Rapid Screen of the Color and Water Content of Fresh-Cut Potato Tuber Slices Using Hyperspectral Imaging Coupled with Multivariate Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Color Measurement and Water Content Measurement
2.3. Hyperspectral Image Acquisition
2.3.1. Hyperspectral Imaging System
2.3.2. Imaging Acquisition and Calibration
2.3.3. Image Preprocessing and Spectral Extraction
2.4. Data Analysis
2.4.1. Regression Models
2.4.2. Wavelength Selection
2.4.3. Model Evaluation
3. Results and Discussion
3.1. Color Parameters and Water Content Distribution
3.2. Spectral Profiles
3.3. Regression Models
3.3.1. Regression Models for Color Prediction
3.3.2. Regression Models for Water Content Prediction
3.4. Visualization
3.4.1. Color Visualization
3.4.2. Water Content Visualization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, H.Q.; Wang, X.L.; Ma, G.S. Nutrition feasibility analysis of development of potato as a staple food. Food Nutr. China 2015, 7, 10–13. [Google Scholar]
- Kanter, M.; Elkin, C. Potato as a source of nutrition for physical performance. Am. J. Potato Res. 2019, 96, 314. [Google Scholar] [CrossRef] [Green Version]
- Lachman, J.; Hamouz, K. Red and purple coloured potatoes as a significant antioxidant source in human nutrition—A review. Plant Soil Environ. 2005, 51, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Cantos, E.; Tudela, J.A.; Gil, M.I.; Espin, J.C. Phenolic compounds and related enzymes are not rate-limiting in browning development of fresh-cut potatoes. J. Agric. Food Chem. 2002, 50, 3015–3023. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Rojas-Graü, M.A.; González, L.A.; Varela, P.; Soliva-Fortuny, R.; Hernando, M.I.H.; Munuera, I.P.; Fiszman, S.; Martín-Bellosoa, O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: A review. Postharvest Biol. Tec. 2010, 57, 139–148. [Google Scholar] [CrossRef]
- Bora, G.C.; Pathak, R.; Ahmadi, M.; Mistry, P. Image processing analysis to track colour changes on apple and correlate to moisture content in drying stages. Food Qual. Saf. 2018, 2, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Mohd Ali, M.; Hashim, N.; Khairunniza-Bejo, S.; Shamsudin, R.; Wan Sembak, W.N.F.H. RGB imaging system for monitoring quality changes of seedless watermelon during storage. Acta Horticult. 2017, 1152, 361–366. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Abdan, K.; Janius, R.; Chen, G.N. Combination of computer vision and backscattering imaging for predicting the moisture content and colour changes of sweet potato (Ipomoea batatas L.) during drying. Comput. Electron Agric. 2018, 150, 178–187. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q.N.; Liu, F.; He, Y.; Xiao, Y.Z. Rapid and non-destructive measurement of spinach pigments content during storage using hyperspectral imaging with chemometrics. Measurement 2017, 97, 149–155. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, H.; Liu, F.; He, Y. Application of near-infrared hyperspectral imaging with variable selection methods to determine and visualize caffeine content of coffee beans. Food Bioprocess Tech. 2017, 10, 213–221. [Google Scholar] [CrossRef]
- Lin, X.H.; Xu, J.L.; Sun, D.W. Investigation of moisture content uniformity of microwave-vacuum dried mushroom (Agaricus bisporus) by NIR hyperspectral imaging. LWT-Food Sci. Technol. 2019, 109, 108–117. [Google Scholar] [CrossRef]
- Liu, Y.W.; Sun, D.W.; Cheng, J.H.; Han, Z. Hyperspectral imaging sensing of changes in moisture content and color of beef during microwave heating process. Food Anal. Method. 2018, 11, 2472–2484. [Google Scholar] [CrossRef]
- Nghia, N.D.T.; Dusabumuremyi, J.C.; Saeys, W. Cross-polarized VNIR hyperspectral reflectance imaging for non-destructive quality evaluation of dried banana slices, drying process monitoring and control. J. Food Eng. 2018, 238, 85–94. [Google Scholar]
- Crichton, S.; Shrestha, L.; Hurlbert, A.; Sturm, B. Use of hyperspectral imaging for the prediction of water content and chromaticity of raw and pretreated apple slices during convection drying. Dry Technol. 2018, 36, 804–816. [Google Scholar] [CrossRef]
- Liu, Y.H.; Sun, Y.; Xie, A.G.; Yu, H.C.; Yin, Y.; Li, X.; Duan, X. Potential of hyperspectral imaging for rapid prediction of anthocyanin content of purple-fleshed sweet potato tuber slices during drying process. Food Anal. Method. 2017, 10, 3836–3846. [Google Scholar] [CrossRef]
- Rady, A.; Guyer, D.; Lu, R. Evaluation of sugar content of potatoes using hyperspectral imaging. Food Bioprocess Tech. 2015, 8, 995–1010. [Google Scholar] [CrossRef]
- Su, W.H.; Sun, D.W. Rapid determination of starch content of potato and sweet potato by using NIR hyperspectral imaging. Hortscience 2019, 54, S38. [Google Scholar]
- Kjaer, A.; Nielsen, G.; Staerke, S.; Clausen, M.R.; Edelenbos, M.; Jorgensen, B. Prediction of starch, soluble sugars and amino acids in potatoes (Solanum tuberosum L.) using hyperspectral imaging, dielectric and LF-NMR methodologies. Potato Res. 2016, 59, 357–374. [Google Scholar] [CrossRef]
- Su, W.H.; Sun, D.W. Potential of hyperspectral imaging for visual authentication of sliced organic potatoes from potato and sweet potato tubers and rapid grading of the tubers according to water proportion. Comput. Electron Agric. 2016, 125, 113–124. [Google Scholar] [CrossRef]
- Gao, Y.W.; Li, Q.W.; Rao, X.Q.; Ying, Y.B. Precautionary analysis of sprouting potato eyes using hyperspectral imaging technology. Int. J. Agric. Biol. Eng. 2018, 11, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Trong, N.N.D.; Tsuta, M.; Nicolai, B.M.; De Baerdemaeker, J.; Saeys, W. Prediction of optimal cooking time for boiled potatoes by hyperspectral imaging. J. Food Eng. 2011, 105, 617–624. [Google Scholar] [CrossRef]
- Ji, Y.M.; Sun, L.J.; Li, Y.S.; Li, J.; Liu, S.C.; Xie, X.; Xu, Y.T. Non-destructive classification of defective potatoes based on hyperspectral imaging and support vector machine. Infrared Phys. Techn. 2019, 99, 71–79. [Google Scholar] [CrossRef]
- Lopez-Maestresalas, A.; Keresztes, J.C.; Goodarzi, M.; Arazuri, S.; Jaren, C.; Saeys, W. Non-destructive detection of blackspot in potatoes by Vis-NIR and SWIR hyperspectral imaging. Food Control. 2016, 70, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, Y.; Yu, H.; Xie, A.; Li, X.; Yin, Y.; Duan, X. Non-destructive prediction of water content and freezable water content of purple-fleshed sweet potato tuber slices during drying process using hyperspectral imaging technique. Food Anal. Method. 2016, 10, 1535–1546. [Google Scholar] [CrossRef]
- Arnold, T.; DeBiasio, M. Study of near-infrared imaging spectroscopy for the inspection of peeled potato tubers. In Sensing for Agriculture and Food Quality and Safety X; International Society for Optics and Photonics: Orlando, FL, USA, 2018. [Google Scholar]
- Amjad, W.; Crichton, S.O.J.; Munir, A.; Hensel, O.; Sturm, B. Hyperspectral imaging for the determination of potato slice water content and chromaticity during the convective hot air drying process. Biosyst. Eng. 2018, 166, 170–183. [Google Scholar] [CrossRef]
- Moscetti, R.; Sturm, B.; Crichton, S.O.J.; Amjad, W.; Massantini, R. Postharvest monitoring of organic potato (cv. Anuschka) during hot-air drying using visible-NIR hyperspectral imaging. J. Sci. Food Agric. 2017, 98, 2507–2517. [Google Scholar] [CrossRef]
- Maskan, M. Kinetics of colour change of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- ElMasry, G.; Wang, N.; Vigneault, C.; Qiao, J.; ElSayed, A. Early detection of apple bruises on different background colors using hyperspectral imaging. LWT-Food Sci. Technol. 2008, 41, 337–345. [Google Scholar] [CrossRef]
- Huang, L.X.; Zhou, Y.B.; Meng, L.W.; Wu, D.; He, Y. Comparison of different CCD detectors and chemometrics for predicting total anthocyanin content and antioxidant activity of mulberry fruit using visible and near infrared hyperspectral imaging technique. Food Chem. 2017, 224, 1–10. [Google Scholar] [CrossRef]
- De Brabanter, K.; Karsmakers, P.; Ojeda, F.; Alzate, C.; De Brabanter, J.; Pelckmans, K.; De Moor, B.; Vandewalle, J.; Suykens, J.A.K. LS-SVMlab Toolbox User’s Guide Version 1.8 (2011). Available online: http://www.esat.kuleuven.be/sista/lssvmlab/ (accessed on 6 November 2019).
- Araujo, M.C.U.; Saldanha, T.C.B.; Galvao, R.K.H.; Yoneyama, T.; Chame, H.C.; Visani, V. The successive projections algorithm for variable selection in spectroscopic multicomponent analysis. Chemometr. Intell. Lab. 2001, 57, 65–73. [Google Scholar] [CrossRef]
- Fan, W.; Shan, Y.; Li, G.Y.; Lv, H.Y.; Li, H.D.; Liang, Y.Z. Application of competitive adaptive reweighted sampling method to determine effective wavelengths for prediction of total acid of vinegar. Food Anal. Method. 2012, 5, 585–590. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Chu, B.; Zhang, C. Determination of total polysaccharides and total flavonoids in chrysanthemum morifolium using near-infrared hyperspectral imaging and multivariate analysis. Molecules 2018, 23, 2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzer, R. Practical Guide to Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2008; Volume 47, pp. 4628–4629. [Google Scholar] [CrossRef]
- Malacara, D. Color Vision and Colorimetry: Theory and Applications, 2nd ed.; SPIE Press: Bellingham, WA, USA, 2011. [Google Scholar]
- Li, M.Z. Spectroscopy Analysis Technology and Application; Science Press: Beijing, China, 2006. [Google Scholar]
- Sasic, S.; Ozaki, Y. Short-wave hear-infrared spectroscopy of biological fluids. 1. Quantitative analysis of fat, protein, and lactose in raw milk by partial least squares regression and band assignment. Anal. Chem. 2001, 73, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.; Knochel, S. Microbiological stability and diversity in raw pre-peeled potatoes packed in different atmospheres. Eur. Food Res. Technol. 2003, 217, 421–426. [Google Scholar] [CrossRef]
- Lund, B.M. A bacteriological study of stored sulphite treated peeled potatoes. J. Appl. Bacteriol. 1968, 31, 479–492. [Google Scholar] [CrossRef] [PubMed]
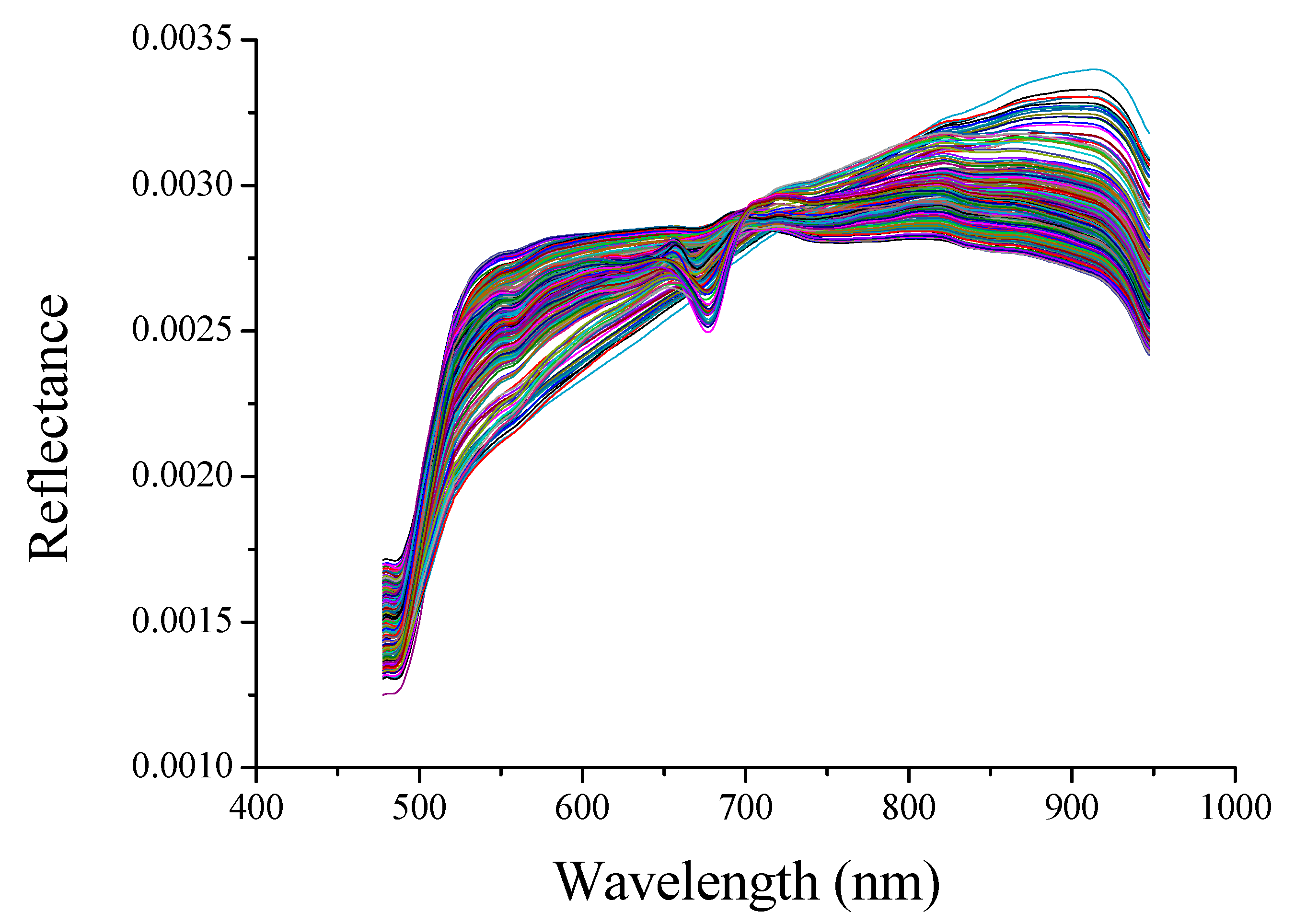
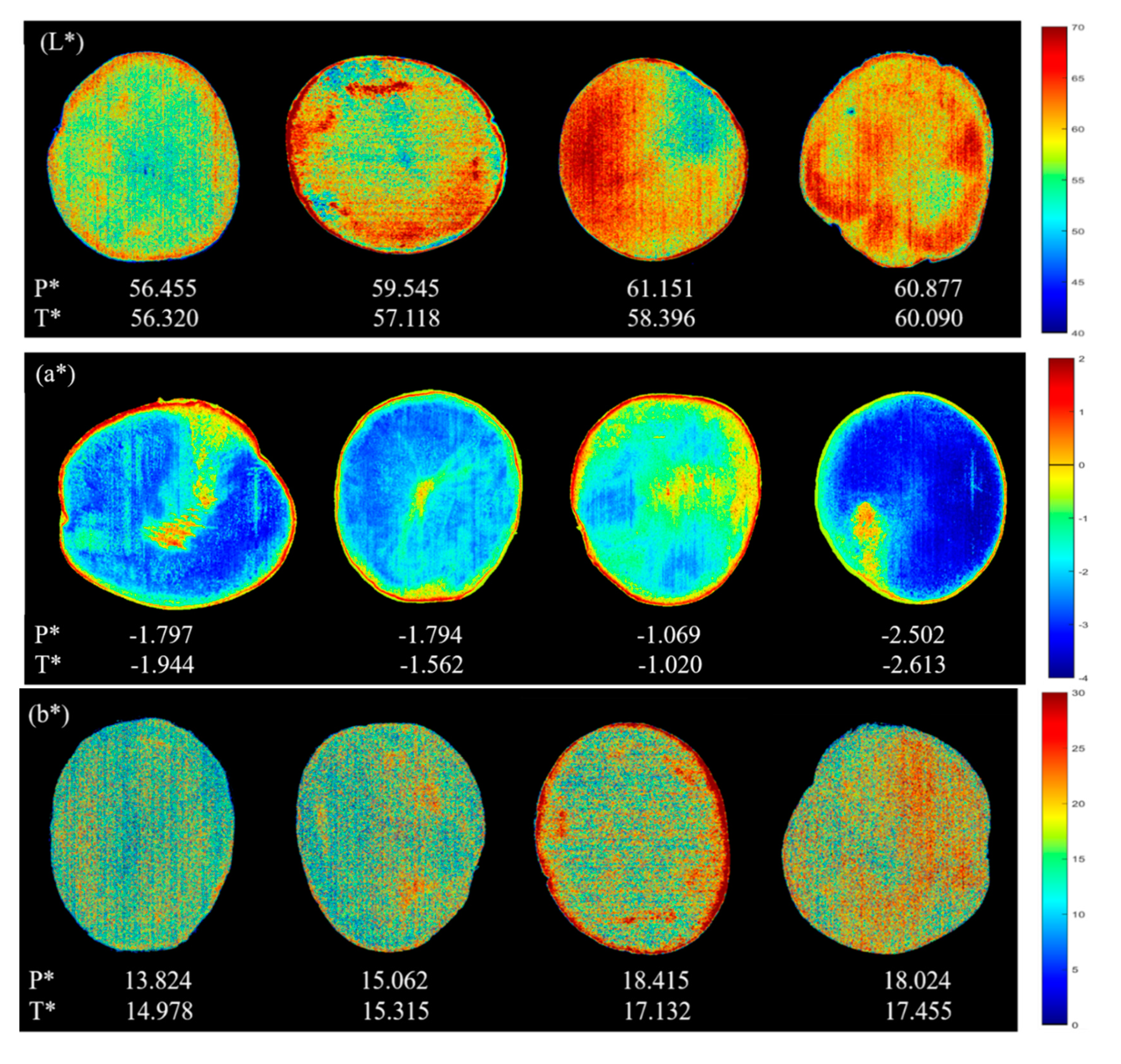
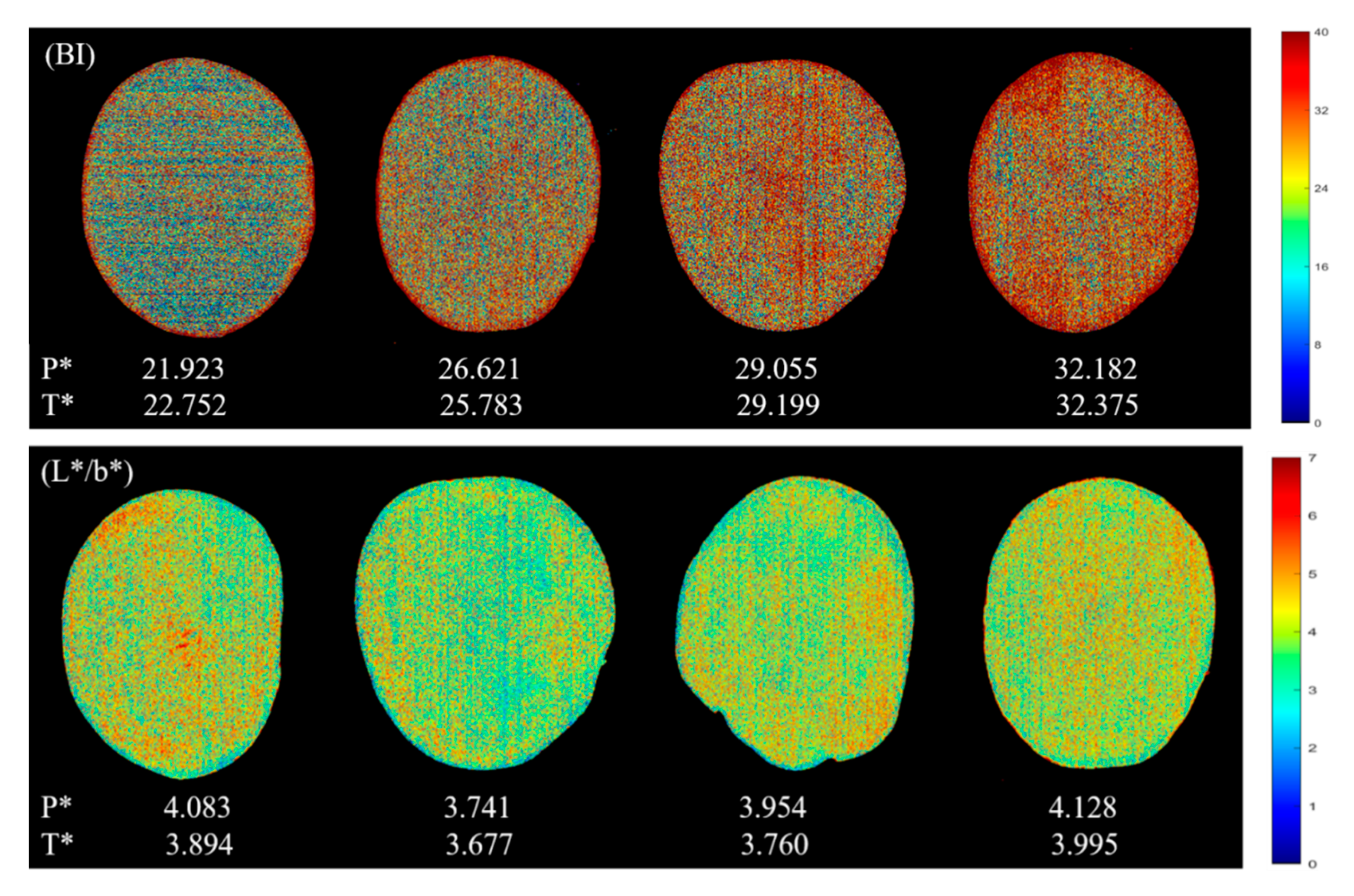
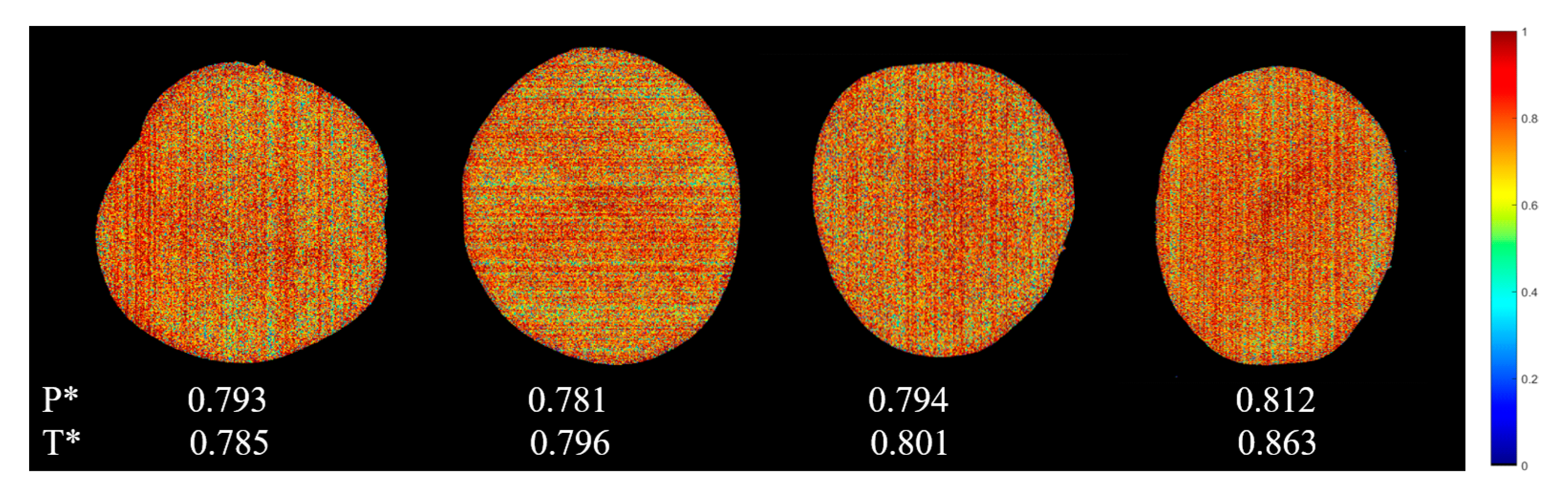
| Indicator | Sample Set | Number | Range | Mean | Standard Deviation |
|---|---|---|---|---|---|
| L* | Cal a | 156 | 43.794–64.738 | 57.548 | 3.328 |
| Pre a | 78 | 45.497–64.681 | 57.561 | 3.301 | |
| a* | Cal | 156 | −3.096–+2.050 | −1.277 | 1.308 |
| Pre | 78 | −3.045–+1.886 | −1.278 | 1.31 | |
| b* | Cal | 156 | 11.247–20.681 | 15.703 | 1.98 |
| Pre | 78 | 11.581–20.567 | 15.704 | 1.977 | |
| BI | Cal | 156 | 22.720–37.097 | 29.106 | 3.343 |
| Pre | 78 | 22.752–36.278 | 29.104 | 3.357 | |
| L*/b* | Cal | 156 | 2.921–4.401 | 3.696 | 0.315 |
| Pre | 78 | 3.011–4.248 | 3.696 | 0.312 | |
| water content | Cal | 156 | 0.753–0.879 | 0.811 | 0.0209 |
| Pre | 78 | 0.758–0.876 | 0.811 | 0.021 |
| Models | Data Type | N.V. b | Calibration | Validation | Prediction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2c | RMSEC | SDC | R2cv | RMSECV | SDCV | R2p | RMSEP | SDP | RPD | |||
| L* value prediction | ||||||||||||
| PLS | Full | 370 | 0.841 | 1.324 | 3.051 | 0.816 | 1.562 | 2.894 | 0.738 | 1.710 | 3.007 | 1.758 |
| SPA | 23 | 0.838 | 1.333 | 3.047 | 0.802 | 1.622 | 2.717 | 0.736 | 1.723 | 2.993 | 1.736 | |
| CARS | 43 | 0.907 | 1.013 | 3.169 | 0.870 | 1.316 | 2.848 | 0.801 | 1.470 | 2.919 | 1.985 | |
| LSSVM | Full | 370 | 0.938 | 0.827 | 3.174 | 0.814 | 1.437 | 3.126 | 0.858 | 1.298 | 3.010 | 2.319 |
| SPA | 23 | 0.937 | 0.832 | 3.177 | 0.828 | 1.377 | 3.116 | 0.848 | 1.336 | 2.913 | 2.181 | |
| CARS | 43 | 0.932 | 0.865 | 3.165 | 0.834 | 1.353 | 3.089 | 0.851 | 1.305 | 3.061 | 2.345 | |
| a* value prediction | ||||||||||||
| PLS | Full | 370 | 0.943 | 0.312 | 1.270 | 0.947 | 0.335 | 1.247 | 0.945 | 0.312 | 1.272 | 4.078 |
| SPA | 15 | 0.928 | 0.351 | 1.260 | 0.931 | 0.381 | 1.255 | 0.941 | 0.318 | 1.255 | 3.949 | |
| CARS | 24 | 0.946 | 0.304 | 1.272 | 0.949 | 0.326 | 1.251 | 0.954 | 0.283 | 1.254 | 4.428 | |
| LSSVM | Full | 370 | 0.976 | 0.201 | 1.281 | 0.949 | 0.294 | 1.276 | 0.956 | 0.274 | 1.289 | 4.704 |
| SPA | 15 | 0.964 | 0.248 | 1.277 | 0.950 | 0.290 | 1.275 | 0.957 | 0.271 | 1.284 | 4.731 | |
| CARS | 24 | 0.966 | 0.239 | 1.281 | 0.950 | 0.292 | 1.279 | 0.957 | 0.272 | 1.275 | 4.686 | |
| b* value prediction | ||||||||||||
| PLS | Full | 370 | 0.887 | 0.663 | 1.865 | 0.858 | 0.825 | 1.674 | 0.881 | 0.689 | 1.949 | 2.827 |
| SPA | 21 | 0.899 | 0.628 | 1.877 | 0.862 | 0.816 | 1.695 | 0.887 | 0.679 | 1.982 | 2.918 | |
| CARS | 24 | 0.929 | 0.526 | 1.908 | 0.910 | 0.658 | 1.839 | 0.900 | 0.623 | 1.874 | 3.008 | |
| LSSVM | Full | 370 | 0.962 | 0.383 | 1.930 | 0.909 | 0.597 | 1.938 | 0.924 | 0.546 | 1.942 | 3.560 |
| SPA | 21 | 0.941 | 0.481 | 1.913 | 0.912 | 0.587 | 1.908 | 0.922 | 0.556 | 1.923 | 3.461 | |
| CARS | 24 | 0.959 | 0.399 | 1.927 | 0.909 | 0.597 | 1.920 | 0.924 | 0.548 | 1.895 | 3.457 | |
| BI value prediction | ||||||||||||
| PLS | Full | 370 | 0.911 | 0.993 | 3.191 | 0.896 | 1.197 | 3.068 | 0.898 | 1.083 | 3.364 | 3.107 |
| SPA | 17 | 0.887 | 1.121 | 3.148 | 0.862 | 1.379 | 3.030 | 0.887 | 1.141 | 3.333 | 2.920 | |
| CARS | 25 | 0.902 | 1.045 | 3.174 | 0.884 | 1.263 | 3.034 | 0.890 | 1.125 | 3.351 | 2.978 | |
| LSSVM | Full | 370 | 0.958 | 0.685 | 3.248 | 0.924 | 0.922 | 3.231 | 0.940 | 0.823 | 3.328 | 4.047 |
| SPA | 17 | 0.950 | 0.742 | 3.242 | 0.923 | 0.924 | 3.243 | 0.932 | 0.875 | 3.353 | 3.831 | |
| CARS | 25 | 0.958 | 0.686 | 3.256 | 0.932 | 0.869 | 3.244 | 0.929 | 0.899 | 3.297 | 3.669 | |
| L*/b* value prediction | ||||||||||||
| PLS | Full | 370 | 0.904 | 0.097 | 0.299 | 0.885 | 0.118 | 0.289 | 0.872 | 0.114 | 0.300 | 2.634 |
| SPA | 18 | 0.915 | 0.092 | 0.301 | 0.905 | 0.107 | 0.289 | 0.883 | 0.111 | 0.299 | 2.706 | |
| CARS | 30 | 0.938 | 0.078 | 0.305 | 0.928 | 0.093 | 0.294 | 0.929 | 0.087 | 0.296 | 3.390 | |
| LSSVM | Full | 370 | 0.957 | 0.065 | 0.305 | 0.919 | 0.089 | 0.305 | 0.947 | 0.073 | 0.292 | 4.023 |
| SPA | 18 | 0.954 | 0.068 | 0.305 | 0.922 | 0.088 | 0.305 | 0.948 | 0.072 | 0.293 | 4.093 | |
| CARS | 30 | 0.948 | 0.072 | 0.304 | 0.927 | 0.085 | 0.304 | 0.940 | 0.078 | 0.299 | 3.847 | |
| Models | Data Type | N.V. b | Calibration | Validation | Prediction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2c | RMSEC | SDC | R2cv | RMSECV | SDCV | R2p | RMSEP | SDP | RPD | |||
| PLS | Full | 370 | 0.777 | 0.010 | 0.018 | 0.620 | 0.014 | 0.018 | 0.718 | 0.011 | 0.020 | 1.781 |
| SPA | 20 | 0.751 | 0.010 | 0.018 | 0.624 | 0.014 | 0.017 | 0.719 | 0.011 | 0.019 | 1.675 | |
| CARS | 22 | 0.788 | 0.010 | 0.019 | 0.692 | 0.013 | 0.017 | 0.721 | 0.011 | 0.019 | 1.700 | |
| LSSVM | Full | 370 | 0.812 | 0.009 | 0.018 | 0.692 | 0.012 | 0.018 | 0.778 | 0.010 | 0.020 | 2.006 |
| SPA | 20 | 0.803 | 0.009 | 0.018 | 0.653 | 0.012 | 0.019 | 0.794 | 0.010 | 0.019 | 2.018 | |
| CARS | 22 | 0.825 | 0.009 | 0.018 | 0.713 | 0.011 | 0.019 | 0.791 | 0.010 | 0.019 | 1.978 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Q.; Bai, X.; He, Y. Rapid Screen of the Color and Water Content of Fresh-Cut Potato Tuber Slices Using Hyperspectral Imaging Coupled with Multivariate Analysis. Foods 2020, 9, 94. https://doi.org/10.3390/foods9010094
Xiao Q, Bai X, He Y. Rapid Screen of the Color and Water Content of Fresh-Cut Potato Tuber Slices Using Hyperspectral Imaging Coupled with Multivariate Analysis. Foods. 2020; 9(1):94. https://doi.org/10.3390/foods9010094
Chicago/Turabian StyleXiao, Qinlin, Xiulin Bai, and Yong He. 2020. "Rapid Screen of the Color and Water Content of Fresh-Cut Potato Tuber Slices Using Hyperspectral Imaging Coupled with Multivariate Analysis" Foods 9, no. 1: 94. https://doi.org/10.3390/foods9010094
APA StyleXiao, Q., Bai, X., & He, Y. (2020). Rapid Screen of the Color and Water Content of Fresh-Cut Potato Tuber Slices Using Hyperspectral Imaging Coupled with Multivariate Analysis. Foods, 9(1), 94. https://doi.org/10.3390/foods9010094






