Comparative Analysis of Lycopene Content from Different Tomato-Based Food Products on the Cellular Activity of Prostate Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Materials
2.2. Samples and Lycopene Extraction
2.3. High Performance Liquid Chromatography (HPLC) Analysis
2.4. Cell Culture Experiments
2.5. Cell Viability Assay
2.6. Cell Cycle Analysis
2.7. Apoptosis Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. HPLC Analysis
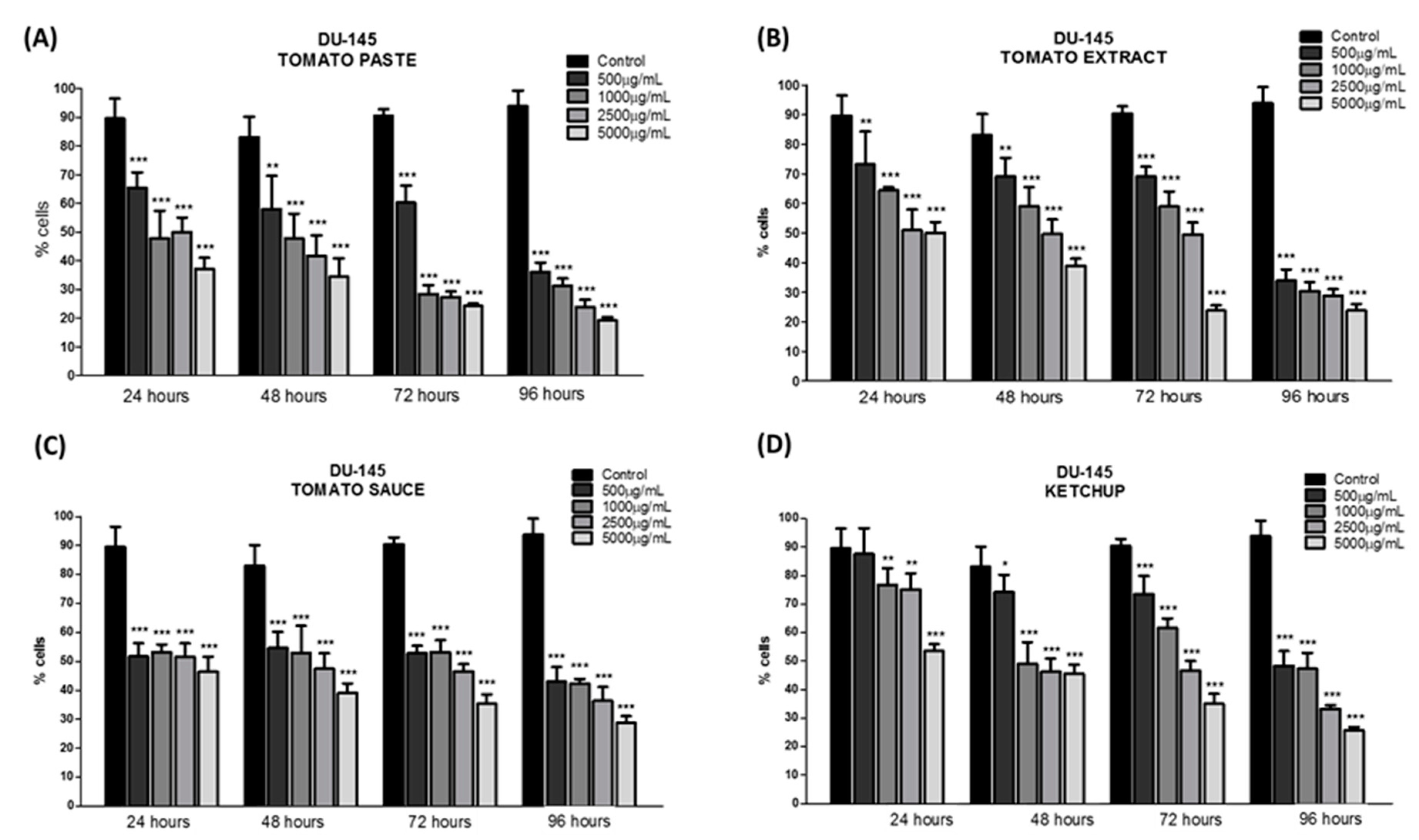
3.2. Effect of Lycopene Extracts on the Number of Viable Cells in Culture
3.3. Effect of Lycopene Extracts on Cell-Cycle Progression
3.4. Apoptosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Aronson, W.; Freedland, S.J. Nutrition, dietary interventions and prostate cancer: The latest evidence. BMC Med. 2015, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Askari, F.; Parizi, M.K.; Jessri, M.; Rashidkhani, B. Fruit and vegetable intake in relation to prostate cancer in Iranian men: A case–control study. Asian Pac. J. Cancer Prev. 2014, 15, 5223–5227. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mao, Q.; Cao, M.; Xie, L. Cruciferous vegetables intake and risk of prostate cancer: A meta-analysis. Int. J. Urol. 2012, 19, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Richman, E.L.; Carroll, P.R.; Chan, J.M. Vegetable and fruit intake after diagnosis and risk of prostate cancer progression. Int. J. Cancer 2012, 131, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, C.J.; Trumbo, P.R.; Ellwood, K.C. The U.S. Food and Drug Administration’s evidence-based review for qualified health claims: Tomatoes, lycopene, and cancer. J. Natl. Cancer Inst. 2007, 99, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Lu, Q.-Y. Overview of mechanism of action of lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, U.; Hussain, M.; Banerjee, M.; Seren, S.; Sarkar, F.H.; Fontana, J.; Forman, J.D.; Cher, M.L.; Powell, I.; Pontes, J.E.; et al. Lycopene and Soy Isoflavones in the Treatment of Prostate Cancer. Nutr. Cancer 2007, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef]
- Fielding, J.M.; Rowley, K.G.; Cooper, P.; O’Dea, K. Increases in plasma lycopene concentration after consumption of tomatoes cooked with olive oil. Asia Pac. J. Clin. Nutr. 2005, 14, 131–136. [Google Scholar]
- Vallverdú-Queralt, A.; Regueiro, J.; de Alvarenga, J.F.; Torrado, X.; Lamuela-Raventos, R.M. Carotenoid profile of tomato sauces: Effect of cooking time and content of extra virgin olive oil. Int. J. Mol. Sci. 2015, 16, 9588–9599. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The Potential Role of Lycopene for the Prevention and Therapy of Prostate Cancer: From Molecular Mechanisms to Clinical Evidence. Int. J. Mol. Sci. 2013, 14, 14620–14646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toma, R.B.; Frank, G.C.; Nakayama, K.; Tawfik, E. Lycopene content in raw tomato varieties and tomato products. J. Foodserv. 2008, 19, 127–132. [Google Scholar] [CrossRef]
- Olajire, A.A.; Ibrahim, A.O.; Adelowo-Imeokparia, F.E.; Abdul-Hammed, M.; Pak, J. Lycopene in tomato and tomato-based products: Levels and their contribution to dietary lycopene. Sci. Ind. Res. 2007, 50, 18–21. [Google Scholar]
- Tan, H.L.; Thomas-Ahner, J.M.; Grainger, E.M.; Wan, L.; Francis, D.M.; Schwartz, S.J.; Erdman, J.W., Jr.; Clinton, S.K. Tomato-based food products for prostate cancer prevention: What have we learned? Cancer Metastasis Rev. 2010, 29, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Gartner, C.; Stahl, W.; SiesLycopene, H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am. J. Clin. Nutr. 1997, 66, 116–122. [Google Scholar] [CrossRef]
- Giovannucci, E.; Rimm, E.B.; Liu, Y.; Stampfer, M.J.; Willett, W.C. A prospective study of tomato products, lycopene, and prostate cancer risk. J. Natl. Cancer Inst. 2002, 94, 391–398. [Google Scholar] [CrossRef]
- Hori, S.; Butler, E.; McLoughlin, J. Prostate cancer and diet: Food for thought? BJU Int. 2011, 107, 1348–1359. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Singh Negi, D.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and Risk of Prostate Cancer. A Systematic Review and Meta-Analysis. Medicine 2015, 94, E1260. [Google Scholar] [CrossRef]
- Venier, N.A.; Colquhoun, A.J.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Lycopene enhances the anti-proliferative and pro-apoptotic effects of capsaicin in prostate cancer in vitro. J. Cancer Ther. Res. 2012. [Google Scholar] [CrossRef]
- Lian, F.; Smith, D.E.; Ernst, H.; Russell, R.M.; Wang, X.D. Apo-10’-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis 2007, 28, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.A.; Elsen, A.C.; Zuniga, K.; Lindshield, B.L.; Erdman, J.W., Jr. Lycopene and apo-12′-lycopenal reduce cell proliferation and alter cell cycle progression in human prostate cancer cells. Nutr. Cancer 2011, 63, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Nahum, A.; Hirsch, K.; Danilenko, M.; Watts, C.K.; Prall, O.W.; Levy, J.; Sharoni, Y. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E-cdk2 complexes. Oncogene 2001, 20, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Kristal, A.R.; Till, C.; Platz, E.A.; Song, X.; King, I.B.; Neuhouser, M.L.; Ambrosone, C.B.; Thompson, I.M. Serum lycopene concentration and prostate cancer risk: Results from the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2011, 20, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.K.; Stroud, C.K.; Nakamura, M.T.; Lila, M.A.; Erdman, J.W., Jr. Serum testosterone is reduced following short-term phytofluene, lycopene, or tomato powder consumption in F344 rats. J. Nutr. 2006, 136, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Sharoni, Y.; Linnewiel-Hermoni, K.; Zango, G.; Khanin, M.; Salman, H.; Veprik, A.; Danilenko, M.; Levy, J. The role of lycopene and its derivatives in the regulation of transcription systems: Implications for cancer prevention. Am. J. Clin. Nutr. 2011, 96, 1173S–1178S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dhakal, I.; Stone, A.; Ning, B.; Greene, G.; Lang, N.P.; Kadlubar, F.F. Plasma carotenoids and prostate cancer: A population-based case-control study in Arkansas. Nutr. Cancer 2007, 59, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Zu, K.; Mucci, L.; Rosner, B.A.; Clinton, S.K.; Loda, M.; Stampfer, M.J.; Giovannucci, E. Dietary lycopene, angiogenesis, and prostate cancer: A prospective study in the prostate-specific antigen era. J. Natl. Cancer Inst. 2014, 106, djt430. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Stacewicz-Sapuntzakis, M.; Duncan, C.; Sharifi, R.; Ghosh, L.; van Breemen, R.; Ashton, D.; Bowen, P.E. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J. Natl. Cancer Inst. 2001, 93, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.C.P.; Teodoro, A.J.; Oliveira, F.L.; Santos, C.A.; Takiya, C.M.; Junior, O.S.; Bianco, M.; Junior, A.P.; Nasciutti, L.E.; Ferreira, L.B.; et al. Influence of lycopene on cell viability, cell cycle, and apoptosis of human prostate cancer and benign hyperplastic cells. Nutr. Cancer 2013, 65, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O.; Sarkar, F.H.; Djuric, Z.; Sakr, W.; Pollak, M.N.; Khachik, F.; Banerjee, M.; Bertram, J.S.; Wood, D.P., Jr. Effects of lycopene supplementation in patients with localized prostate cancer. Exp. Biol. Med. 2002, 227, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Tan, H.; Thomas-Ahner, J.M.; Pearl, D.K.; Erdman, J.W., Jr.; Moran, N.E.; Clinton, S.K. Dietary tomato and lycopene impact androgen signaling- and carcinogenesis-related gene expression during early TRAMP prostate carcinogenesis. Cancer Prev. Res. 2014, 7, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Sharifi, R.; Viana, M.; Pajkovic, N.; Zhu, D.; Yuan, L.; Yang, Y.; Bowen, P.E.; Stacewicz-Sapuntzakis, M. Antioxidant effects of lycopene in African American men with prostate cancer or benign prostate hyperplasia: A randomized, controlled trial. Cancer Prev. Res. 2011, 4, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Dini, L.I.; Koff, W.J. Perfil do câncer de próstata no hospital de clínicas de Porto Alegre. Rev. Assoc. Med. Bras. 2006, 52, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.K.; Beeson, W.L.; Phillips, R.L.; Fraser, G.E. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 1989, 64, 598–604. [Google Scholar] [CrossRef]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Hantz, H.L.; Young, L.F.; Martin, K.R. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp. Biol. Med. 2005, 230, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Fridman, J.S.; Yang, M.; Baranov, E.; Hoffman, R.M.; Lowe, S.W. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 2002, 1, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Erhardt, J.G.; Meisner, C.; Bode, J.C.; Bode, C. Lycopene, beta-carotene, and colorectal adenomas. Am. J. Clin. Nutr. 2003, 78, 1219–1224. [Google Scholar] [CrossRef]
- Nunes, I.L.; Mercadante, A.Z. Obtenção de cristais de licopeno a partir de descarte de tomate. Ciênc Tecnol Aliment Campinas 2004, 24, 440–447. [Google Scholar] [CrossRef]
- Pacheco, S.; Peixoto, F.; Borguini, R.G.; Nascimento, L.S.M.; Bobeda, C.R.R.; Santiago, M.C.P.A.; Godoy, R.L.O. Microscale extraction method for HPLC carotenoid analysis in vegetable matrices. Sci. Agric. 2014, 71, 416–419. [Google Scholar] [CrossRef] [Green Version]
- Barber, N.J.; Barber, J. Lycopene and prostate cancer. Prostate Cancer Prostatic Dis. 2002, 5, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Waliszewski, K.N.; Blasco, G. Propriedades nutraceúticas del licopeno. Salud Publica Mex. 2010, 52, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Boileau, A.C.; Merchen, N.R.; Wasson, K.; Atkinson, C.A.; Erdman, J.W., Jr. Cis-lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets. J. Nutr. 1999, 129, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Unlu, N.Z.; Bohn, T.; Francis, D.M.; Nagaraja, H.N.; Clinton, S.K.; Schwartz, S.J. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br. J. Nutr. 2007, 98, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W., Jr. How do nutritional and hormonal status modify the bioavailability, uptake, and distribution of different isomers of lycopene? J. Nutr. 2005, 135, 2046S–2047S. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Russett, M.D.; Handelman, G.J.; Snodderly, D.M. Structural and geometrical isomers of carotenoids in human plasma. J. Nutr. 1990, 120, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Von Doering, W.; Sotirious-Leventis, C.; Roth, W.R. Thermal interconversion among 15-cis-, 13-cis-, and all-trans-beta-carotene: Kinectics, Arrhenius parameters, thermochemistry, and potential relevance to anticarcinogenicity of all-trans-beta-carotene. J. Am. Chem. Soc. 1995, 117, 2747–2757. [Google Scholar] [CrossRef]
- Renju, G.L.; Muraleedhara, K.G.; Bandugula, V.R. Effect of lycopene isolated from Chlorella marina on proliferation and apoptosis in human prostate cancer cell line PC-3. Tumour Biol. 2014, 35, 10747–10758. [Google Scholar] [CrossRef]
- Prakash, P.; Russel, R.M.; Krinsky, N.I. In vitro inhibition ofproliferation of strogen-dependent and estrogen-independent human breast cancer cells treated with carotenoids or retinoids. Nutr. Cancer 2001, 131, 1574–1580. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Hadley, C.W.; Miller, E.C.; Schwartz, S.J.; Clinton, S.K. Tomatoes, lycopene, and prostate cancer: Progress and promise. Exp. Biol. Med. 2002, 227, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An Update on the Health Effects of Tomato Lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacewicz-Sapuntzakis, M.; Bowen, P.E. Role of lycopene and tomato products in prostate health. Biochim. Biophys. Acta 2005, 1740, 202–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacanli, M.; Bașaran, N.; Bașaran, A.A. Lycopene: Is it Beneficial to Human Health as an Antioxidant? Turk. J. Pharm. Sci. 2017, 14, 311–318. [Google Scholar] [CrossRef]
- Moran, N.E.; Erdman, J.W., Jr.; Clinton, S.K. Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch. Biochem. Biophys. 2013, 539, 171–180. [Google Scholar] [CrossRef] [Green Version]




| Sample | Total Carotenoids | Cis-Lycopene | Trans-Lycopene | Lycopene Content |
|---|---|---|---|---|
| (μg/g) | (μg/g) | (μg/g) | (%) | |
| Ketchup | 147.81 ± 8.35 a | 9.20 ± 0.89 a | 133.39 ± 6.64 a | 96.48 ± 0.36 a |
| Tomato Extract | 85.60 ± 1.09 b | 6.48 ± 0.88 b | 74.94 ± 1.73 b | 95.12 ± 0.22 b |
| Tomato Sauce | 168.95 ± 5.36 c | 7.40 ± 0.49 b | 155.94 ± 7.45 c | 96.65 ± 1.63 a,b,c |
| Tomato Paste | 77.57 ± 1.81 d | 5.05 ± 0.40 b | 70.80 ± 2.09 b | 97.78 ± 0.10 c |
| Compounds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell Type | Cell Death Stages | Untreated Cells (Control) | Tomato Paste | Tomato Extract | Tomato Sauce | Ketchup | ||||
| 500 μg/mL | 5000 μg/mL | 500 μg/mL | 5000 μg/mL | 500 μg/mL | 5000 μg/mL | 500 μg/mL | 5000 μg/mL | |||
| DU-145 | Viable cells | 93.33 ± 1.23 a | 89.10 ± 2.71 b | 64.30 ± 1.56 c | 89.36 ± 1.48 b | 70.93 ± 2.02 d | 88.45 ± 1.48 b | 78.45 ± 1.20 e | 91.32 ± 1.24 a | 89.80 ± 0.32 b |
| Early apoptosis | 0.49 ± 0.12 a | 2.32 ± 0.53 b | 13.20 ± 1.70 c | 2.12 ± 0.29 b | 8.71 ± 0.40 d | 3.56 ± 0.56 b | 6.65 ± 0.35 e | 1.14 ± 0.28 f | 2.90 ± 0.30 b | |
| Late apoptosis | 0.57 ± 0.14 a | 4.35 ± 1.09 b | 21.05 ± 0.64 c | 4.82 ± 0.54 b | 19.00 ± 0.85 c | 3.50 ± 0.34 b | 14.25 ± 0.64 d | 3.22 ± 0.46 b | 5.90 ± 0.25 e | |
| Necrosis | 5.61 ± 0.97 a | 4.23 ± 1.06 b | 1.45 ± 0.49 b | 3.70 ± 0.64 c | 1.36 ± 0.38 b | 4.49 ± 0.59 a | 0.65 ± 0.21 d | 4.32 ± 0.50 a | 1.40 ± 0.27 b | |
| PC-3 | Viable cells | 97.64 ± 0.08 a | 90.26 ± 0.23 b | 37.37 ± 0.04 c | 87.67 ± 0.89 b | 40.05 ± 0.49 d | 93.39 ± 0.04 b | 42.78 ± 0.37 c | 93.73 ± 0.37 b | 94.14 ± 0.18 b |
| Early apoptosis | 0.84 ± 0.07 a | 1.87 ± 0.15 b | 21.53 ± 0.33 c | 3.07 ± 0.22 d | 16.35 ± 0.35 e | 1.87 ± 0.20 b | 18.35 ± 0.35 e | 1.64 ± 0.28 b | 2.86 ± 0.29 d | |
| Late apoptosis | 0.82 ± 0.07 a | 5.54 ± 0.35 b | 39.00 ± 0.57 c | 8.63 ± 0.48 d | 42.52 ± 0.35 c | 2.55 ± 0.49 e | 38.15 ± 0.64 c | 3.58 ± 0.49 e | 2.36 ± 0.32 e | |
| Necrosis | 0.7 ± 0.06 a | 2.33 ± 0.39 b | 2.10 ± 0.28 b | 0.63 ± 0.19 a | 1.08 ± 0.21 c | 2.19 ± 0.25 b | 0.72 ± 0.21 a | 1.05 ± 0.16 a | 0.64 ± 0.13 a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, N.d.C.P.; Elias, M.d.B.; Machado, C.L.; Trindade, B.B.; Borojevic, R.; Teodoro, A.J. Comparative Analysis of Lycopene Content from Different Tomato-Based Food Products on the Cellular Activity of Prostate Cancer Cell Lines. Foods 2019, 8, 201. https://doi.org/10.3390/foods8060201
Soares NdCP, Elias MdB, Machado CL, Trindade BB, Borojevic R, Teodoro AJ. Comparative Analysis of Lycopene Content from Different Tomato-Based Food Products on the Cellular Activity of Prostate Cancer Cell Lines. Foods. 2019; 8(6):201. https://doi.org/10.3390/foods8060201
Chicago/Turabian StyleSoares, Nathalia da Costa Pereira, Monique de Barros Elias, Clara Lima Machado, Bruno Boquimpani Trindade, Radovan Borojevic, and Anderson Junger Teodoro. 2019. "Comparative Analysis of Lycopene Content from Different Tomato-Based Food Products on the Cellular Activity of Prostate Cancer Cell Lines" Foods 8, no. 6: 201. https://doi.org/10.3390/foods8060201
APA StyleSoares, N. d. C. P., Elias, M. d. B., Machado, C. L., Trindade, B. B., Borojevic, R., & Teodoro, A. J. (2019). Comparative Analysis of Lycopene Content from Different Tomato-Based Food Products on the Cellular Activity of Prostate Cancer Cell Lines. Foods, 8(6), 201. https://doi.org/10.3390/foods8060201