Effect of Different Processing Methods on the Accumulation of the Phenolic Compounds and Antioxidant Profile of Broomcorn Millet (Panicum miliaceum L.) Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Broomcorn Millet
2.2. Sample Preparation
2.3. Extraction of Millet Flour
2.4. Estimation of Total Phenolic Content
2.5. Determination of Total Flavonoid Content
2.6. Chromatographic Analysis of Phenolic Acids and Catechin
2.7. Antioxidant Capacity Analysis
2.7.1. DPPH Free Radical Scavenging Capacity
2.7.2. Ferric Reducing Antioxidant Power Assay
2.7.3. Phosphomolybdenum Method
2.7.4. Hydroxyl Radical Scavenging Capacity
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chethan, S.; Malleshi, N.G. Finger millet polyphenols: Optimization of extraction and the effect of pH on their stability. Food Chem. 2007, 105, 862–870. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MS. J. Functional Foods 2011, 3, 144–158. [Google Scholar] [CrossRef]
- Kumari, K.S.; Thayumanavan, B. Characterization of starches of proso, foxtail, barnyard, kodo and little millets. Plant Foods Human Nutr. 1998, 53, 47–56. [Google Scholar] [CrossRef]
- Denery-Papini, S.; Nicolas, Y.; Popineau, Y. Efficiency and limitation of immunochemical assays for the testing of gluten-free foods. J. Cereal Sci. 1999, 30, 121–131. [Google Scholar] [CrossRef]
- Nishizawa, N.; Sato, D.; Ito, Y.; Nagasawa, T.; Hatakeyama, Y.; Choi, M.R.; Choi, Y.Y.; Wei, Y.M. Effects of dietary protein of proso millet on liver injury induced by D-galactosamine in rats. Biosci. Biotech. Biochem. 2002, 66, 92–96. [Google Scholar] [CrossRef]
- Viswanath, V.; Urooj, A.; Malleshi, N.G. Evaluation of antioxidant and antimicrobial properties of finger millet polyphenols (Eleusine coracana). Food Chem. 2009, 114, 340–346. [Google Scholar] [CrossRef]
- Choi, Y.; Jeong, H.S.; Lee, J. Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chem. 2007, 103, 130–138. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Liu, R.H. Phenolic and carotenoid profiles and antiproliferative activity of foxtail millet. Food Chem. 2015, 174, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.H.; Rooney, L.W.; Earp, C.F. Tanin and phenol of sorghum. Cereal Foods World 1984, 29, 776–779. [Google Scholar]
- Shahidi, F.; Naczk, M. Phenolics in food and nutraceuticals. CRC Press: Boca Raton, FL, USA, 2004; pp. 1–82. [Google Scholar]
- Zhang, L.; Liu, R.; Niu, W. Phytochemical and antiproliferative activity of prosso millet. PLoS ONE 2014, 9, e104058. [Google Scholar]
- Chandrasekara, A.; Shahidi, F. The content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Chethan, S.; Sreerama, Y.N.; Malleshi, N.G. Mode of inhibition of finger millet malt amylases by the millet phenolics. Food Chem. 2008, 111, 187–191. [Google Scholar] [CrossRef]
- Hithamani, G.; Krishnapura, S. Effect of domestic processing on the polyphenol content and bioaccessibility in finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Chem. 2014, 164, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.A.R.; Miller, N.J.; Paganga, C. Structure antioxidant activity relationship of flavonoids and phenolic acids. Free Radicals Biol. Medicine 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Koshihara, Y.; Neichi, T.; Murota, S.; Lao, A.; Fujimoto, Y.; Tatsuno, T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochimica Biophysica Acta 1984, 792, 92–97. [Google Scholar]
- Wang, T.; He, F.; Cheng, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Functional Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Taylor, J.R.; Duodu, K.G. Effects of processing sorghum and millets on their phenolic phytochemicals and the implications of this to the health-enhancing properties of sorghum and millet food and beverage products. J. Sci. Food Agri. 2015, 95, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Han, F.; Ding, Z.; Fan, L. Effect of different processing method on the antioxidant activity of 6 cultivars of foxtile millet. J. Food Quality 2017. [Google Scholar] [CrossRef]
- Hotz, C.; Gibson, R.S. Traditional food processing and preparation practives to ehnhaces the bioavilaibity of micronutrients in plant based diets. J. Nutri. 2007, 37, 1097–1100. [Google Scholar] [CrossRef]
- Geetha, R.; Mishra, H.N.; Srivastav, P.P. Twin screw extrusion of Kodo millet-chickpea been: process parameter optimization, physicochemical and functional properties. J. Food Sci. Technol. 2014, 51, 3144–3153. [Google Scholar] [CrossRef]
- Fei, H.; Lu, Z.; Wenlong, D.; Aike, L. Effect of roasting on phenolics content and antioxidant activity of proso millet. Inter. J. Food Eng. 2018, 4, 110–116. [Google Scholar] [CrossRef]
- Deprez, S.; Mila, I.; HUneau, J.; Tome, D.; Scalbert, A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid. Redox. Signaling 2001, 3, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Towo, E.E.; Svanberg, U.; Ndossi, G.D. Effect of grain pre-treatment on different extractable phenolic groups in cereals and legumes commonly consumed in Tanzania. J. Sci. Food Agri. 2003, 83, 980–986. [Google Scholar] [CrossRef]
- Zielinski, H.; Michalska, A.; Piskula, M.K.; Kozlowska, H. Antioxidants in thermally treated buckwheat groats. Mole. Nutri. Food Res. 2006, 50, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, S.R.; Guha, M. Effect of processing methods on the nutraceutical and antioxidant properties of little millet (Panicum sumatrense) extracts. Food Chem. 2014, 126, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–458. [Google Scholar]
- Ghimeray, A.K.; Sharma, P.; Phoutaxay, P.; Salitxay, T.; Woo, S.H.; Park, S.U.; Park, C.H. Far infrared irradiation alters total polyphenol, total flavonoid, antioxidant property and quercetin production in Tartary buckwheat sprout powder. J. Cereal Sci. 2014, 59, 167–172. [Google Scholar] [CrossRef]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 1, 361–368. [Google Scholar] [CrossRef]
- Braca, A.; Fico, G.; Morelli, I.; Simone, F.; Tome, F.; Tommasi, N. Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species. J. Ethnopharma. 2003, 86, 63–678. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura, C.F. Antioxidant activity of dietary polyphenols as determined by modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Analyt. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Guttridge, J.M.C.; Aruoma, O.I. The deoxyribose method: a simple test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Bio. 1987, 165, 215–162. [Google Scholar] [CrossRef]
- Gallegos-Infante, J.A.; Rocha-Guzman, N.E.; Gonzalez-Laredo, R.F.; Pulido-Alonso, J. Effect of processing on the antioxidant properties of extracts from Mexican barley (Hordeum vulgare) cultivar. Food Chem. 2010, 119, 903–906. [Google Scholar] [CrossRef]
- Duenas, M.; Hernandez, T.; Estrella, I.; Fernandez, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Sartelet, H.; Serghat, S.; Lobstein, A.; Ingenbleek, Y.; Anton, R.; Petitfrère, E.; Aguie-Aguie, G.; Martiny, L.; Haye, B. Flavonoids extracted from fonio millet (Digitaria exilis) reveal potent antithyroid properties. Nutrition 1996, 12, 100–106. [Google Scholar] [CrossRef]
- Gaitan, E. Goitrogens in food and water. Annual Review Nutri. 1990, 10, 21–39. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.W.; Kim, B.; Kim, H.T.; Ko, J.M.; Baek, I.-Y.; Seo, W.T.; Kang, Y.M.; Cho, K.M. Changes in phenolic compounds (isoflavones and phenolic acids) and antioxidant properties in high-protein soybean (Glycine max L., cv. Saedanbaek) for different roasting conditions. J. Korean Soc. Appl. Bi. Chem. 2013, 56, 605–612. [Google Scholar] [CrossRef]
- Zielinski, H.; Anna, M.; Miryam, A.B.; Mari, D.C.; Mariusz, K.P. Changes in protein quality and antioxidant properties of buckwheat seeds and groats induced by roasting. J. Agri. Food Chem. 2009, 57, 4771–4776. [Google Scholar] [CrossRef]
- Gujral, H.S.; Sharma, P.; Sharma, R. Antioxidant properties of sand roasted and steam cooked Bengal gram (Cicer arietinum). Food Sci. Biotech. 2013, 22, 183–188. [Google Scholar] [CrossRef]
- Siah, S.; Konczak, I.; Wood, J.A.; Agboola, S.; Blanchard, C.L. Effects of roasting on phenolic composition and in vitro antioxidant capacity of Australian grown faba beans (Vicia faba L.). Plant Foods Human Nutri. 2014, 69, 85–91. [Google Scholar] [CrossRef]
- Duarte-Almeida, J.M.; Negri, G.; Salatino, A.; de Carvalho, J.E.; Lajolo, F.M. Anti-proliferative and antioxidant activities of an acylated tricin glycocide from sugarcane (Saccharum officinarum) juice. J. Phytochem. 2007, 68, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, V.S.; Sareen, N.; Daniel, M.; Gallego, E.B. Flavonoids and phenolic acids from pearl millet (Pennisetum glaucum) based foods and their functional implications. Func. Foods Health Disease 2012, 2, 251–264. [Google Scholar] [CrossRef]
- Kampa, M.; Alexaki, V.I.; Notas, G.; Nifli, A.P.; Nistikaki, A. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004, 6, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Dykes, L.; Gu, L.W.; Rooney, L.W.; Prior, R.L. Processing of sorghum (Sorghum bicolor) and sorghum products alters procyanidin oligomer and polymer distribution and content. J. Agric. Food Chem. 2003, 51, 5516–5521. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Sonia, C.; Dino, M.; Maria, C.N.; Carlo, R.L. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Techno. 2000, 11, 340–346. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufian-Henares, J.A.; Morales, F.J. Study of fluorescence of Maillard reaction compounds in breakfast cereals. Mol. Nutr. Food Res. 2006, 50, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sha, X.; Rahman, E.; Wang, Y.; Ji, B.; Wu, W.; Zhou, F. Antioxidant capacity and amino acid profile of millet bran wine and the synergistic interaction between major polyphenols. J. Food Sci. Tech. 2018, 55, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.O.K.; Piao, J.P.; Park, C.H.; Cho, D.H. Far infrared irradiation enhances nutraceutical compounds and antioxidant properties in Angelica gigas Nakai powder. Antioxidants 2018, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Vaher, M.; Matso, K.; Levandi, T.; Helmja, K.; Kaljurand, M. Phenolic compounds and the antioxidant activity of the bran, flour and whole grain of different wheat varieties. Procedia Chem. 2010, 2, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Ivanisova, E.; Ondrejovic, M.; Silhar, S. Antioxidant activity of milling fractions of selected cereals. Nova Biotechnologica et Chimica 2012, 11, 45–56. [Google Scholar] [CrossRef]
- John, J.A.; Shahidi, F. Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). J. Funct. Foods. 2010, 2, 196–209. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S. Antioxidant and polyphenols oxidase activity of germinated barley and its milling fractions. Food Chem. 2010, 120, 673–678. [Google Scholar] [CrossRef]
- Rocha-Guzmán, N.E.; González-Laredom, R.F.; Ibarra-Pérez, F.J.; Nava-Berúmen, C.A.; Gallegos-Infante, J.A. Effect of pressure cooking on the antioxidant activity of extracts from three common bean (Phaseolus vulgaris L.) cultivars. Food Chem. 2007, 100, 31–35. [Google Scholar]
- Chandrasekara, N.; Shahidi, F. Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and testa. J. Agr. Food Chem. 2009, 59, 5006–5014. [Google Scholar] [CrossRef] [PubMed]
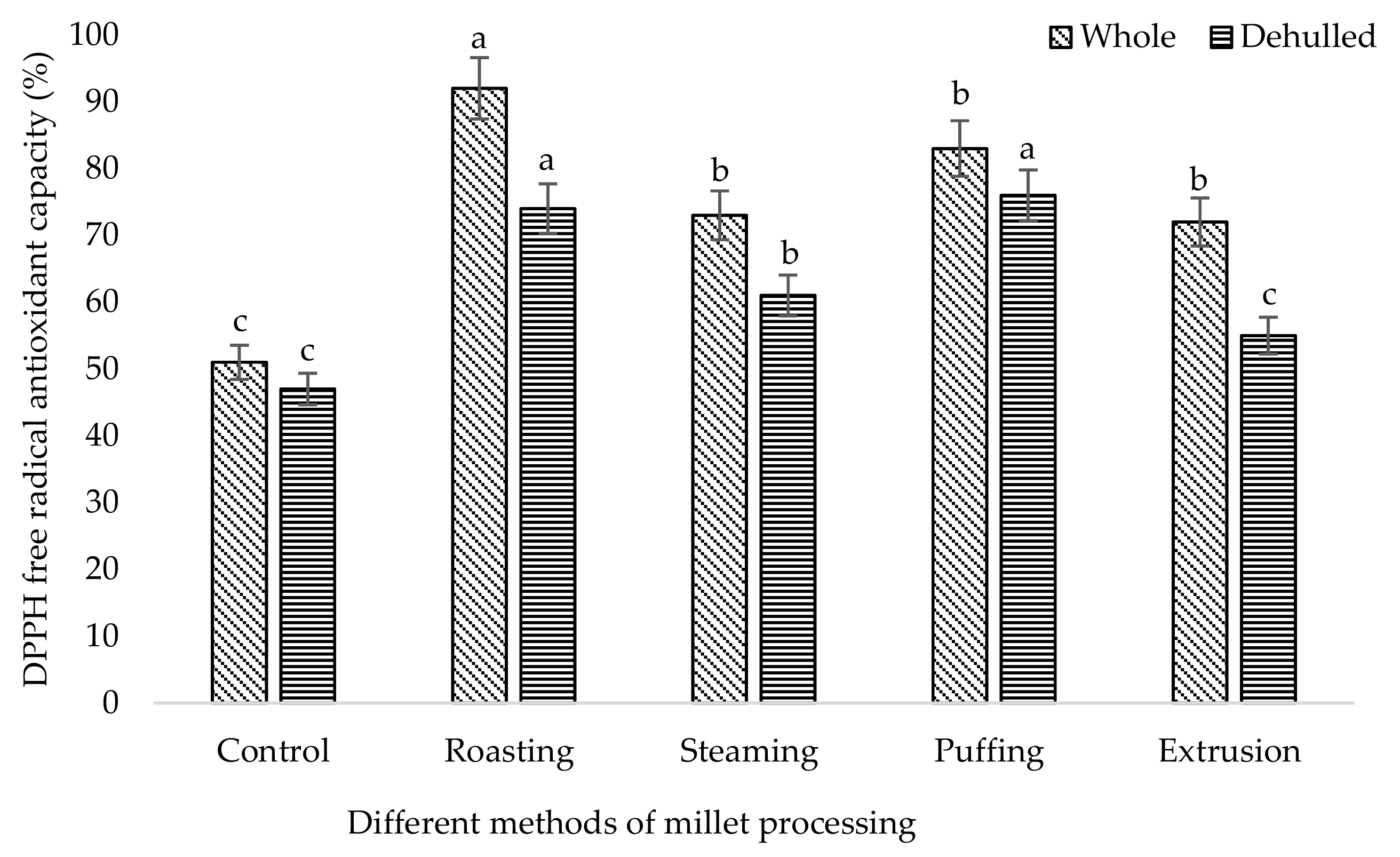
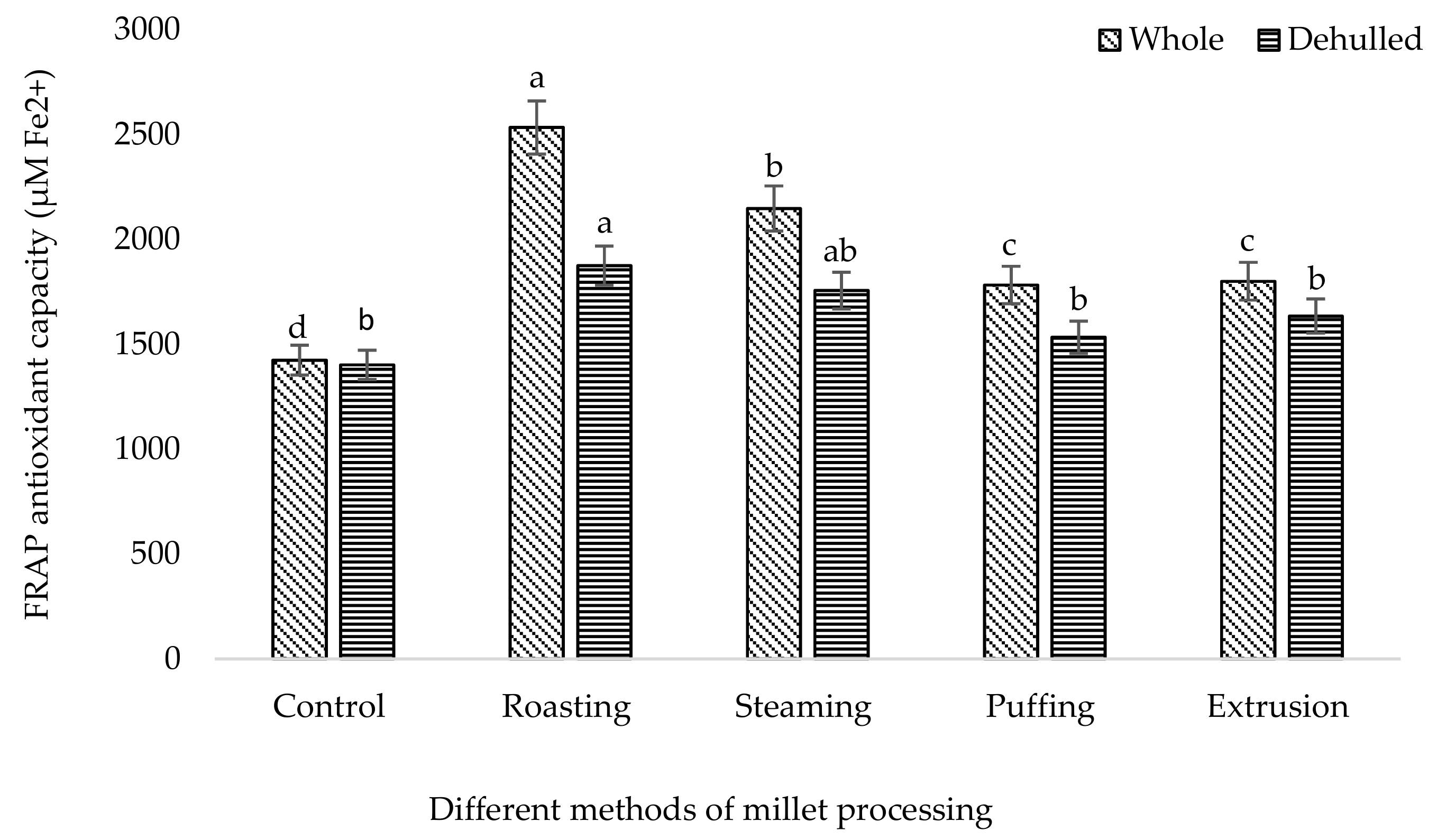
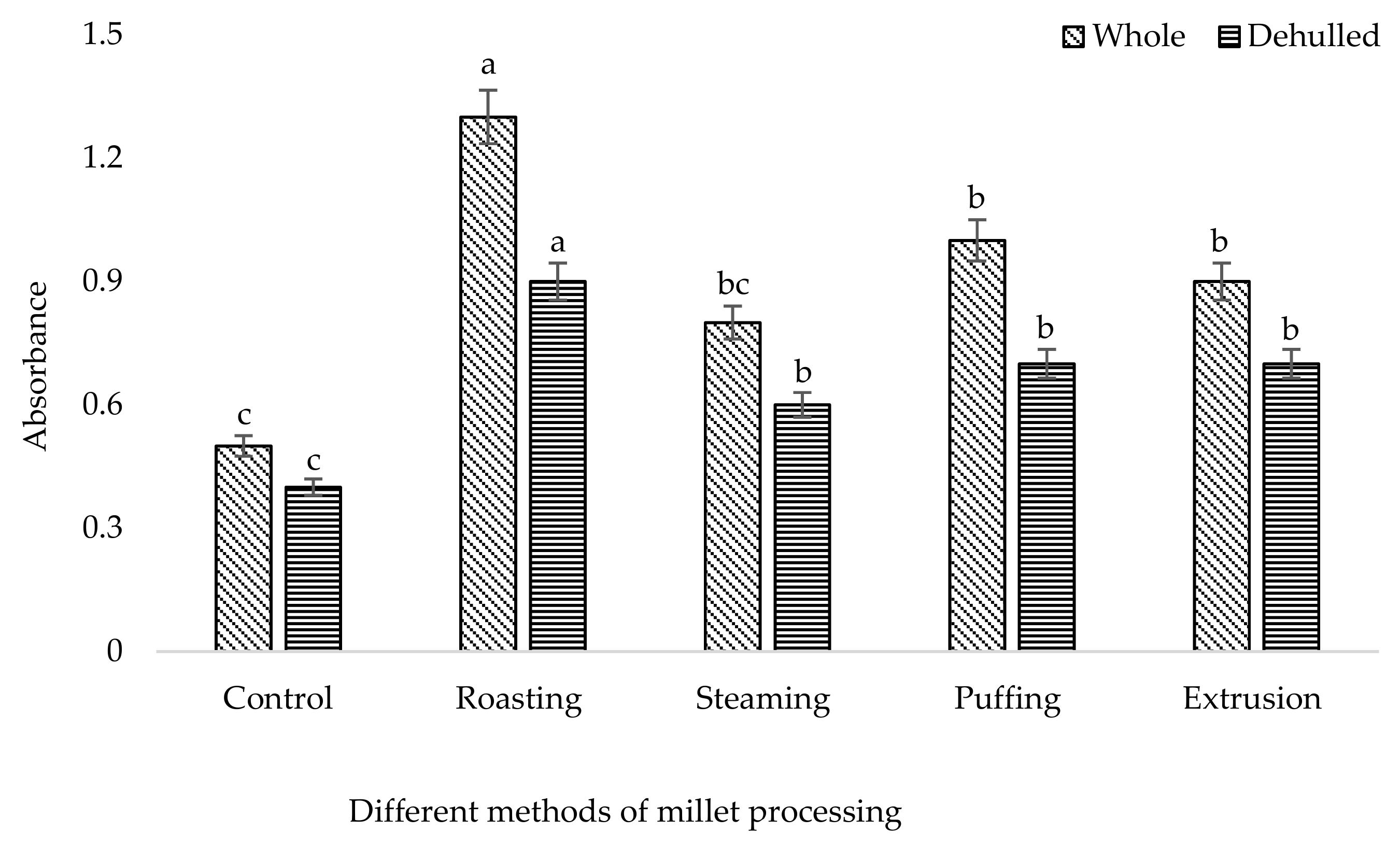
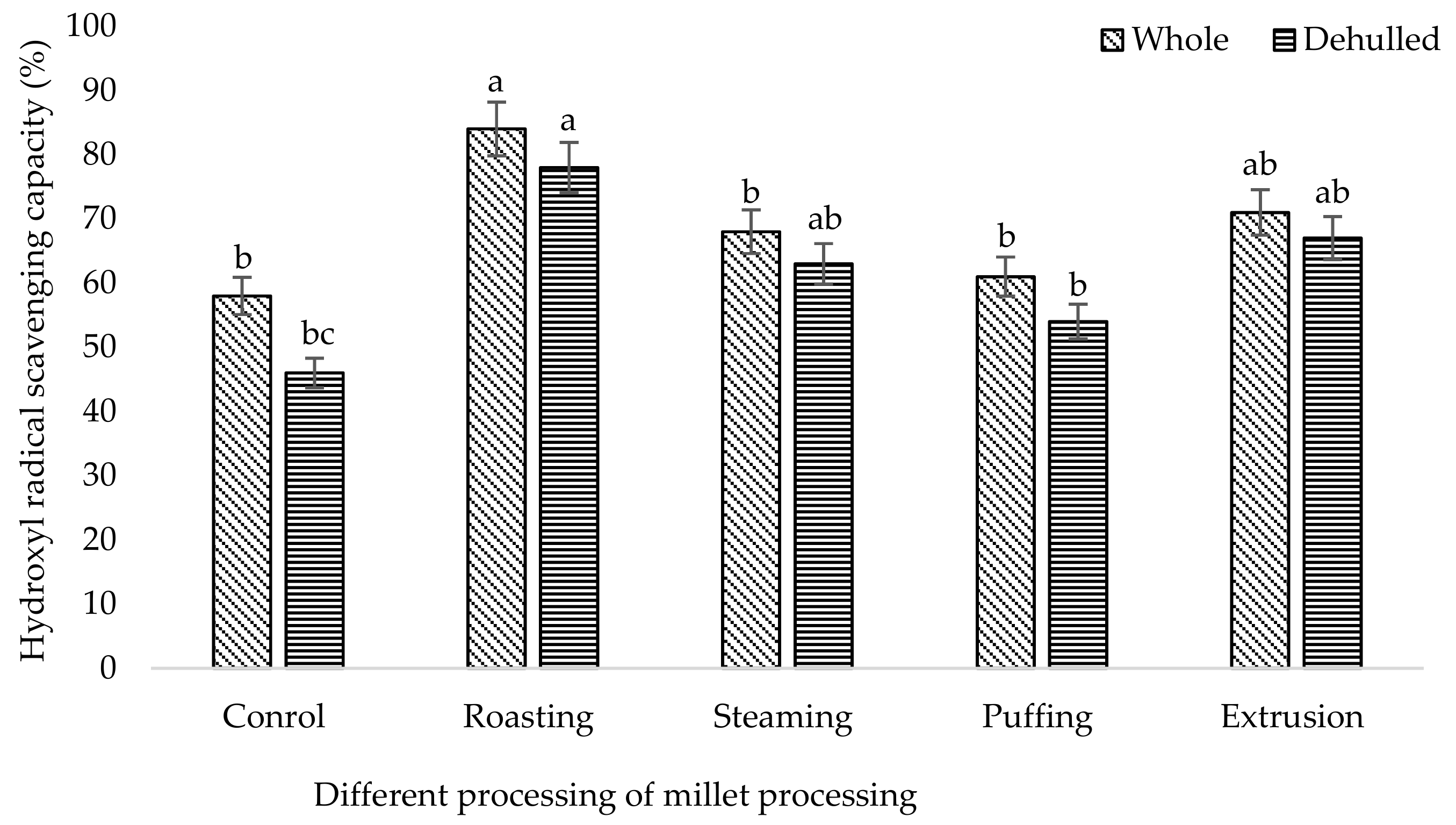
| Treatments | Total Phenolic Content (mg/100 g Ferulic Acid Equi.) dw | Total Flavonoid Content (mg/100 g Rutin Equi.) dw | ||
|---|---|---|---|---|
| Whole | Dehulled | Whole | Dehulled | |
| Control | 295 ± 2.34 d | 167 ± 1.23 e | 183 ± 3.57 c | 154 ± 4.53 d |
| Roasting | 670 ± 1.57 a | 587 ± 1.87 a | 391 ± 3.26 a | 301 ± 5.31 a |
| Steaming | 315 ± 3.48 d | 274 ± 2.14 d | 282 ± 2.58 b | 212 ± 5.21 c |
| Puffing | 645 ± 2.35 b | 547 ± 2.56 a | 304 ± 4.25 b | 256 ± 3.25 b |
| Extrusion | 455 ± 1.64 c | 315 ± 1.26 c | 219 ± 3.68 bc | 167 ± 5.14 d |
| Treatments | Hydroxybenzoic Acid Derivatives | Hydroxycinnamic Acid Derivatives | Flavonoid (Condensed Tannin) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Syringic Acid (μg/100 g) | Gallic Acid (µg/100 g) | 4 Hydroxy Benzoic Acid (µg/100 g) | Ferulic Acid (µg/100 g) | Sinapic Acid (µg/100 g) | Catechin (µg/100 g) | |||||||
| Whole | Dehulled | Whole | Dehulled | Whole | Dehulled | Whole | Dehulled | Whole | Dehulled | Whole | Dehulled | |
| Control | 11.27 ± 2.7 c | 5.34 ± 0.8 d | 9.37 ± 4.1 c | 11.1 ± 0.8 c | 9.11 ± 3.4 c | 6.87 ± 0.4 c | 68.63± 9.4 c | 38.36 ± 1.8 d | 9.32 ± 1.9 d | 12.87 ± 3.5 d | 88.48 ± 12.3 b | 90.7 ± 11.4 a |
| Roasting | 53.71 ± 2.2 a | 32.45 ± 5.9 b | 62.34 ± 3.4 a | 25.72 ± 4.3 b | 54.19 ± 5.1 a | 24.11 ± 4.6 a | 118.79 ± 13.7 a | 94 ± 5.4 a | 73.25 ± 11.8 a | 56.2 ± 8.2 a | 134.24 ± 11.3 a | 94 ± 7.9 a |
| Steaming | 49.03 ± 6.5 a | 56.37 ± 6.6 a | 39.86 ± 7.7 b | 39 ± 7.1 a | 8.1 ± 3.5 c | 6.9 ± 0.5 b | 57.37 ± 6.3 c | 47.75 ± 4.6 c | 43 ± 9.2 b | 29 ± 7.4 b | 52.94 ± 13.7 c | 88 ± 13.7 b |
| Puffing | 31.42 ± 3.19 b | 34.6 ± 5.7 b | 28.69 ± 5.8 b | 23.06 ± 6.4 b | 49.76 ± 3.7 a | 27.16 ± 7.3 a | 88.6 ± 13.7 b | 93.47 ± 9.2 a | 27 ± 6.3 c | 19 ± 3.7 c | 104.4 ± 14.8 b | 73.3 ± 15.3 b |
| Extrusion | 28.21 ± 2.4 b | 19.01 ± 7.3 c | 39.29 ± 3.4 b | 23.62 ± 6.2 b | 23.63 ± 6.8 b | 6.52 ± 7.9 b | 79.22 ± 8.3 b | 63 ± 9.3 b | 22 ± 3.8 c | 17 ± 2.8 c | 84.78 ± 9.7 b | 98.48 ± 11.7 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalam Azad, M.O.; Jeong, D.I.; Adnan, M.; Salitxay, T.; Heo, J.W.; Naznin, M.T.; Lim, J.D.; Cho, D.H.; Park, B.J.; Park, C.H. Effect of Different Processing Methods on the Accumulation of the Phenolic Compounds and Antioxidant Profile of Broomcorn Millet (Panicum miliaceum L.) Flour. Foods 2019, 8, 230. https://doi.org/10.3390/foods8070230
Kalam Azad MO, Jeong DI, Adnan M, Salitxay T, Heo JW, Naznin MT, Lim JD, Cho DH, Park BJ, Park CH. Effect of Different Processing Methods on the Accumulation of the Phenolic Compounds and Antioxidant Profile of Broomcorn Millet (Panicum miliaceum L.) Flour. Foods. 2019; 8(7):230. https://doi.org/10.3390/foods8070230
Chicago/Turabian StyleKalam Azad, Md Obyedul, Da In Jeong, Md Adnan, Timnoy Salitxay, Jeong Won Heo, Most Tahera Naznin, Jung Dae Lim, Dong Ha Cho, Byoung Jae Park, and Cheol Ho Park. 2019. "Effect of Different Processing Methods on the Accumulation of the Phenolic Compounds and Antioxidant Profile of Broomcorn Millet (Panicum miliaceum L.) Flour" Foods 8, no. 7: 230. https://doi.org/10.3390/foods8070230
APA StyleKalam Azad, M. O., Jeong, D. I., Adnan, M., Salitxay, T., Heo, J. W., Naznin, M. T., Lim, J. D., Cho, D. H., Park, B. J., & Park, C. H. (2019). Effect of Different Processing Methods on the Accumulation of the Phenolic Compounds and Antioxidant Profile of Broomcorn Millet (Panicum miliaceum L.) Flour. Foods, 8(7), 230. https://doi.org/10.3390/foods8070230








