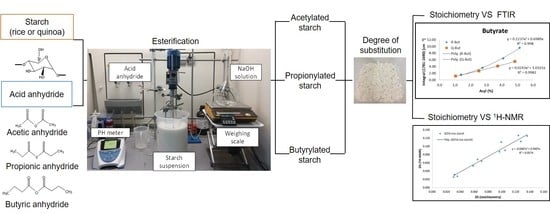
Comparison of Three Methods to Determine the Degree of Substitution of Quinoa and Rice Starch Acetates, Propionates, and Butyrates: Direct Stoichiometry, FTIR, and 1H-NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
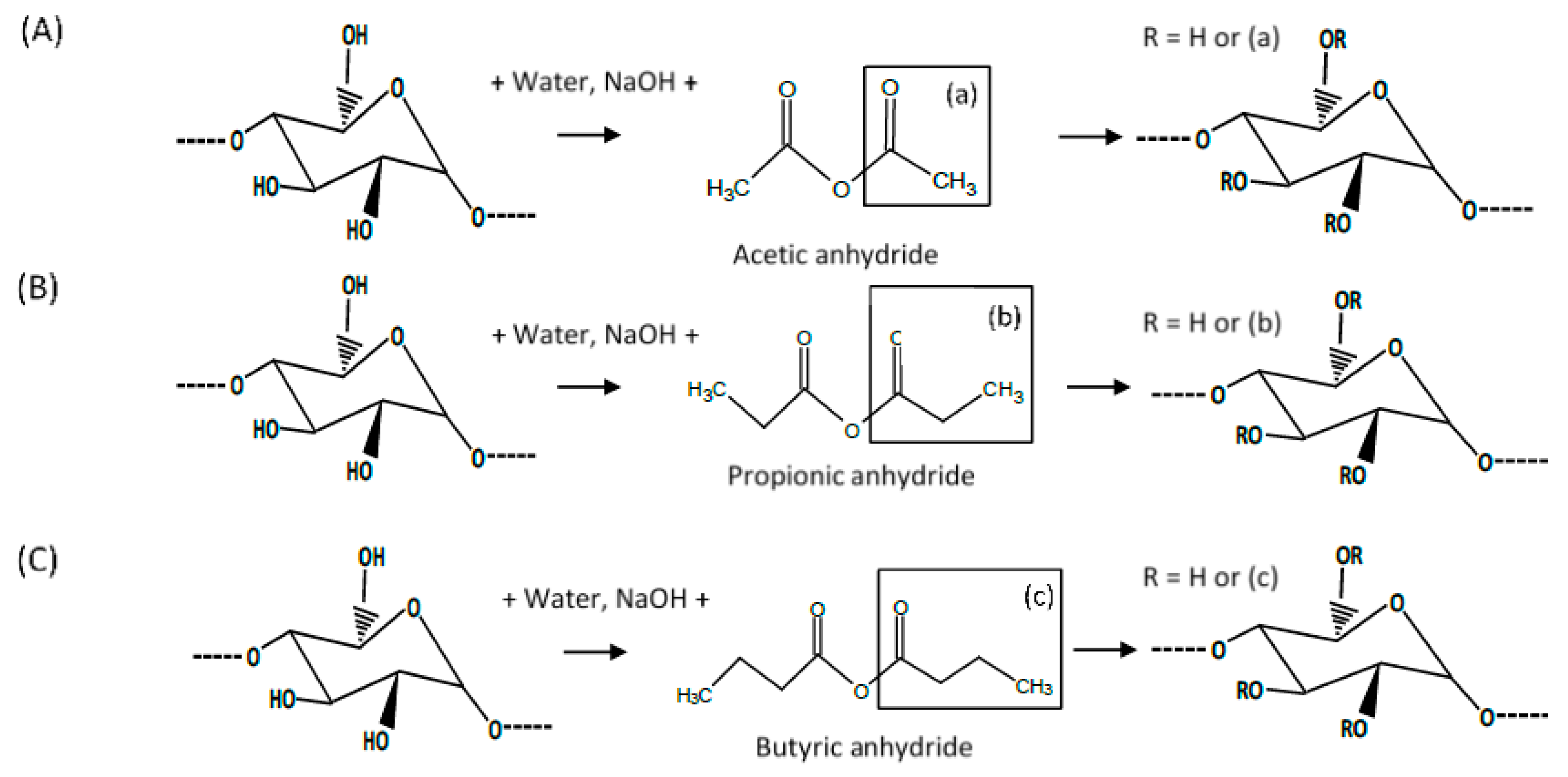
2.2. Acetylation, Propionylation, and Butyrylation of Quinoa and Rice Starches
2.3. Determination of the Degree of Substitution of Esterified Starches
2.3.1. Stoichiometric Calculation from the Molar Amount of Educts
- St–OH = starch,
- (CH3CO)2O = acetic anhydride,
- St–OCOCH3 = starch acetate.
2.3.2. FTIR Spectroscopy
2.3.3. Nuclear Magnetic Resonance (1H-NMR)
3. Results and Discussion
3.1. Determination of Degree of Substitution
3.1.1. Stoichiometric Calculation from the Molar Amount of Educts
3.1.2. Fourier Transform Infrared Spectroscopy
3.1.3. Nuclear Magnetic Resonance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hall, D.M.; Sayre, J.G. A scanning electron-microscope study of starches:Part i: Root and tuber starches1. Text. Res. J. 1969, 39, 1044–1052. [Google Scholar] [CrossRef]
- Jane, J.L.; Kasemsuwan, T.; Leas, S.; Zobel, H.; Robyt, J.F. Anthology of starch granule morphology by scanning electron microscopy. Starch Stärke 1994, 46, 121–129. [Google Scholar] [CrossRef]
- Marefati, A.; Wiege, B.; Haase, N.U.; Matos, M.; Rayner, M. Pickering emulsifiers based on hydrophobically modified small granular starches–part i: Manufacturing and physico-chemical characterization. Carbohydr. Polym. 2017, 175, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Timgren, A.; Sjöö, M.; Dejmek, P.; Rayner, M. Preparation and encapsulation properties of double pickering emulsions stabilized by quinoa starch granules. Colloids Surf. Physicochem. Eng. Asp. 2013, 423, 147–153. [Google Scholar] [CrossRef]
- Rayner, M.; Timgren, A.; Sjöö, M.; Dejmek, P. Quinoa starch granules: A candidate for stabilising food-grade pickering emulsions. J. Sci. Food Agric. 2012, 92, 1841–1847. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Li, L.; Guo, S. Acetylated starch-based biodegradable materials with potential biomedical applications as drug delivery systems. Curr. Appl. Phys. 2007, 7, e90–e93. [Google Scholar] [CrossRef]
- Marefati, A.; Bertrand, M.; Sjöö, M.; Dejmek, P.; Rayner, M. Storage and digestion stability of encapsulated curcumin in emulsions based on starch granule pickering stabilization. Food Hydrocoll. 2017, 63, 309–320. [Google Scholar] [CrossRef]
- Marefati, A.; Wiege, B.; Abdul Hadi, N.; Dejmek, P.; Rayner, M. In vitro intestinal lipolysis of emulsions based on starch granule pickering stabilization. Food Hydrocoll. 2019, 95, 468–475. [Google Scholar] [CrossRef]
- Song, X.; He, G.; Ruan, H.; Chen, Q. Preparation and properties of octenyl succinic anhydride modified early indica rice starch. Starch Stärke 2006, 58, 109–117. [Google Scholar] [CrossRef]
- Zainal Abiddin, N.F.; Yusoff, A.; Ahmad, N. Effect of octenylsuccinylation on physicochemical, thermal, morphological and stability of octenyl succinic anhydride (osa) modified sago starch. Food Hydrocoll. 2018, 75, 138–146. [Google Scholar] [CrossRef]
- Hong, L.F.; Cheng, L.H.; Gan, C.Y.; Lee, C.Y.; Peh, K.K. Evaluation of starch propionate as emulsion stabiliser in comparison with octenylsuccinate starch. LWT 2018, 91, 526–531. [Google Scholar] [CrossRef]
- Martins, P.C.; Gutkoski, L.C.; Martins, V.G. Impact of acid hydrolysis and esterification process in rice and potato starch properties. Int. J. Biol. Macromol. 2018, 120, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Borah, P.K.; Rappolt, M.; Duary, R.K.; Sarkar, A. Effects of folic acid esterification on the hierarchical structure of amylopectin corn starch. Food Hydrocoll. 2019, 86, 162–171. [Google Scholar] [CrossRef]
- Fang, J.M.; Fowler, P.A.; Tomkinson, J.; Hill, C.A.S. The preparation and characterisation of a series of chemically modified potato starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Nuñez-Santiago, C.; Garcia-Suarez, F.J.L.; Roman-Gutierrez, A.D.; Bello-Pérez, L.A. Effect of reagent type on the acetylation of barley and maize starches. Starch Stärke 2010, 62, 489–497. [Google Scholar] [CrossRef]
- Barrios, S.E.; Giammanco, G.; Contreras, J.M.; Laredo, E.; López-Carrasquero, F. Characterization of esterified cassava starch with long alkyl side chains and different substitution degrees. Int. J. Biol. Macromol. 2013, 59, 384–390. [Google Scholar] [CrossRef]
- García-Tejeda, Y.V.; Leal-Castañeda, E.J.; Espinosa-Solis, V.; Barrera-Figueroa, V. Synthesis and characterization of rice starch laurate as food-grade emulsifier for canola oil-in-water emulsions. Carbohydr. Polym. 2018, 194, 177–183. [Google Scholar] [CrossRef]
- Leal-Castañeda, E.J.; García-Tejeda, Y.; Hernández-Sánchez, H.; Alamilla-Beltrán, L.; Téllez-Medina, D.I.; Calderón-Domínguez, G.; García, H.S.; Gutiérrez-López, G.F. Pickering emulsions stabilized with native and lauroylated amaranth starch. Food Hydrocoll. 2018, 80, 177–185. [Google Scholar] [CrossRef]
- Agostoni, C.; Bruzzese, M.G. Fatty acids: Their biochemical and functional classification. La Pediatr. Med. E Chir. Med Surg. Pediatrics 1992, 14, 473–479. [Google Scholar]
- Annison, G.; Illman, R.J.; Topping, D.L. Acetylated, propionylated or butyrylated starches raise large bowel short-chain fatty acids preferentially when fed to rats. J. Nutr. 2003, 133, 3523–3528. [Google Scholar] [CrossRef]
- Plate, S.; Diekmann, S.; Steinhäuser, U.; Drusch, S. Determination of the degree of substitution of hydrolysed octenylsuccinate-derivatised starch. LWT Food Sci. Technol. 2012, 46, 580–582. [Google Scholar] [CrossRef]
- Zhong, J.F.; Chai, X.S.; Hu, H.C.; Fu, S.Y. Determination of degree of substitution in succinic anhydride modified cellulose by headspace gas chromatography. J. Chromatogr. A 2012, 1229, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Nmr spectroscopy of starch systems. Food Hydrocoll. 2017, 63, 611–624. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.-C.; Herrera, A.; Prakash, O. Study of octenyl succinic anhydride-modified waxy maize starch by nuclear magnetic resonance spectroscopy. Carbohydr. Polym. 2011, 83, 407–413. [Google Scholar] [CrossRef]
- Pisklak, D.M.; Zielińska-Pisklak, M.A.; Szeleszczuk, Ł.; Wawer, I. 13c solid-state nmr analysis of the most common pharmaceutical excipients used in solid drug formulations, part i: Chemical shifts assignment. J. Pharm. Biomed. Anal. 2016, 122, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schäfer, C.; Wu, P.; Deng, B.; Yang, G.; Li, E.; Gilbert, R.G.; Li, C. Mechanistic understanding of the relationships between molecular structure and emulsification properties of octenyl succinic anhydride (osa) modified starches. Food Hydrocoll. 2018, 74, 168–175. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by ftir and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Namazi, H.; Fathi, F.; Dadkhah, A. Hydrophobically modified starch using long-chain fatty acids for preparation of nanosized starch particles. Sci. Iran. 2011, 18, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Colussi, R.; El Halal, S.L.M.; Pinto, V.Z.; Bartz, J.; Gutkoski, L.C.; da Rosa Zavareze, E.; Dias, A.R.G. Acetylation of rice starch in an aqueous medium for use in food. LWT Food Sci. Technol. 2015, 62, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Winkler, H.; Vorwerg, W.; Wetzel, H. Synthesis and properties of fatty acid starch esters. Carbohydr. Polym. 2013, 98, 208–216. [Google Scholar] [CrossRef]




| Anhydride | Purity | Density (g/mL) | Molar Mass (g/mol) | Molarity of NaOH Used during Acylation (mol/L) | |
|---|---|---|---|---|---|
| Quinoa Starch | Rice Starch | ||||
| Acetic | ≥99% | 1.082 | 102.1 | 0.7095 | 0.7095 |
| Propionic | ≥98.5 | 1.015 | 130.1 | 0.7148 | 0.7170 |
| Butyric | ≥97.0% | 0.967 | 158.2 | 0.7148 | 0.7170 |
| Volume Anhydride (mL) | Anhyd (mol) | NaOH (g) | NaOH (mol) | Anhyd.–St. (mol) | Acyl (%) | DS (stoichiometry) | DS (1H-NMR) |
|---|---|---|---|---|---|---|---|
| Ac-A 1.60 | 0.01679 | 28.63 | 0.01980 | 0.01378 | 0.95 | 0.0360 | 0.0274 ± 0.0012 |
| Prop-A 2.19 | 0.01683 | 31.43 | 0.02196 | 0.01170 | 1.06 | 0.0305 | 0.0255 ± 0.0017 |
| But-A 2.84 | 0.01684 | 30.74 | 0.02148 | 0.01220 | 1.38 | 0.0319 | 0.0314 ± 0.0025 |
| Ac-A 3.20 | 0.03357 | 57.88 | 0.04002 | 0.02712 | 1.85 | 0.0708 | 0.0642 ± 0.0048 |
| Prop-A 4.37 | 0.03358 | 64.03 | 0.04475 | 0.02241 | 2.02 | 0.0586 | 0.0527 ± 0.0026 |
| But-A 5.67 | 0.03362 | 60.72 | 0.04243 | 0.02481 | 2.76 | 0.0648 | 0.0681 ± 0.0010 |
| Ac-A 4.80 | 0.05036 | 87.84 | 0.06074 | 0.03998 | 2.70 | 0.1044 | 0.0906 ± 0.0030 |
| Prop-A 6.56 | 0.05042 | 91.42 | 0.06389 | 0.03695 | 3.29 | 0.0965 | 0.0869 ± 0.0039 |
| But-A 8.51 | 0.05046 | 91.19 | 0.06373 | 0.03718 | 4.09 | 0.0971 | 0.1102 ± 0.0029 |
| Ac-A 6.40 | 0.06715 | 118.85 | 0.08219 | 0.05211 | 3.49 | 0.1361 | 0.1251 ± 0.0053 |
| Prop-A 8.75 | 0.06724 | 122.88 | 0.08587 | 0.04865 | 4.28 | 0.1270 | 0.1121 ± 0.0007 |
| But-A 11.34 | 0.06724 | 124.74 | 0.08717 | 0.04731 | 5.14 | 0.1235 | 0.1267 ± 0.0026 |
| Volume Anhydride (mL) | Anhyd. (mol) | NaOH (g) | NaOH (mol) | Anhyd.–St. (mol) | Acyl (%) | DS (stoichiometry) | DS (1H-NMR) |
|---|---|---|---|---|---|---|---|
| Ac-A 1.60 | 0.01679 | 30.24 | 0.02091 | 0.01267 | 0.87 | 0.0331 | 0.0269 ± 0.0026 |
| Prop-A 2.19 | 0.01683 | 31.94 | 0.02225 | 0.01141 | 1.04 | 0.0298 | 0.0250 ± 0.0015 |
| But-A 2.84 | 0.01684 | 31.90 | 0.02222 | 0.01146 | 1.30 | 0.0300 | 0.0331 ± 0.0040 |
| Ac-A 3.20 | 0.03357 | 60.72 | 0.04199 | 0.02515 | 1.72 | 0.0657 | 0.0562 ± 0.0020 |
| Prop-A 4.37 | 0.03358 | 61.40 | 0.04277 | 0.02439 | 2.19 | 0.0637 | 0.0579 ± 0.0019 |
| But-A 5.67 | 0.03362 | 64.03 | 0.04461 | 0.02263 | 2.53 | 0.0591 | 0.0598 ± 0.0053 |
| Ac-A 4.80 | 0.05036 | 91.35 | 0.06317 | 0.03755 | 2.54 | 0.0981 | 0.0852 ± 0.0019 |
| Prop-A 6.56 | 0.05042 | 92.38 | 0.06436 | 0.03648 | 3.25 | 0.0953 | 0.0943 ± 0.0002 |
| But-A 8.51 | 0.05046 | 96.35 | 0.06713 | 0.03379 | 3.73 | 0.0883 | 0.0978 ± 0.0025 |
| Ac-A 6.40 | 0.06715 | 120.45 | 0.08330 | 0.05100 | 3.42 | 0.1333 | 0.1105 ± 0.0058 |
| Prop-A 8.75 | 0.06724 | 126.06 | 0.08783 | 0.04665 | 4.12 | 0.1219 | 0.1143 ± 0.0061 |
| But-A 11.34 | 0.06724 | 129.36 | 0.09012 | 0.04436 | 4.84 | 0.1159 | 0.1229 ± 0.0065 |
| Volume Anhydride (mL) | Reaction Efficiency (%) | |
|---|---|---|
| Rice Starch | Quinoa Starch | |
| Native | not defined | not defined |
| Ac-A 1.60 | 82.1 | 75.5 |
| Prop-A 2.19 | 69.5 | 67.7 |
| But-A 2.84 | 72.5 | 68.1 |
| Ac-A 3.20 | 80.8 | 74.9 |
| Prop-A 4.37 | 66.7 | 72.6 |
| But-A 5.67 | 73.8 | 67.3 |
| Ac-A 4.80 | 79.4 | 74.6 |
| Prop-A 6.56 | 73.3 | 72.4 |
| But-A 8.51 | 74.6 | 67.0 |
| Ac-A 6.40 | 77.6 | 76.0 |
| Prop-A 8.75 | 72.4 | 69.4 |
| But-A 11.34 | 70.4 | 66.0 |
| Rice Starch | |||||
| Acetate | Propionate | Butyrate | |||
| Acyl (%) | Integral (1781–1690) (cm−1) | Acyl (%) | Integral (1781–1690) (cm−1) | Acyl (%) | Integral (1781–1690) (cm−1) |
| 0.95 | 2.31 ± 0.17 | 1.06 | 1.65 ± 0.14 | 1.38 | 1.55 ± 0.09 |
| 1.85 | 4.67 ± 0.62 | 2.02 | 3.47 ± 0.07 | 2.76 | 3.63 ± 0.11 |
| 2.70 | 7.16 ± 0.23 | 3.29 | 5.84 ± 0.17 | 4.09 | 6.41 ± 0.41 |
| 3.49 | 9.52 ± 0.38 | 4.28 | 7.89 ± 0.65 | 5.14 | 9.61 ± 0.45 |
| Quinoa Starch | |||||
| Acetate | Propionate | Butyrate | |||
| Acyl (%) | Integral (1781–1690) (cm−1) | Acyl (%) | Integral (1781–1690) (cm−1) | Acyl (%) | Integral (1781–1690) (cm−1) |
| 0.87 | 1.41 ± 0.12 | 1.04 | 0.96 ± 0.06 | 1.03 | 1.19 ± 0.09 |
| 1.72 | 3.39 ± 0.32 | 2.19 | 2.51 ± 0.05 | 2.53 | 2.66 ± 0.03 |
| 2.54 | 5.00 ± 0.52 | 3.25 | 4.07 ± 0.15 | 3.73 | 4.12 ± 0.09 |
| 3.42 | 6.41 ± 0.08 | 4.12 | 5.34 ± 0.06 | 4.84 | 5.51 ± 0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Hadi, N.; Wiege, B.; Stabenau, S.; Marefati, A.; Rayner, M. Comparison of Three Methods to Determine the Degree of Substitution of Quinoa and Rice Starch Acetates, Propionates, and Butyrates: Direct Stoichiometry, FTIR, and 1H-NMR. Foods 2020, 9, 83. https://doi.org/10.3390/foods9010083
Abdul Hadi N, Wiege B, Stabenau S, Marefati A, Rayner M. Comparison of Three Methods to Determine the Degree of Substitution of Quinoa and Rice Starch Acetates, Propionates, and Butyrates: Direct Stoichiometry, FTIR, and 1H-NMR. Foods. 2020; 9(1):83. https://doi.org/10.3390/foods9010083
Chicago/Turabian StyleAbdul Hadi, Nabilah, Berthold Wiege, Sebastian Stabenau, Ali Marefati, and Marilyn Rayner. 2020. "Comparison of Three Methods to Determine the Degree of Substitution of Quinoa and Rice Starch Acetates, Propionates, and Butyrates: Direct Stoichiometry, FTIR, and 1H-NMR" Foods 9, no. 1: 83. https://doi.org/10.3390/foods9010083
APA StyleAbdul Hadi, N., Wiege, B., Stabenau, S., Marefati, A., & Rayner, M. (2020). Comparison of Three Methods to Determine the Degree of Substitution of Quinoa and Rice Starch Acetates, Propionates, and Butyrates: Direct Stoichiometry, FTIR, and 1H-NMR. Foods, 9(1), 83. https://doi.org/10.3390/foods9010083