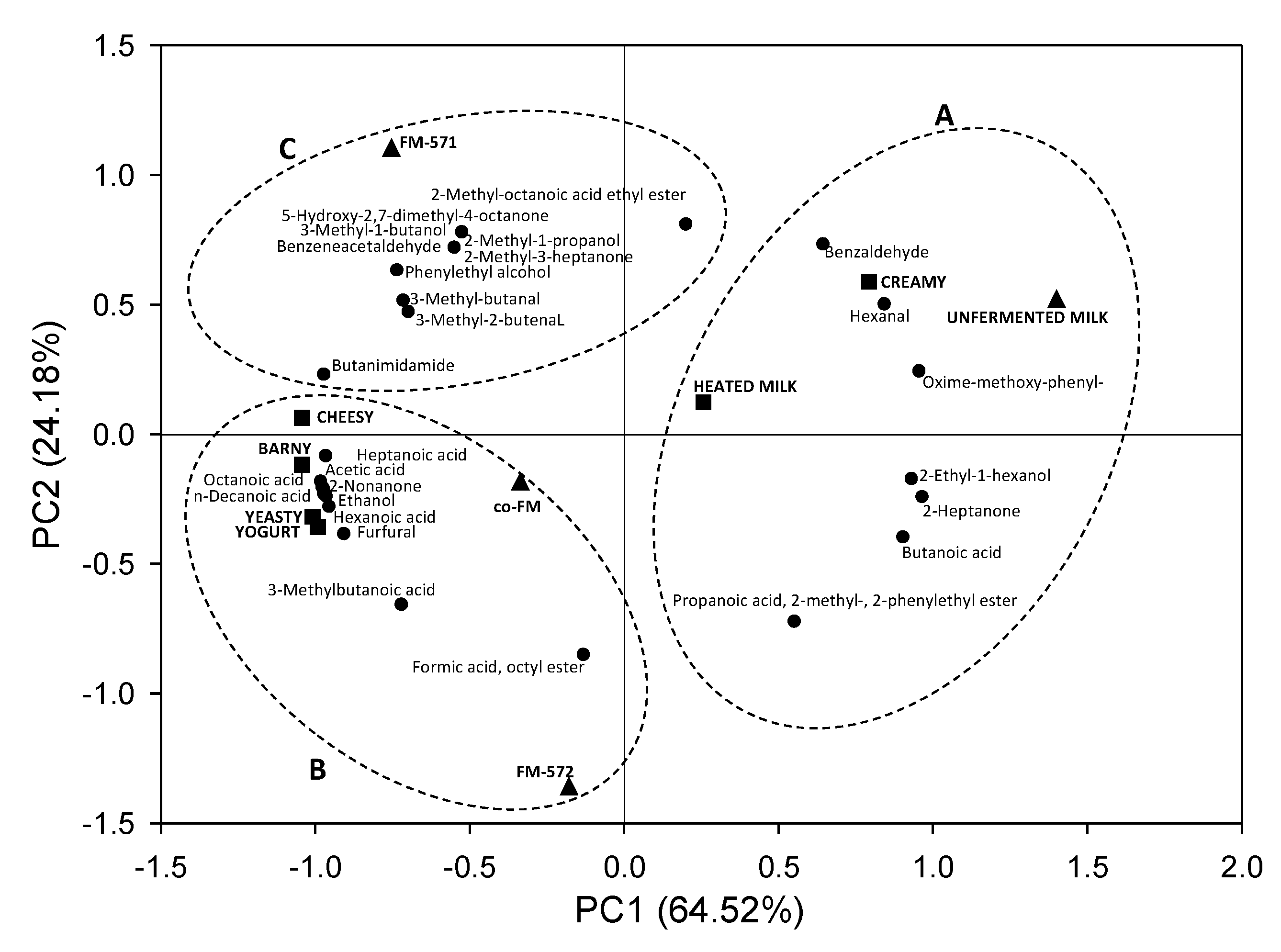
3.4. Aroma Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayra-Makinen, A.; Bigret, M. Industrial Use and Production of Lactic Acid Bacteria. In Lactic Acid Bacteria Microbiological and Functional Aspects; Salminen, S., von Wright, A., Ouwehand, A., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 175–198. [Google Scholar]
- Klijn, N.; Weerkamp, A.H.; de Vos, W.M. Detection and characterization of lactose- utilizing Lactococcus spp. in natural ecosystems. Appl. Environ. Microbiol. 1995, 61, 788–792. [Google Scholar] [PubMed]
- Ward, H.J.L.; Davey, G.P.; Heap, H.A.; Kelly, J.W. Lactococcus lactis. In Encyclopedia of Dairy Science; Rogniski, H., Fuquay, J.W., Fox, P.F., Eds.; Elsevier Science Ltd.: London, UK, 2002; pp. 1511–1516. [Google Scholar]
- Del Río, B.; Linares, D.M.; Ladero, V.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Putrescine production via the agmatine deiminase pathway increases the growth of Lactococcus. Lactococcus lactis and causes the alkalinization of the culture medium. Appl. Microbiol. Blot. 2015, 99, 897–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Huo, R.; Kwok, L.; Li, C.; Ma, Y.; Mi, Z.; Chen, Y. Effects of applying Lactobacillus helveticus H9 as adjunct starter culture in yogurt fermentation and storage. J. Dairy Sci. 2019, 102, 223–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Figueroa, J.C.; González-Córdova, A.F.; Torres-Llanez, M.J.; Garcia, H.S.; Vallejo-Cordoba, B. Novel angiotensin I-converting enzyme inhibitory peptides produced in fermented milk by specific wild Lactococcus lactis strains. J. Dairy Sci. 2012, 93, 5032–5038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epigmenio, A. Evaluación de la Actividad Inhibidora de la Enzima Convertidora de Angiotensina en Leches Fermentadas con Cepas Específicas de Lactococcus Lactis en un Modelo Gastrointestinal Simulado. Master’s Thesis, Centro de Investigación en Alimentación y Desarrollo, A.C., Hermosillo, Sonora, Mexico, August 2018. [Google Scholar]
- Rodríguez-Figueroa, J.C.; González-Córdova, A.F.; Astiazarán-Gacía, H.; Vallejo-Córdoba, B. Hypotensive and heart rate lowering effects in rats receiving milk fermented by specific Lactococcus lactis strains. Br. J. Nutr. 2013, 109, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Figueroa, J.C.; González-Córdova, A.F.; Astiazarán-Gacía, H.; Vallejo-Cordoba, B. Antihypertensive and hypolipidemic effect of milk fermented by specific Lactococcus lactis strains. J. Dairy Sci. 2013, 96, 4094–4099. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; González-Córdova, A.F.; Astiazarán-García, H.; Esparza-Romero, J.; Vallejo-Córdoba, B. Mechanistic pathways underlying the antihypertensive effect of fermented milk with Lactococcus lactis NRRL B-50571 in spontaneously hypertensive rats. Nutrients 2018, 10, 262. [Google Scholar] [CrossRef] [Green Version]
- Pihlanto, A. Lactic Fermentation and Bioactive Peptides. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; Kongo, M., Ed.; IntechOpen: London, UK, 2013; pp. 309–332. [Google Scholar]
- Omae, M.; Maeyama, Y.; Nishimura, T. Sensory properties and taste compounds of fermented milk produced by Lactococcus lactis and Streptococcus thermophilus. Food Sci. Technol. Res. 2008, 14, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Smid, E.J.; Kleerebezem, M. Production of aroma compounds in lactis fermentations. Annu. Rev. Food Sci. Technol. 2014, 5, 313–326. [Google Scholar] [CrossRef]
- Ayad, E.H.E.; Verheul, A.; Engels, W.J.M.; Wouters, J.T.M.; Smit, G. Enhanced flavour by combination of selected lactococci from industrial and artisanal origin with focus on completion of a metabolic pathway. J. Appl. Microbiol. 2001, 90, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, D.D.; Wu, Z.; Peng, T.; Zeng, X.Q.; Li, H. Volatile organic compounds profile during milk fermentation by Lactobacillus pentosus and correlations between volatiles flavor and carbohydrate metabolism. J. Dairy Sci. 2014, 97, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, T.; Jin, R.; Li, T.; Chen, H.; Sun, T. Characteristics of Milk Fermented by Streptococcus thermophilus MGA45-4 and the Profiles of Associated Volatile Compounds during Fermentation and Storage. Molecules 2018, 23, 878. [Google Scholar]
- Gutiérrez-Méndez, N.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Nevárez-Moorillón, G.V.; Rivera-Chavira, B. Evaluation of aroma generation of Lactococcus lactis with an electronic nose and sensory analysis. J. Dairy Sci. 2008, 91, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Amárita, F.; de la Plaza, M.; Fernández de Placencia, P.; Requena, T.; Pleáez, C. Cooperation between wild lactococcal strains for cheese aroma formation. Food Chem. 2006, 94, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Proteolytic activity of dairy lactic acid bacteria and probiotics as determinant of growth and in vitro angiotensin-converting enzyme inhibitory activity in fermented milk. Lait 2007, 86, 21–28. [Google Scholar] [CrossRef]
- Bartolomeo, M.P.; Maisano, F. Validation of a reversed-phase hplc method for quantitative amino acid analysis. J. Biomol. Tech. 2006, 17, 131–137. [Google Scholar]
- González-Córdova, G.; Vallejo-Cordoba, B. Quantitative determination of short-chain free fatty acids in milk using solid-phase microextraction and gas chromatography. J. Agric. Food Chem. 2001, 49, 4603–4608. [Google Scholar] [CrossRef]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Descriptive Analysis Techniques. In Sensory Evaluation Techniques, 5th ed.; Meilgaard, M., Civille, G.V., Carr, B.T., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2016; pp. 201–222. [Google Scholar]
- Heller, K.J. Probiotic bacteria in fermented foods: Product characteristics and starter organisms. Am. J. Clin. Nutr. 2001, 73, 374S–379S. [Google Scholar] [CrossRef]
- Dodd, H.M.; Gasson, M.J. Bacteriocins of lactic acid bacteria. In Genetics and Biotechnology of Lactic Acid Bacteria; Gasson, M.J., de Vos, W.M., Eds.; Blackie Academic and Professional: London, UK, 1994; pp. 211–252. [Google Scholar]
- Rodríguez-Figueroa, J.C.; Reyes-Díaz, R.; González-Córdova, A.F.; Troncoso-Rojas, R.; Vargas-Arispuro, I.; Vallejo-Cordoba, B. Angiotensin-converting enzyme inhibitory activity of milk fermented by wild and industrial Lactococcus lactis strains. J. Dairy Sci. 2010, 93, 5032–5038. [Google Scholar] [CrossRef] [Green Version]
- Widyastuti, Y.; Febrisiantosa, A. The role of lactic acid bacteria in milk fermentation. Food Nutr. Sci. 2014, 5, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Gobbetti, M.; Di Cagno, R.; Calasso, M.; Neviani, E.; Fox, P.F.; De Angelis, M. Drivers that establish and assembly the lactic acid bacteria biota in cheeses. Trends Food Sci. Technol. 2018, 78, 244–254. [Google Scholar] [CrossRef]
- Sulejmani, E.; Hayaloglu, A.A.; Rafajlovska, V. Study of the chemical composition, proteolysis, volatile compounds, and textural properties of industrial and traditional Beaten (Bieno sirenje) ewe milk cheese. J. Dairy Sci. 2014, 97, 1210–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudley, E.G.; Steele, J.L. Lactococcus lactis LM0230 contains a single aminotransferase involved in aspartate biosynthesis, which is essential in milk. Microbiology 2001, 147, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziadi, M.; Bergot, G.; Courtin, P.; Chambellon, E.; Hamdi, M.; Yvon, M. Amino acid catabolism by Lactococcus lactis during milk fermentation. Int. Dairy J. 2010, 20, 25–31. [Google Scholar] [CrossRef]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J.M. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef]
- Tanaous, C.; Kieronczyk, A.; Helinck, S.; Chambellon, E.; Yvon, M. Glutamate dehydrogenase activity: A major criterion for the selection of flavour-producing lactic acid bacteria strains. Antonie van Leeuwenhoek. 2002, 82, 271–278. [Google Scholar] [CrossRef]
- Tavaria, F.K.; Dahl, S.; Carballo, J.F.; Malcata, F.X. Amino acid catabolism and generation of volatiles by lactic acid bacteria. J. Dairy Sci. 2002, 85, 2462–2470. [Google Scholar] [CrossRef]
- Frank, D.C.; Owen, C.M.; Patterson, J. Solid phase micro-extraction (SPME) combined with gas-chromatography and olfactometry-mass spectrometry for characterization of cheese aroma compounds. LWT-Food Sci. Technol. 2004, 37, 139–154. [Google Scholar] [CrossRef]
- Bao, Z.; Xiong, J.; Lin, W.; Ye, J. Profiles of free fatty acids, free amino acids, and volatile compounds of milk bases fermented by Lactobacillus casei GBHM-21 with different fat levels. CyTA-J. Food. 2016, 14, 10–17. [Google Scholar] [CrossRef]
- Zareba, D.; Ziarno, M.; Scibisz, I.; Gawron, J. The importance of volatile compound profile in the assessment of fermentation conducted by Lactobacillus casei DN-114 001. Int. Dairy J. 2014, 35, 11–14. [Google Scholar] [CrossRef]
- Dursun, A.; Güler, Z.; Şekerli, Y.E. Characterization of volatile compounds and organic acids in ultra-high-temperature milk packaged in tetra brik cartons. Int. J. Food Prop. 2017, 20, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Dan, T.; Wang, D.; Jin, R.L.; Zhang, H.P.; Zhou, T.T.; Sun, T.S. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. J. Dairy Sci. 2016, 100, 2488–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, T.; Wang, D.; Wu, S.; Jin, R.; Ren, W.; Sun, T.S. Profiles of Volatile Flavor Compounds in Milk Fermented with Different Proportional Combinations of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. Molecules 2017, 22, 1633. [Google Scholar]
- Sfakianakis, P.; Tzia, C. Flavour profiling by gas chromatography-mass spectrometry and sensory analysis of yoghurt derived from ultrasonicated and homogenised milk. Int. Dairy J. 2017, 75, 120–128. [Google Scholar] [CrossRef]
- Kilcawley, K.N.; Faulkner, H.; Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P. Factors influencing the flavour of bovine milk and cheese from grass based versus non-grass based milk production systems. Foods 2018, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Qian, M.C.; Burbank, H.M. Hard Italian cheeses: Parmigiano-reggiano and grana-padano. In Improving the Flavour of Cheese; Weimer, B.C., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition: London, UK, 2007; pp. 421–443. [Google Scholar]
- Vagenas, G.; Roussis, I.G. Fat-derived volatiles of crious products of cows’, ewes’, and goats’ milk. Int. J. Food Prop. 2012, 15, 665–682. [Google Scholar] [CrossRef]
- Yue, J.; Zheng, Y.; Liu, Z.; Deng, Y.; Jing, Y.; Luo, Y.; Yu, W.; Zhao, Y. Characterization of volatile compounds in microfiltered pasteurized milk using solid-phase microextraction and GCxGC-TOFMS. Int. J. Food Prop. 2015, 18, 2193–2212. [Google Scholar] [CrossRef]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; García-Parra, J.; Ramírez, R. Characterisation by SPME-GC-MS of the volatile profile of a Spanish soft cheese PDO Torta del Casar during ripening. Food Chem. 2010, 118, 182–189. [Google Scholar] [CrossRef]
- Jensen, K.A.; Evans, K.M.; Kirk, T.K.; Hammel, K.E. Biosynthetic pathway for veratryl alcohol in the ligninolytic fungus phanerochaete chrysosporium. Appl. Environ. Microb. 1994, 60, 709–714. [Google Scholar]
- Smit, B.A.; Engels, W.J.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broome, M.C. Starter culture development for improved cheese flavour. In Improving the Flavour of Cheese; Weimer, B.C., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition: London, UK, 2007; pp. 157–174. [Google Scholar]
- Moio, L.; Langlois, D.; Etievant, P.X.; Addeo, F. Powerful odorants in water buffalo and bovine Mozzarella cheese by use of extract dilution sniffing analysis. Ital. J. Food Sci. 1993, 3, 227–237. [Google Scholar]
- Cadwallader, K.R.; Singh, T.K. Flavours and Off-Flavours in Milk and Dairy Products. In Advanced Dairy Chemistry: Lactose, Water, Salts and Minor Constituents; McSweeney, P.L.H., Fox, P.F., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 631–690. [Google Scholar]
- Van der Schaft, P. Approaches to production of natural flavours. In Flavour Development, Analysis and Perception in Food and Beverages; Parker, J.K., Elmore, J.S., Methven, L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition: Sawston, Cambridge, UK, 2015; pp. 235–248. [Google Scholar]
- Horwood, J.F.; Stark, W.; Hull, R.R. A ‘fermented yeasty’ flavour defect in Cheddar cheese. Aust. J. Dairy Technol. 1987, 42, 25–26. [Google Scholar]
- Cheng, F. Volatile flavor compounds in yogurt: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Turnbaugh, J.D.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Rychlik, M.; Sax, M.; Schieberle, P. On the role of short chain fatty acids for the development of a cheese-like off-note in pasteurized yoghurt. Lebensmitt. Wiss. Technol. 2013, 39, 521–527. [Google Scholar] [CrossRef]
- Afzal, M.I.; Boulahya, K.A.; Jacquot, M.; Delaunay, S.; Cailliezgrimal, C. Biosynthesis and role of 3-methylbutanal in cheese by lactic acid bacteria: Major metabolic pathways, enzymes involved, and strategies for control. Crit. Rev. Food Sci. 2017, 57, 399–406. [Google Scholar] [CrossRef]
- Dan, T.; Chen, H.; Li, T.; Tian, J.; Ren, W.; Zhang, H.; Sun, T. Influence of Lactobacillus plantarum P-8 on fermented milk flavor and storage stability. Front. Microbiol. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Velazquez, G.; Torres, J.A.; Qian, M.C. Quantitative Determination of Thermally Derived Off-Flavor Compounds in Milk Using Solid-Phase Microextraction and Gas Chromatography. J. Dairy Sci. 2005, 88, 3764–3772. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Holland, R.; Crow, V.L. Esters and their biosynthesis in fermented dairy products: A review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Alemayehu, D.; Hannon, J.A.; Mcauliffe, O.; Ross, R.P. Characterization of plant-derived lactococci on the basis of their volatile compounds profile when grown in milk. Int. J. Food Microbiol. 2014, 172, 57–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solano Lopez, C.E.; Taehyun, J.; Alvarez, V.B. Volatile Compounds and Chemical Changes in Ultrapasteurized Milk Packaged in Polyethylene Terephthalate Containers. J. Food Sci. 2005, 70, 407–412. [Google Scholar] [CrossRef]
- Gaze, L.V.; Costa, M.P.; Monteiro, M.L.; Lavorato, J.A.; Conte Júnior, C.A.; Raices, R.S.; Cruz, A.G.; Freitas, M.Q. Dulce de Leche, a typical product of Latin America: Characterization by physicochemical, optical and instrumental methods. Food Chem. 2015, 169, 471–477. [Google Scholar] [CrossRef] [PubMed]

| Amino Acid (μg/mL) | FM-571 | FM-572 | Co-FM | |
|---|---|---|---|---|
| Aspartic acid | N | 0.67 ± 0.17 a | 0.2 ± 0.01 b | 0.26 ± 0.07 b |
| Glutamic acid | E | 11.06 ± 0.50 a | 142 ± 1.3 b | 13.83 ± 0.53 c |
| Serine | S | 2.95 ± 0.19 a | 3.93 ± 0.24 b | 3.69 ± 0.5 b |
| Histidine | H | 14.33 ± 0.34 a | 21.09 ± 0.42 b | 18.94 ± 0.29 c |
| Arginine | R | 9.03 ± 0.78 a | 8.09 ± 0.14 a | 12.39 ± 0.19 b |
| Threonine | T | 10.10 ± 0.15 a | 10.57 ± 0.10 a | 10.21 ± 0.28 a |
| Glycine | G | 5.51 ± 0.21 a | 4.13 ± 0.14 b | 5.27 ± 0.82 ab |
| Tyrosine | Y | 38.86 ± 2.68 a | 17.69 ± 0.14 b | 38.92 ± 0.02 a |
| Alanine | A | 58.68 ± 1.07 a | 12.88 ± 0.11 b | 68.36 ± 0.37 c |
| Tryptophan | W | 0.36 ± 0.09 a | 0.47 ± 0.10 a | 0.87 ± 0.08 b |
| Methionine | M | 1.69 ± 0.08 a | 0.91 ± 0.10 b | 3.78 ± 0.23 c |
| Valine | V | ND | 3.97 ± 0.24 a | 1.73 ± 0.05 b |
| Phenylalanine | F | 5.84 ± 0.39 a | 23.30 ± 0.39 b | 12.56 ± 0.23 c |
| Isoleucine | I | 0.97 ± 0.02 a | 5.89 ± 0.68 b | 0.19 ± 0.03 a |
| Leucine | L | 3.34 ± 0.10 a | 15.30 ± 0.32 b | 2.85 ± 0.04 a |
| Lysine | K | 13.87 ± 0.33 a | 22.52 ± 0.41 b | 17.11 ± 0.78 c |
| Asparagine | N | ND | ND | ND |
| Glutamine | Q | ND | ND | ND |
| Volatile Compound | RT 1 (min) | Relative Abundance (μg/L) | |||
|---|---|---|---|---|---|
| Unfermented Milk | FM-571 | FM-572 | Co-FM | ||
| Acids | |||||
| Acetic acid ** | 37.92 | 4.09 ± 2.30 a | 90.53 ± 12.97 b | 69.39 ± 24.79 b | 72.25 ± 58.92 b |
| Butanoic acid ** | 45.35 | 45.81 ± 31.22 a | 24.98 ± 3.64 a | 36.12 ± 15.76 a | 33.84 ± 7.03 a |
| 3-Methylbutanoic acid * | 47.71 | ND | 2.88 ± 0.58 a | 7.19 ± 6.86 a | 3.06 ± 1.94 a |
| Hexanoic acid ** | 58.68 | 34.08 ± 2.45 a | 163.96 ± 26.07 b | 171.75 ± 39.22 b | 186.28 ± 1.52 b |
| Heptanoic acid ** | 64.89 | ND | 2.25 ± 0.48 a | 1.89 ± 0.12 a | 2.44 ± 0.26 a |
| Octanoic acid ** | 73.23 | 31.47 ± 3.58 a | 184.32 ± 26.40 b | 164.50 ± 6.59 b | 178.31 ± 7.81 b |
| n-Decanoic acid ** | 102.49 | ND | 68.79 ± 13.33 a | 60.12 ± 2.72 a | 63.85 ± 4.01 a |
| Alcohols | |||||
| Ethanol ** | 11.19 | ND | 16.73 ± 5.21 a | 15.62 ± 2.11 a | 20.02 ± 7.57 a |
| 2-Methyl-1-propanol * | 20.28 | ND | 2.92 ± 0.73 | ND | ND |
| 3-Methyl-1-butanol * | 26.85 | 5.37 ± 5.76 a | 273.14 ± 37.52 b | ND | 76.41 ± 104.76 ab |
| 2-Ethyl-1-hexanol * | 39.44 | 22.69 ± 0.65 a | 2.57 ± 0.44 b | 6.00 ± 3.72 b | 8.02 ± 4.87 b |
| Phenylethyl alcohol ** | 56.70 | ND | 277.73 ± 58.84 a | ND | 49.07 ± 62.68 a |
| Aldehydes | |||||
| 3-Methyl-butanal * | 10.20 | ND | 41.95 ± 8.50 a | ND | 44.28 ± 5.21 a |
| Hexanal ** | 19.52 | 23.71 ± 7.37 a | 2.52 ± 1.61 b | ND | 2.94 ± 2.06 b |
| 3-Methyl-2-butenal * | 25.28 | ND | 1.88 ± 0.16 a | ND | 2.05 ± 0.29 a |
| Furfural ** | 38.38 | 1.58 ± 0.04 a | 2.84 ± 0.75 a | 3.18 ± 0.23 a | 2.63 ± 0.36 a |
| Benzaldehyde ** | 40.99 | 31.19 ± 31.55 a | 20.07 ± 3.69 a | 9.95 ± 6.29 a | 11.09 ± 6.99 a |
| Phenyl acetaldehyde * | 46.31 | ND | 46.60 ± 6.75 | ND | ND |
| Ketones | |||||
| 2-Methyl-3-heptanone * | 23.15 | ND | 34.34 ± 7.74 | ND | ND |
| 2-Heptanone * | 24.78 | 13.42 ± 0.16 a | 7.10 ± 0.07 a | 9.61 ± 4.33 a | 9.05 ± 1.01 a |
| 2-Nonanone * | 30.45 | 1.52 ± 0.25 a | 4.95 ± 0.52 b | 4.68 ± 0.20 b | 4.18 ± 0.30 b |
| 5-Hydroxy-2,7-dimethyl-4-octanone * | 39.44 | ND | 74.29 ± 11.21 | ND | ND |
| Esters | |||||
| 2-Methyl-octanoic acid ethyl ester * | 36.36 | 8.21 ± 0.08 a | 6.63 ± 0.05 b | 0.94 ± 0.38 b | 8.14 ± 0.17 a |
| Formic acid, octyl ester * | 42.18 | ND | ND | 1.72 ± 0.70 a | 0.99 ± 0.10 a |
| Propanoic acid, 2-methyl-, 2-phenylethyl ester * | 65.59 | ND | 0.68 ± 0.08 | ND | ND |
| Nitrogenous | |||||
| Oxime-methoxy-phenyl- * | 53.02 | 48.70 ± 9.45 a | 5.83 ± 1.19 b | 6.04 ± 0.27 b | 4.34 ± 0.33 b |
| Other compounds | |||||
| Butanimidamide * | 44.02 | ND | 1.29 ± 0.23 a | 1.14 ± 0.05 a | 1.20 ± 0.03 a |
| Aroma Descriptors | Reference Sample |
|---|---|
| Creamy | Heavy cream |
| Yogurt | Unflavored yogurt |
| Yeasty | Raw yeast dough |
| Cheesy | Commercial raw milk Mexican Fresco cheese |
| Barny | Stable hay |
| Heated milk | Condensed milk |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán-Barrientos, L.M.; Garcia, H.S.; Reyes-Díaz, R.; Estrada-Montoya, M.C.; Torres-Llanez, M.J.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B. Cooperation between Lactococcus lactis NRRL B-50571 and NRRL B-50572 for Aroma Formation in Fermented Milk. Foods 2019, 8, 645. https://doi.org/10.3390/foods8120645
Beltrán-Barrientos LM, Garcia HS, Reyes-Díaz R, Estrada-Montoya MC, Torres-Llanez MJ, Hernández-Mendoza A, González-Córdova AF, Vallejo-Cordoba B. Cooperation between Lactococcus lactis NRRL B-50571 and NRRL B-50572 for Aroma Formation in Fermented Milk. Foods. 2019; 8(12):645. https://doi.org/10.3390/foods8120645
Chicago/Turabian StyleBeltrán-Barrientos, Lilia M., Hugo S. Garcia, Ricardo Reyes-Díaz, María C. Estrada-Montoya, María J. Torres-Llanez, Adrián Hernández-Mendoza, Aarón F. González-Córdova, and Belinda Vallejo-Cordoba. 2019. "Cooperation between Lactococcus lactis NRRL B-50571 and NRRL B-50572 for Aroma Formation in Fermented Milk" Foods 8, no. 12: 645. https://doi.org/10.3390/foods8120645






