The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. PEF Treatement
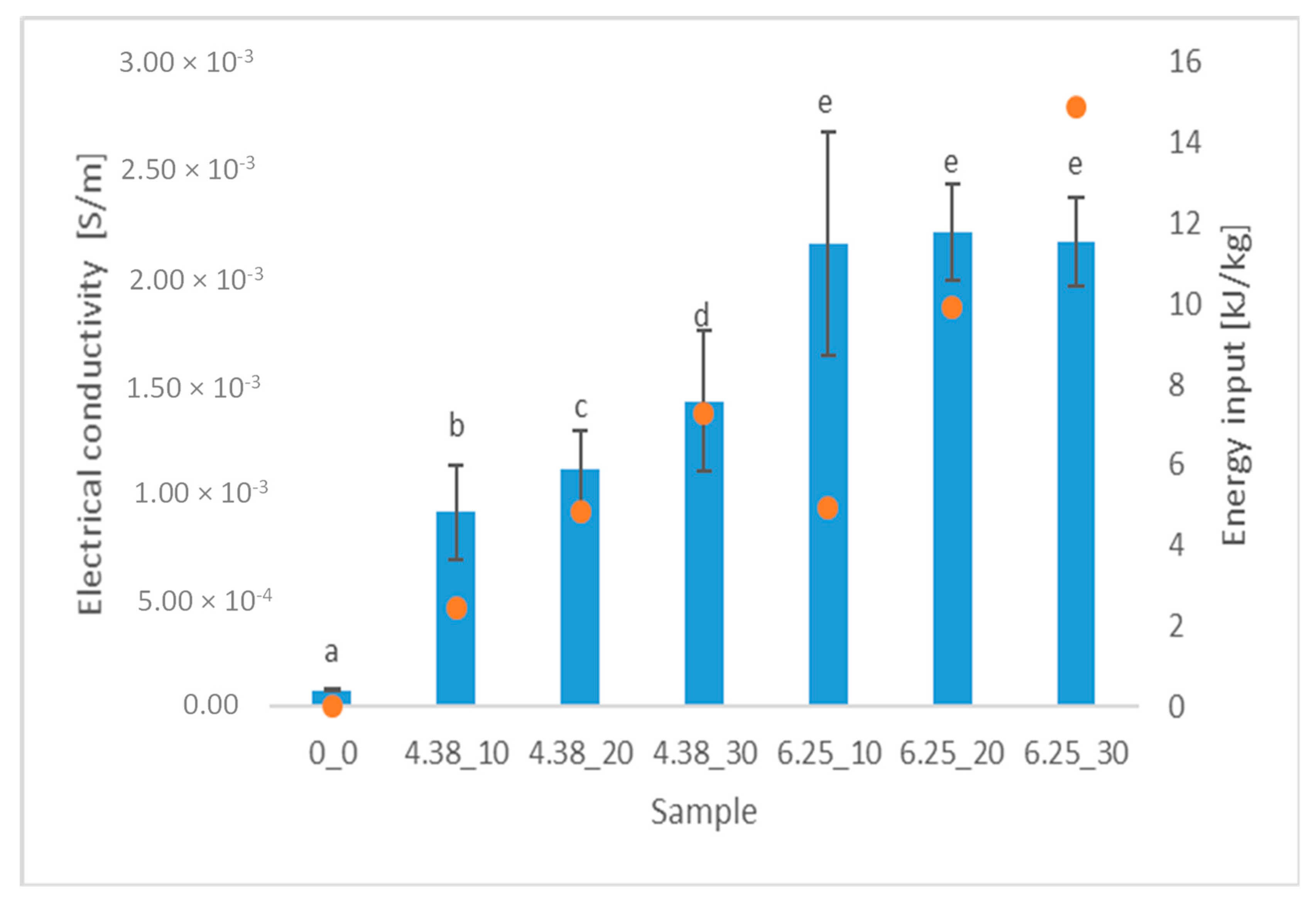
2.3. Electrical Conductivity
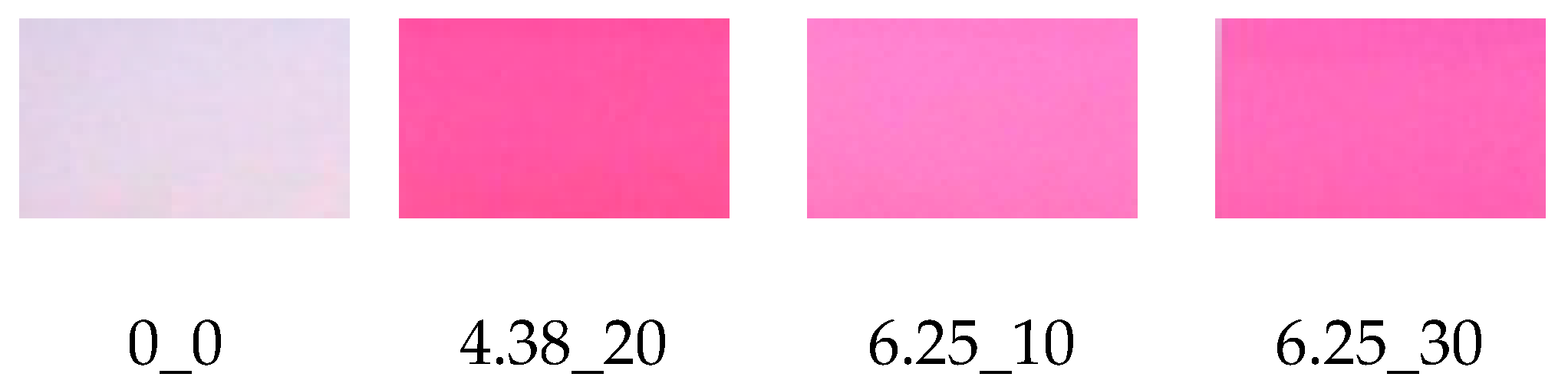
2.4. Color Determination
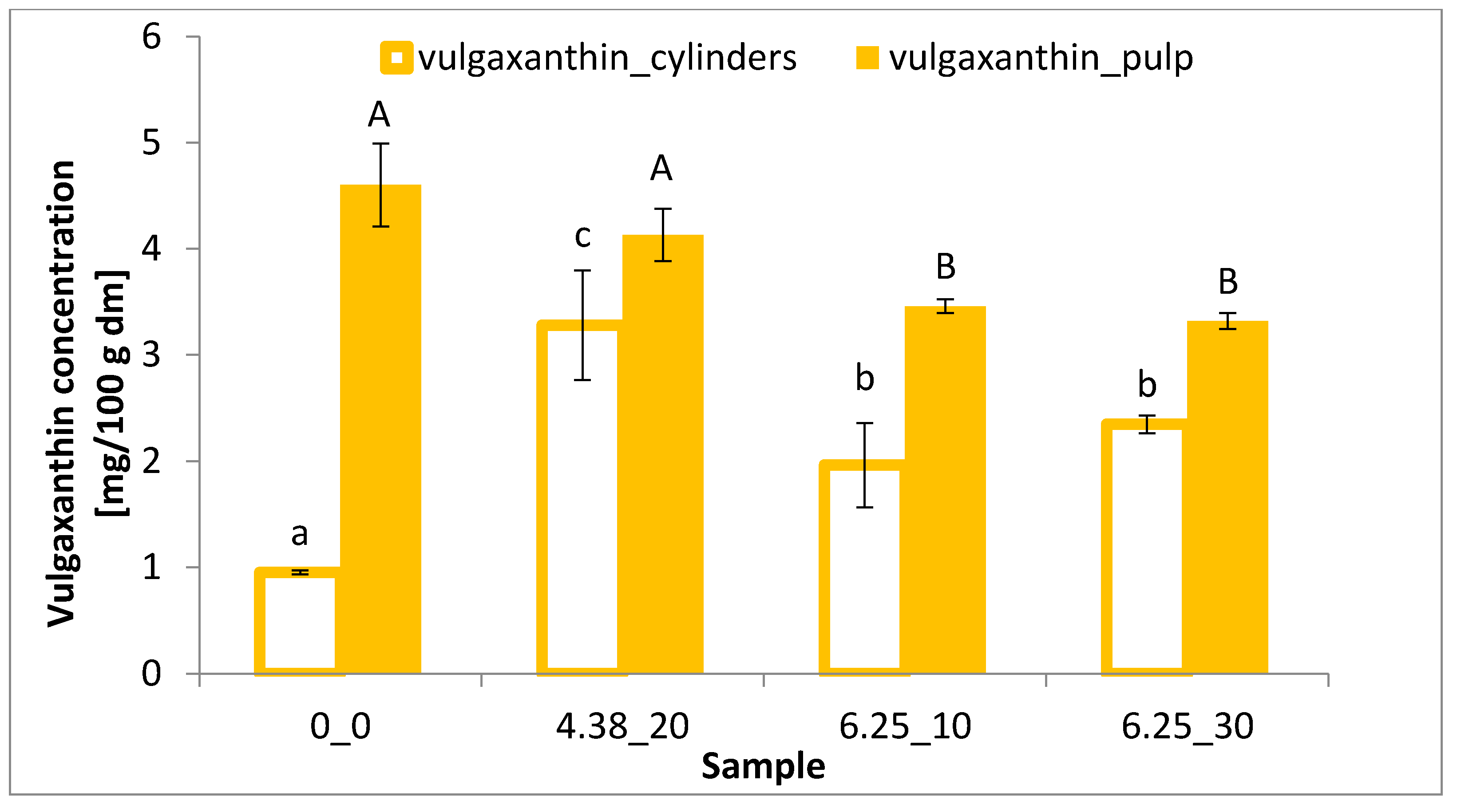
2.5. Betalain Content
2.6. Statitical Analysis—PCA and Pearson’s Correlation
3. Results and Discussion
3.1. Electical Conductivity
3.2. Color Determination
3.3. Betalain Content
3.4. HCA and Pearson’s Correlation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Slavov, A.; Karagyozov, V.; Denev, P.; Kratchanova, M.; Kratchanov, C. Antioxidant activity of red beet juices obtained after microwave and thermal pretreatments. Czech J. Food Sci. 2013, 31, 139–147. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT—Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Szopinska, A.A.; Gawęda, M. Comparison of yield and quality of red beet roots cultivated using conventional, integrated and organic method. J. Hort. Res. 2013, 21, 107–114. [Google Scholar] [CrossRef]
- Tanabtabzadeh, M.S.; Javanbakht, V.; Golshirazi, A.H. Extraction of Betacyanin and Betaxanthin Pigments from Red Beetroots by Chitosan Extracted from Shrimp Wastes. Waste Biomass Valoriz. 2019, 10, 641–653. [Google Scholar] [CrossRef]
- Celli, G.B.; Brooks, M.S.-L. Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins—A current review. Food Res. Int. 2017, 100, 501–509. [Google Scholar] [CrossRef]
- Singh, A.; Ganesapillai, M.; Gnanasundaram, N. Optimizaton of extraction of betalain pigments from beta vulgaris peels by microwave pretreatment. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E.; Ballard, T.; Liceaga, A.; San Martín-González, M.F. Microwave-assisted extraction of betalains from red beet (Beta vulgaris). LWT—Food Sci. Technol. 2014, 59, 276–282. [Google Scholar] [CrossRef]
- Ramli, N.S.; Ismail, P.; Rahmat, A. Influence of conventional and ultrasonic-assisted extraction on phenolic contents, betacyanin contents, and antioxidant capacity of red dragon fruit (Hylocereus polyrhizus). The Sci. World J. 2014, 2014, 964731. [Google Scholar] [CrossRef]
- Laqui-Vilca, C.; Aguilar-Tuesta, S.; Mamani-Navarro, W.; Montaño-Bustamante, J.; Condezo-Hoyos, L. Ultrasound-assisted optimal extraction and thermal stability of betalains from colored quinoa (Chenopodium quinoa Willd) hulls. Ind. Crop. Prod. 2018, 111, 606–614. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Dalla Rosa, M. Effect of pulsed electric field (PEF) pre-treatment coupled with osmotic dehydration on physico-chemical characteristics of organic strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Chudoba, T.; Lojkowski, W.; Witrowa-Rajchert, D. The impact of pulsed electric field treatment on selected bioactive compound content and color of plant tissue. Innov. Food Sci. Emerg. Technol. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N.I. Enhanced extraction from solid foods and biosuspensions by pulsed electrical energy. Food Eng. Rev. 2010, 2, 95–108. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Fincan, M.; DeVito, F.; Dejmek, P. Pulsed electric field treatment for solid-liquid extraction of red beetroot pigment. J. Food Eng. 2004, 64, 381–388. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Raso, J.; Alvarez, I. Enhancement of the extraction of betanine from red beetroot by pulsed electric fields. J. Food Eng. 2009, 90, 60–66. [Google Scholar] [CrossRef]
- Luengo, E.; Martinez, J.M.; Álvarez, I.; Raso, J. Comparison of the efficacy of pulsed electric fields treatments in the millisecond and microsecond range for the extraction of betanine from red beetroot. In Proceedings of the 1st World Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine and Food & Environmental Technologies (WC 2015), Portorož, Slovenia, 6–10 September 2015; pp. 375–378. [Google Scholar]
- Chalermchat, Y.; Fincan, M.; Dejmek, P. Pulsed electric field treatment for solid-liquid extraction of red beetroot pigment: mathematical modelling of mass transfer. J. Food Eng. 2004, 64, 229–236. [Google Scholar] [CrossRef]
- Wiktor, A.; Iwaniuk, M.; Śledź, M.; Nowacka, M.; Chudoba, T.; Witrowa-Rajchert, D. Drying kinetics of apple tissue treated by pulsed electric field. Dry Technol. 2013, 31, 112–119. [Google Scholar] [CrossRef]
- Fijalkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The physical, optical and reconstitution properties of apples subjectes to ultrasound before drying. Ital. J. Food Sci. 2017, 29, 343–356. [Google Scholar]
- Fijalkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The influence of ultrasound pre-treatment on drying kinetics and the colour and betalains content in beetroot. Zeszyty Problemowe Postępów Nauk Rolniczych 2015, 581, 11–20. [Google Scholar]
- Lebovka, N.; Bazhal, M.; Vorobiev, E. Estimation of characteristic damage time of food materials in pulsed electric fields. J. Food Eng. 2002, 54, 337–346. [Google Scholar] [CrossRef]
- Tylewicz, U.; Aganovic, K.; Vannini, M.; Toepfl, S.; Bortolotti, V.; Dalla Rosa, M.; Oey, I.; Heinz, V. Effect of pulsed electric field treatment on water distribution of freeze-dried apple tissue evaluated with DSC and TD-NMR techniques. Innov. Food Sci. Emerg. Technol. 2016, 37, 352–358. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Bazhal, M.I.; Vorobiev, E. Simulation and experimental investigation of food material breakage using pulsed electric field treatment. J. Food Eng. 2000, 44, 213–223. [Google Scholar] [CrossRef]
- Bazhal, M.; Lebovka, N.; Vorobiev, E. Optimisation of pulsed electric field strength for electroplasmolysis of vegetable tissues. Biosyst. Eng. 2003, 86, 339–345. [Google Scholar] [CrossRef]
- Puértolas, E.; Saldaña, G.; Raso, J. Pulsed electric field treatment for fruit and vegetable processing. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer International Publishing AG: Basel, Switzerland, 2017. [Google Scholar]
- Manchali, S.; Murthy, K.N.C.; Nagaraju, S.; Neelwarne, B. Stability of Betalain Pigments of Red Beet. In Red Beet Biotechnology: Food and Pharmaceutical Applications; Neelwarne, B., Ed.; Springer Science and Business Media: New York, NY, USA, 2013. [Google Scholar]
- Donsì, F.; Ferrari, G.; Pataro, G. Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Eng. Rev. 2010, 2, 109–130. [Google Scholar] [CrossRef]
- Canatella, P.J.; Karr, J.F.; Petros, J.A.; Prausnitz, M.R. Quantitative study of electroporation-mediated molecular uptake and cell viability. Biophys. J. 2001, 80, 755–764. [Google Scholar] [CrossRef]
- Antigo, J.L.D.; Bergamasco, R.D.C.; Madrona, G.S. Effect of pH on the stability of red beet extract (Beta vulgaris L.) microcapsules produced by spray drying or freeze drying. Food Sci. Technol. 2018, 38, 72–77. [Google Scholar] [CrossRef]


| Sample Code | Electric Field Intensity (kV/cm) | Pulse Number (-) | Energy (kJ/kg) |
|---|---|---|---|
| 0_0 | 0 | 0 | 0 |
| 4.38_10 | 4.38 | 10 | 2.43 |
| 4.38_20 | 4.38 | 20 | 4.86 |
| 4.38_30 | 4.38 | 30 | 7.28 |
| 6.25_10 | 6.25 | 10 | 4.96 |
| 6.25_20 | 6.25 | 20 | 9.92 |
| 6.25_30 | 6.25 | 30 | 14.88 |
| Sample | L* | a* | b* | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| 0_0 | 21.1 ± 0.8 a | 21 ± 1 AB | 27 ± 2 a | 29 ± 2 A | 7.74 ± 0.6 a | 8.8 ± 0.9 A |
| 4.38_20 | 18.9 ± 0.9 c | 20.7 ± 0.7 A | 18 ± 3 c | 20 ± 2 B | 4.6 ± 0.9 c | 4.9 ± 0.7 C |
| 6.25_10 | 20 ± 1 b | 24.5 ± 0.8 C | 23 ± 3 b | 31 ± 2 A | 6 ± 1 b | 6.1 ± 0.9 BC |
| 6.25_30 | 18 ± 1 c | 23 ± 1 BC | 18 ± 2 c | 29 ± 3 A | 4.6 ± 0.8 c | 7 ± 1 B |
| Sample | L* | a* | b* | |||
|---|---|---|---|---|---|---|
| Cylinders | Pulp | Cylinders | Pulp | Cylinders | Pulp | |
| 0_0 | 96.2 ± 0.2 a | 72 ± 1 A | 7.2 ± 0.4 a | 52 ± 2 A | −0.6 ± 0.1 a | −1.7 ± 0.4 A |
| 4.38_20 | 76 ± 3 b | 70 ± 2 A | 43 ± 5 b | 55 ± 4 A | 1.5 ± 1.6 a | −1.4 ± 0.5 A |
| 6.25_10 | 81 ± 2 b | 70 ± 1 A | 36 ± 4 b | 57 ± 2 A | −5 ± 2 b | −6.16 ± 0.06 B |
| 6.25_30 | 79 ± 2 b | 70 ± 2 A | 41 ± 3 b | 58 ± 4 A | −5.1 ± 0.7 b | −9.3 ± 06 C |
| Variable | L* | a* | b* | BC | VC |
|---|---|---|---|---|---|
| L* | - | r = −0.9977 | r = 0.3593 | r = −0.9760 | r = −0.8853 |
| p < 0.001 | p = 0.382 | p < 0.001 | p = 0.003 | ||
| a* | r = −0.9977 | - | r = −0.4214 | r = 0.9821 | r = 0.8594 |
| p < 0.001 | p = 0.298 | p < 0.001 | p = 0.006 | ||
| b* | r = 0.3593 | r = −0.4214 | - | r = −0.4692 | r = 0.0077 |
| p = 0.382 | p = 0.298 | p = 0.241 | p = 0.986 | ||
| BC | r = −0.9760 | r = 0.9821 | r = −0.4692 | - | r = 0.8623 |
| p < 0.001 | p<0.001 | p = 0.241 | p = 0.006 | ||
| VC | r = −0.8853 | r = 0.8594 | r = 0.0077 | r = 0.8623 | - |
| p = 0.003 | p = 0.006 | p = 0.986 | p = 0.006 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, M.; Tappi, S.; Wiktor, A.; Rybak, K.; Miszczykowska, A.; Czyzewski, J.; Drozdzal, K.; Witrowa-Rajchert, D.; Tylewicz, U. The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot. Foods 2019, 8, 244. https://doi.org/10.3390/foods8070244
Nowacka M, Tappi S, Wiktor A, Rybak K, Miszczykowska A, Czyzewski J, Drozdzal K, Witrowa-Rajchert D, Tylewicz U. The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot. Foods. 2019; 8(7):244. https://doi.org/10.3390/foods8070244
Chicago/Turabian StyleNowacka, Malgorzata, Silvia Tappi, Artur Wiktor, Katarzyna Rybak, Agnieszka Miszczykowska, Jakub Czyzewski, Kinga Drozdzal, Dorota Witrowa-Rajchert, and Urszula Tylewicz. 2019. "The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot" Foods 8, no. 7: 244. https://doi.org/10.3390/foods8070244
APA StyleNowacka, M., Tappi, S., Wiktor, A., Rybak, K., Miszczykowska, A., Czyzewski, J., Drozdzal, K., Witrowa-Rajchert, D., & Tylewicz, U. (2019). The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot. Foods, 8(7), 244. https://doi.org/10.3390/foods8070244









