Effect of a Milk-Based Fruit Beverage Enriched with Plant Sterols and/or Galactooligosaccharides in a Murine Chronic Colitis Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. PS Milk-Based Fruit Beverage Formulation
2.2. Animals and Treatment
2.3. Disease Activity Index (DAI)
2.4. Colon Shortening and Presence of Edema
2.5. Histological Analysis
2.6. Myeloperoxidase (MPO) Assay
2.7. Statistical Analysis
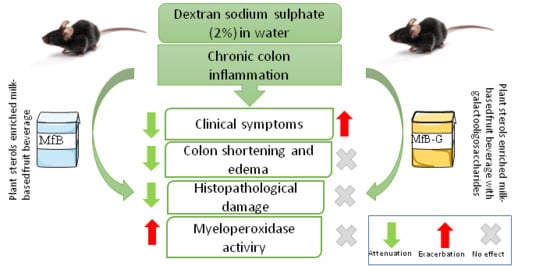
3. Results
3.1. Evaluation of Clinical Symptoms of Induced Chronic Colitis in Mice
3.2. Colon Length Shortening and Presence of Edema
3.3. Histopathological Analysis
3.4. Presence of MPO in Colonic Tissue
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vanga, R.; Long, M.D. Contemporary management of ulcerative colitis. Gastroenterol. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Charlebois, A.; Rosenfeld, G.; Bressler, B. The impact of dietary interventions on the symptoms of inflammatory bowel disease: A systematic review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Al Mijan, M.; Lim, B.O. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: Present status and future trends. World J. Gastroenterol. 2018, 24, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E.; Armini, V.; Ritieni, A. State of the art of Ready-to Use Therapeutic Food: A tool for nutraceuticals addition to foodstuff. Food Chem. 2013, 140, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Wang, W.; Zhang, D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Banerjee, N.; Barnes, R.C.; Pfent, C.M.; Talcott, S.T.; Dashwood, R.H.; Mertens-Talcott, S.U. Mango polyphenolics reduce inflammation in intestinal colitis—Involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo. Mol. Carcinog. 2017, 56, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.P.; Chichlowski, M.; Trinh, C.T.; Greer, P.K. Dietary supplementation with fresh pineapple juice decreases inflammation and colonic neoplasia in IL-10-deficient mice with colitis. Inflamm. Bowel Dis. 2010, 16, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cirmi, S.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. A flavonoid-rich extract of orange juice reduced oxidative stress in an experimental model of inflammatory bowel disease. J. Funct. Foods 2017, 30, 168–178. [Google Scholar] [CrossRef]
- Pacheco, M.T.; Vezza, T.; Diez-Echave, P.; Utrilla, P.; Villamiel, M.; Moreno, F.J. Anti-inflammatory bowel effect of industrial orange by-products in DSS-treated mice. Food Funct. 2018, 9, 4888–4896. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, G.; Mazzone, G.; Tuccillo, C.; Ribecco, M.T.; Graziani, G.; Gravina, A.G.; Caserta, S.; Guido, S.; Fogliano, V.; Caporaso, N.; et al. Apple polyphenols extract (APE) improves colon damage in a rat model of colitis. Dig. Liver Dis. 2012, 44, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; Cárdeno, A.; Aparicio-Soto, M.; Sánchez-Fidalgo, S.; Villegas, I.; de la Lastra, C.A. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol. Res. 2012, 66, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hana, K.; Nemoto, N.; Yoshida, T.; Saegusa, M.; Yokota-Nakatsuma, A.; Song, S.Y.; Iwata, M. Vitamin A inhibits development of dextran sulfate sodium-induced colitis and colon cancer in a mouse model. Biomed. Res. Int. 2016. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Jena, G.B. Mechanistic insight into beta-carotene-mediated protection against ulcerative colitis-associated local and systemic damage in mice. Eur. J. Nutr. 2015, 54, 639–652. [Google Scholar] [CrossRef]
- Mencarelli, A.; Renga, B.; Palladino, G.; Distrutti, E.; Fiorucci, S. The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease. Biochem. Pharmacol. 2009, 78, 1214–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.A.; Kim, E.J.; Kim, D.H. Inhibitory effect of β-sitosterol on TNBS-induced colitis in mice. Planta Med. 2012, 78, 896–898. [Google Scholar] [CrossRef]
- Aldini, R.; Micucci, M.; Cevenini, M.; Fato, R.; Bergamini, C.; Nanni, C.; Cont, M.; Camborata, C.; Spinozzi, S.; Montagnani, M.; et al. Antiinflammatory effect of phytosterols in experimental murine colitis model: Prevention, induction, remission study. PLoS ONE 2014, 9, e108112. [Google Scholar] [CrossRef]
- Kim, K.A.; Lee, I.A.; Gu, W.; Hyam, S.R.; Kim, D.H. β-Sitosterol attenuates high-fat diet-induced intestinal inflammation in mice by inhibiting the binding of lipopolysaccharide to toll-like receptor 4 in the NF-κB pathway. Mol. Nutr. Food Res. 2014, 58, 963–972. [Google Scholar] [CrossRef]
- Te Velde, A.A.; Brüll, F.; Heinsbroek, S.E.; Meijer, S.L.; Lütjohann, D.; Vreugdenhil, A.; Plat, J. Effects of dietary plant sterols and stanol esters with low-and high-fat diets in chronic and acute models for experimental colitis. Nutrients 2015, 7, 8518–8531. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.; Wang, H.; Chen, J.; Luo, Z.; Yang, C.S. β-Sitosterol and stigmasterol ameliorate dextran sulphate sodium-induced colitis in mice fed a high fat Western-style diet. Food Funct. 2017, 8, 4179–4186. [Google Scholar] [CrossRef]
- European Commission. Decision 2004/336/EC of 31 March 2004 authorizing the placing on the market of yellow fat spreads, milk based fruit drinks, yoghurt type products and cheese type products with added phytosterols/phytostanols as novel foods or novel food ingredients under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union 2004, L105, 49–51. [Google Scholar]
- European Commission. Regulation (EU) No 686/2014 of 20 June 2014 amending Regulations (EC) No 983/2009 and (EU) No 384/2010 as regards the conditions of use of certain health claims related to the lowering effect of plant sterols and plant stanols on blood LDL-cholesterol. Off. J. Eur. Union 2014, L182, 27–30. [Google Scholar]
- Islam, M.S.; Murata, T.; Fujisawa, M.; Nagasaka, R.; Ushio, H.; Bari, A.M.; Hori, M.; Ozaki, H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol. 2008, 154, 812–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannidis, O.; Varnalidis, I.; Paraskevas, G.; Botsios, D. Nutritional modulation of the inflammatory bowel response. Digestion 2011, 84, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Laurell, A.; Sjöberg, K. Prebiotics and symbiotic in ulcerative colitis. Scand. J. Gastroenterol. 2017, 52, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.R. A Mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar]
- Holma, R.; Juvonen, P.; Asmawi, M.Z.; Vapaatalo, H.; Korpela, R. Galacto-oligosaccharides stimulate the growth of bifidobacteria but fail to attenuate inflammation in experimental colitis in rats. Scand. J. Gastroenterol. 2002, 37, 1042–1047. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Clinthorne, J.F.; Rondini, E.A.; McCaskey, S.J.; Gurzell, E.A.; Langohr, I.M.; Gardner, E.M.; Fenton, J.I. Supplementation with galacto-oligosaccharides increases the percentage of NK cells and reduces colitis severity in smad3-deficient mice. J. Nutr. 2012, 142, 1336–1342. [Google Scholar] [CrossRef]
- López-García, G.; Cilla, A.; Barberá, R.; Alegría, A. Protective effect of antioxidants contained in milk-based fruit beverages against sterol oxidation products. J. Funct. Foods 2017, 30, 81–89. [Google Scholar] [CrossRef]
- Alvarez-Sala, A.; Blanco-Morales, V.; Cilla, A.; Silvestre, R.A.; Hernández-Álvarez, E.; Granado-Lorencio, F.; Barberá, R.; Garcia-Llatas, G. A positive impact on the serum lipid profile and cytokines after the consumption of a plant sterol-enriched beverage with a milk fat globule membrane: A clinical study. Food Funct. 2018, 9, 5209–5219. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.; Giner, R.M.; Ríos, J.L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Cerdá-Nicolás, J.M.; Giner, R.M. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in C57BL/6 mice. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Almero, J. Modelos experimentales in vivo de enfermedad inflamatoria intestinal y cáncer colorrectal: Conceptos, modelos actuales y aplicabilidad. Nutr. Hosp. 2007, 22, 178–189. [Google Scholar]
- Perŝe, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Gottlieb, Y.; Elhasid, R.; Berger-Achituv, S.; Brazowski, E.; Yerushalmy-Feler, A.; Cohen, S. Neutrophil extracellular traps in paediatric inflammatory bowel disease. Pathol. Int. 2018, 68, 517–523. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulphate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Largarda, M.J.; García-López, F.J.; Sánchez-Siles, L.M.; Blanco- Navarro, I.; Alegría, A.; Pérez-Sacristán, B.; Garcia-Llatas, G.; Donoso-Navarro, E.; Silvestre-Mardomingo, R.A.; et al. Effect of β-cryptoxanthin plus phytosterols on cardiovascular risk and bone turnover markers in postmenopausal women: A randomized crossover trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Ni, Y.; Takahashi, Y.; Watanabe, N.; Sugiura, M.; Ogawa, K.; Nagashimada, M.; Kaneko, S.; Naito, S.; Ota, T. β-Cryptoxanthin alleviates diet-induced nonalcoholic steatohepatitis by suppressing inflammatory gene expression in mice. PLoS ONE 2014, 9, e98294. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Sahin, N.; Yılmaz, I.; Juturu, V. β-Cryptoxanthin ameliorates metabolic risk factors by regulating NF-κB and Nrf2 pathways in insulin resistance induced by high-fat diet in rodents. Food Chem. Toxicol. 2017, 107, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Sabeva, N.S.; McPhaul, C.M.; Li, X.; Cory, T.J.; Feola, D.J.; Graf, G.A. Phytosterols differentially influence ABC transporter expression, cholesterol efflux and inflammatory cytokine secretion in macrophage foam cells. J. Nutr. Biochem. 2011, 22, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.F.; Grispan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 2018, 154, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Geier, M.S.; Butler, R.N.; Giffard, P.M.; Howarth, G.S. Prebiotic and symbiotic fructooligosaccharide administration fail to reduce the severity of experimental colitis in rats. Dis. Colon Rectum 2007, 50, 1061–1069. [Google Scholar] [CrossRef]
- Campbell, J.M.; Fahey, G.C., Jr.; Wolf, B.W. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J. Nutr. 1997, 127, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Bruggencate, S.J.T.; Bovee-Oudenhoven, I.M.; Lettink-Wissink, M.L.; Van der Meer, R. Dietary fructooligosaccharides increase intestinal permeability in rats. J. Nutr. 2005, 135, 837–842. [Google Scholar] [CrossRef]
- Maathuis, A.J.; van den Heuvel, E.G.; Schoterman, M.H.; Venema, K. Galacto-oligosaccharides have prebiotic activity in a dynamic in vitro colon model using a 13C-labeling technique. J. Nutr. 2012, 142, 1205–1212. [Google Scholar] [CrossRef]





| Score | Weight Loss (%) | Stool Consistency | Visible Blood in Feces |
|---|---|---|---|
| 0 | None | Normal | None |
| 1 | 1–5 | ||
| 2 | 6–10 | Loose | Slight bleeding |
| 3 | 11–20 | ||
| 4 | <20 | Diarrhea | Gross bleeding |
| Histological Scoring System for DSS-Induced Colitis | ||
|---|---|---|
| Feature | Score | Description |
| Severity of inflammation | 0 | None |
| 1 | Mild | |
| 2 | Moderate | |
| 3 | Severe | |
| Extent of inflammation | 0 | None |
| 1 | Mucosa | |
| 2 | Mucosa and submucosa | |
| 3 | Transmural | |
| Crypt damage | 0 | None |
| 1 | 1/3 damages | |
| 2 | 2/3 damaged | |
| 3 | Crypts lost, surface and epithelium present | |
| 4 | Crypt and surface epithelium lost | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-García, G.; Cilla, A.; Barberá, R.; Alegría, A.; Recio, M.C. Effect of a Milk-Based Fruit Beverage Enriched with Plant Sterols and/or Galactooligosaccharides in a Murine Chronic Colitis Model. Foods 2019, 8, 114. https://doi.org/10.3390/foods8040114
López-García G, Cilla A, Barberá R, Alegría A, Recio MC. Effect of a Milk-Based Fruit Beverage Enriched with Plant Sterols and/or Galactooligosaccharides in a Murine Chronic Colitis Model. Foods. 2019; 8(4):114. https://doi.org/10.3390/foods8040114
Chicago/Turabian StyleLópez-García, Gabriel, Antonio Cilla, Reyes Barberá, Amparo Alegría, and María C. Recio. 2019. "Effect of a Milk-Based Fruit Beverage Enriched with Plant Sterols and/or Galactooligosaccharides in a Murine Chronic Colitis Model" Foods 8, no. 4: 114. https://doi.org/10.3390/foods8040114
APA StyleLópez-García, G., Cilla, A., Barberá, R., Alegría, A., & Recio, M. C. (2019). Effect of a Milk-Based Fruit Beverage Enriched with Plant Sterols and/or Galactooligosaccharides in a Murine Chronic Colitis Model. Foods, 8(4), 114. https://doi.org/10.3390/foods8040114