Accreditation Procedure for Trichinella spp. Detection in Slaughterhouses: The Experience of an Internal Laboratory in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preliminary Information
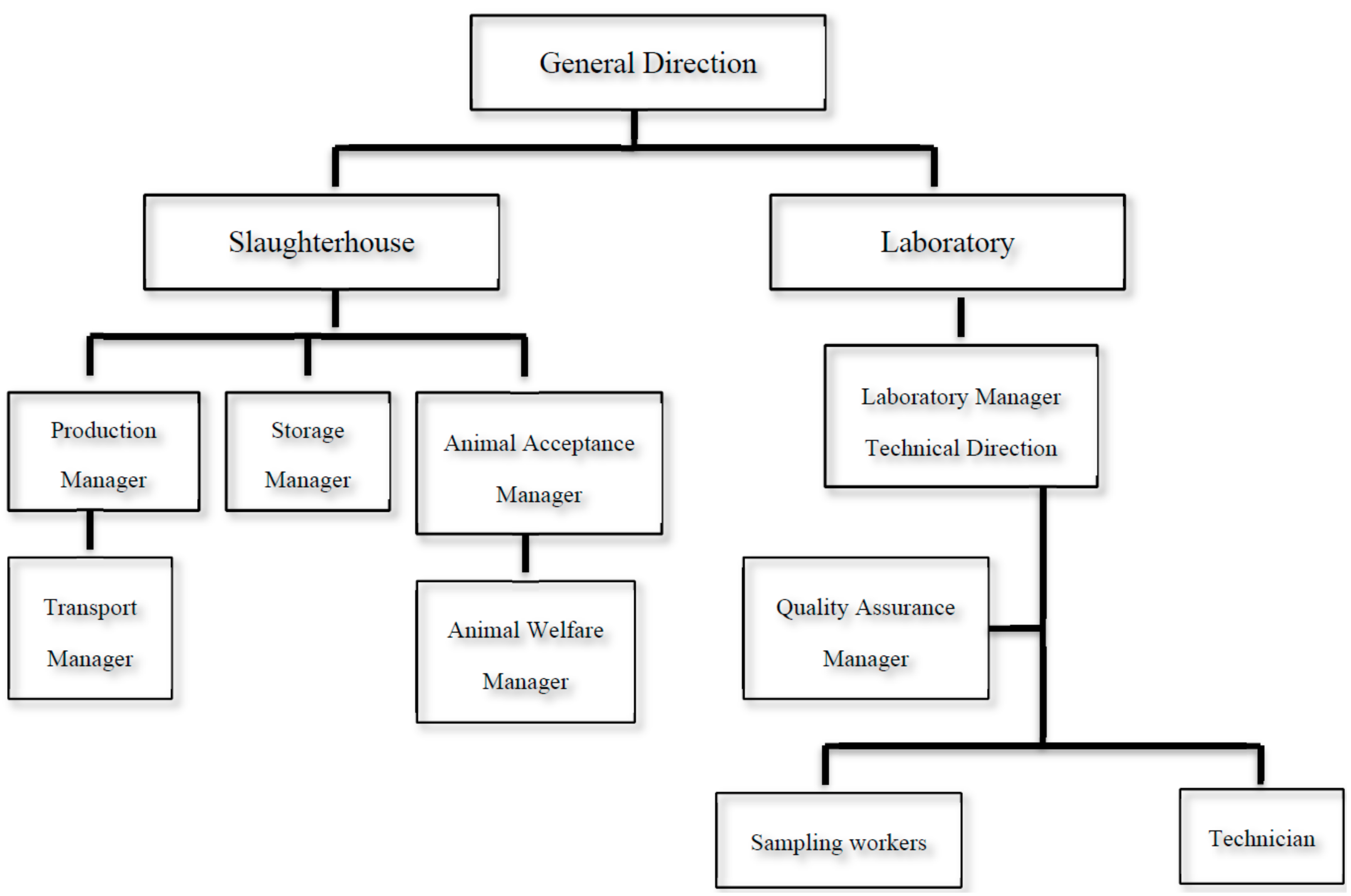
2.2. Preparation of the Quality Assurance Manual
2.3. First Accreditation
2.4. Maintenance of the Accreditation Certificate
2.5. Reference Method of Trichinella spp. Detection
3. Results and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Dimzas, D.; Diakou, A.; Koutras, C.; Gómez-Morales, M.A.; Psalla, D.; Keryttopoulos, P.; Deligianni, D.; Kontotasios, K.; Pozio, E. Human trichinellosis caused by Trichinella britovi in Greece, and literature review. J. Helminthol. 2019, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Gamble, H.R.; Dupouy-Camet, J.; Khazan, H.; Bruschi, F. Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol. 2017, 64, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Morales, M.A.; Ludovisi, A.; Amati, M.; Cherchi, S.; Tonanzi, D.; Pozio, E. Differentiation of Trichinella species (Trichinella spiralis/Trichinella britovi versus Trichinella pseudospiralis) using western blot. Parasite Vectors 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Turiac, I.A.; Cappelli, M.G.; Olivieri, R.; Angelillis, R.; Martinelli, D.; Prato, R.; Fortunato, F. Trichinellosis outbreak due to wild boar meat consumption in southern Italy. Parasite Vectors 2017, 10, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E. Adaptation of Trichinella spp. for survival in cold climates. Food Waterborne Parasitol. 2016, 4, 4–12. [Google Scholar] [CrossRef]
- Zarlenga, D.; Wang, Z.; Mitreva, M. Trichinella spiralis: Adaptation and parasitism. Vet. Parasitol. 2016, 231, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Saracino, M.P.; Calcagno, M.A.; Beauche, E.B.; Garnier, A.; Vila, C.C.; Granchetti, H.; Taus, M.R.; Venturiello, S.M. Trichinella spiralis infection and transplacental passage in human pregnancy. Vet. Parasitol. 2016, 231, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, F.; Yang, D.Q.; Jiang, P.; Liu, R.D.; Zhang, X.; Cui, J.; Wang, Z.Q. Molecular characterization of Trichinella spiralis galectin and its participation in larval invasion of host’s intestinal epithelial cells. Vet. Res. 2018, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Kang, Y.J.; Jo, J.O.; Baek, K.W.; Yu, H.S.; Choi, Y.H.; Cha, H.J.; Ock, M.S. Effect of muscle strength by Trichinella spiralis infection during chronic phase. Int. J. Med. Sci. 2018, 15, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Dupouy-Camet, J.; Kociecka, W.; Bruschi, F.; Bolas-Fernandez, F.; Pozio, E. Opinion on the diagnosis and treatment of human trichinellosis. Expert Opin. Pharmacother. 2002, 3, 1117–1130. [Google Scholar] [PubMed]
- Gottstein, B.; Pozio, E.; Nöckler, K. Epidemiology, diagnosis, treatment and control of trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- Badagliacca, P.; Di Sabatino, D.; Salucci, S.; Romeo, G.; Cipriani, M.; Sulli, N.; Dall’Acqua, F.; Ruggieri, M.; Calistri, P.; Morelli, D. The role of the wolf in endemic sylvatic Trichinella britovi infection in the Abruzzi region of Central Italy. Vet. Parasitol. 2016, 231, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 2075/2005 of 5 December 2005 Laying Down Specific Rules on Official Controls for Trichinella in Meat. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:338:0060:0082:EN:PDF (accessed on 5 June 2019).
- Franssen, F.; Swart, A.; van der Giessen, J.; Havelaar, A.; Takumi, K. Parasite to patient: A quantitative risk model for Trichinella spp. pork and wild boar meat. Int. J. Food Microbiol. 2017, 241, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Regulation (EU) No 1375/2015 of 10 August 2015 Laying Down Specific Rules on Official Controls for Trichinella in Meat. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R1375&from=EN (accessed on 5 June 2019).
- Regulation (EC) No 882/2004 of the European Parliament and of the Council of 29 April 2004 on Official Controls Performed to Ensure the Verification of Compliance with Feed and Food Law, Animal Health and Animal Welfare Rules. Available online: http://www.cefas.co.uk/media/1550/extract_reg_no_882_2004.pdf (accessed on 5 June 2019).
- Zhai, P.; Wang, R.; Zhou, Y.; Hu, D.; Ii, J.; Zhou, Y. Enhancing the capabilities of biosafety laboratories through the established accreditation system: Development of the biosafety laboratory accreditation system in China. J. Biosaf. Biosecur. 2019. [Google Scholar] [CrossRef]
- Regulation (EC) No 765/2008 of the European Parliament and of the Council of 9 July 2008 Setting out the Requirements for Accreditation and Market Surveillance Relating to the Marketing of Products and Repealing Regulation (ECC) No 339/1993. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:218:0030:0047:EN:PDF (accessed on 5 June 2019).
- Ricci, U.; De Sanzo, C.; Carboni, I.; Iozzi, S.; Nutini, A.L.; Torricelli, F. Accreditation of a forensic genetics laboratory in Italy. Forensic Sci. Int.: Gen. Suppl. Ser. 2013, 4, e294–e295. [Google Scholar] [CrossRef]
- Ministry of Health. Esame Trichinoscopico Delle Carni di Suini Domestici—Deroga ai Sensi del Regolamento (UE) No. 1375/2015. Available online: https://www.anmvioggi.it/images/CIRCOLARE_DEL_MINISTERO_DELLA_SALUTE_copy_copy.pdf (accessed on 5 June 2019).
- Ministry of Health. Monitoraggio per il Controllo Delle Trichine ai Sensi Dell’articolo 11 del Regolamento (UE) No 1375/2015. Available online: http://www.ulss4.veneto.it/web/ulss4/Prevenzione/dfsa/normative/norme_nazionali/note_circolari_minsal/0_pagina_iniziale/all/2016/monit_contr_trichine_Reg_UE_2015_1375.pdf (accessed on 5 June 2019).
- Joint ILAC/ISO Communiqué on the Recognition of ISO/IEC 17025 during a Three-Year Transition, 01 November 2017. Available online: http://www.esyd.gr/pweb/s/20/files/anakoinoseis/Joint_ILAC-ISO_Com_-_ISO_IEC_17025_transition.pdf (accessed on 5 June 2019).
- Marucci, G.; Tonanzi, D.; Cherchi, S.; Galati, F.; Bella, A.; Interisano, M.; Ludovisi, A.; Amati, A.; Pozio, E. Proficiency testing to detect Trichinella larvae in meat in the European Union. Vet. Parasitol. 2016, 231, 145–149. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Trichinellosis. Recommendations for Quality Assurance in Digestion Testing Programs for Trichinella, ICT Quality Assurance Committee (Appendix 1) Part 3, 2012; pp. 1–9. Available online: http://www.trichinellosis.org/uploads/PART_3__final__-_PT_7Feb2012.pdf (accessed on 5 June 2019).
- EFSA Panels on Biological Hazards (BIOHAZ), on Contaminants in the Food Chain (CONTAM), and on Animal Health and Welfare (AHAW). Scientific Opinion on the public health hazards to be covered by inspection of meat (swine). EFSA J. 2011, 9, 2351. [Google Scholar]

| Process | Responsible | Input | Output | Activities |
|---|---|---|---|---|
| Customer management | QAM | Sample identification | Sample to be analyzed | Draft acceptance sheet |
| Supplying | TD | Stock management, calibration plan | Products and services | Selection and control of suppliers and products |
| Sampling | QAM | Competent authority | Laboratory | Sampling from carcasses and identification |
| Analysis | TD | Sample delivery | Test report | Application of analytical method |
| Result communication | TD | Test report | Customer and/or competent authority delivery | Registration of delivery and original copy |
| Resource management | TD | Maintenance, setting and operator qualification plan | Setting, training, proficiency testing, and quality control | Ordinary and special maintenance, setting of equipment, personal training |
| Revision | GD | Documents | Review report and improvement actions | Process performance analysis |
| Number | Title | Scope and Application |
|---|---|---|
| P1 | Protection of confidential information | The laboratory guarantees the confidentiality of customer data |
| P2 | Management of documentation of Quality Assurance System | The external or internal documents are identified, registered, and listed in a specific archive |
| P3 | Definition of the contract | The customers accept and sign the proposal of the contract with the Laboratory |
| P4 | Selection of suppliers | The Laboratory selects a specific list for qualified suppliers of reagents, instruments, and equipment and periodically updates it |
| P5 | Check of reagents and equipment | The Laboratory verifies the correct status of equipment and expiry date and the suitability for use of reagents (e.g., pepsine) for the analysis |
| P6 | Resolution of customers’ complaints | The customers’ complaints are registered in a database and analyzed in order to be solved by corrective actions |
| P7 | Management of non-compliance | If the Laboratory verifies a non-compliance, it prepares a document named “Risk Assessment” with corrective actions to be applied |
| P8 | Management of corrective and preventive actions | The Laboratory evaluates the cause of non-compliance and implements corrective/preventive actions in order to avoid the recurring of such event |
| P9 | Management of audit | The audits are conducted annually aiming at verifying if the Quality Assurance System is compliant with ISO/IEC 17025:2005 |
| P10 | Revision by General Direction | The General Direction makes an annual revision of the Quality Assurance System generally after the visit by ACCREDIA |
| P11 | Training of personnel | The Technical Direction guarantees the training of personnel and confirms its effectiveness |
| P12 | Monitoring and maintenance of environmental conditions | The procedures of sanitation are applied daily after the analysis and more accurately each month |
| P13 | Validation of analysis | The validation involves only the good practice of both equipment and technician because the applied method is already officially recognized |
| P14 | Control of data | The Technical Direction examines and signs the test report |
| P15 | Management of equipment | The Laboratory tests the equipment before the analysis in order to check its suitability |
| P16 | Management and setting of instruments | The Laboratory performs the setting of instruments when required |
| P17 | Sampling | The sampling is carried out by qualified personnel under the supervision of the competent authority |
| P18 | Handling and storage of sample | The Laboratory controls the integrity, temperature, and quantity of samples at their arrival |
| P19 | Quality Assurance | The Laboratory assures the quality of the analysis by using spiked meatballs as a reference certified material and participating in inter-laboratory tests |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirone, M.; Visciano, P.; Olivastri, A.M.A.; Sgalippa, M.P.; Paparella, A. Accreditation Procedure for Trichinella spp. Detection in Slaughterhouses: The Experience of an Internal Laboratory in Italy. Foods 2019, 8, 195. https://doi.org/10.3390/foods8060195
Schirone M, Visciano P, Olivastri AMA, Sgalippa MP, Paparella A. Accreditation Procedure for Trichinella spp. Detection in Slaughterhouses: The Experience of an Internal Laboratory in Italy. Foods. 2019; 8(6):195. https://doi.org/10.3390/foods8060195
Chicago/Turabian StyleSchirone, Maria, Pierina Visciano, Alberto Maria Aldo Olivastri, Maria Paola Sgalippa, and Antonello Paparella. 2019. "Accreditation Procedure for Trichinella spp. Detection in Slaughterhouses: The Experience of an Internal Laboratory in Italy" Foods 8, no. 6: 195. https://doi.org/10.3390/foods8060195
APA StyleSchirone, M., Visciano, P., Olivastri, A. M. A., Sgalippa, M. P., & Paparella, A. (2019). Accreditation Procedure for Trichinella spp. Detection in Slaughterhouses: The Experience of an Internal Laboratory in Italy. Foods, 8(6), 195. https://doi.org/10.3390/foods8060195






