Conversion of Exhausted Sugar Beet Pulp into Fermentable Sugars from a Biorefinery Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Enzyme Production by Solid-State Fermentation on Fresh ESBPPs
2.3. Enzyme Extraction and Activity Assays
2.4. Enzymatic Hydrolysis of Fresh ESBPPs by Adding Fermented Solid
High-Solid Hydrolysis in Fed-Batch Mode
2.5. Enzymatic Hydrolysis by SSF Enzymatic Extract
2.6. Sample Analyses
2.7. Parameter Estimation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Enzymatic Hydrolysis of Fresh ESBPPs by Adding Fermented Solid
3.1.1. Effect of Temperature
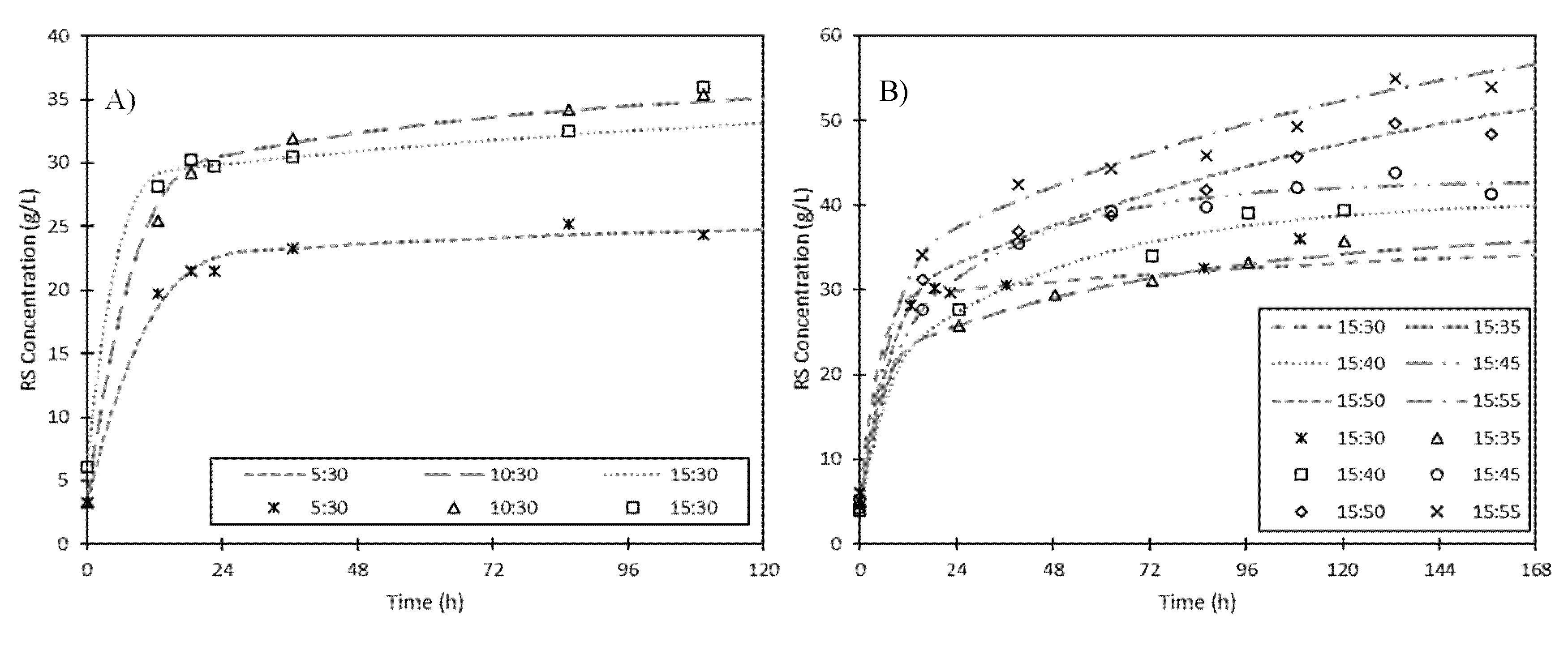
3.1.2. Effect of Fermented to Fresh Solid Ratio
3.1.3. Supplementation of Fermented ESBPPs with Commercial Cellulase
3.1.4. High-Solid Fed-Batch Enzymatic Hydrolysis
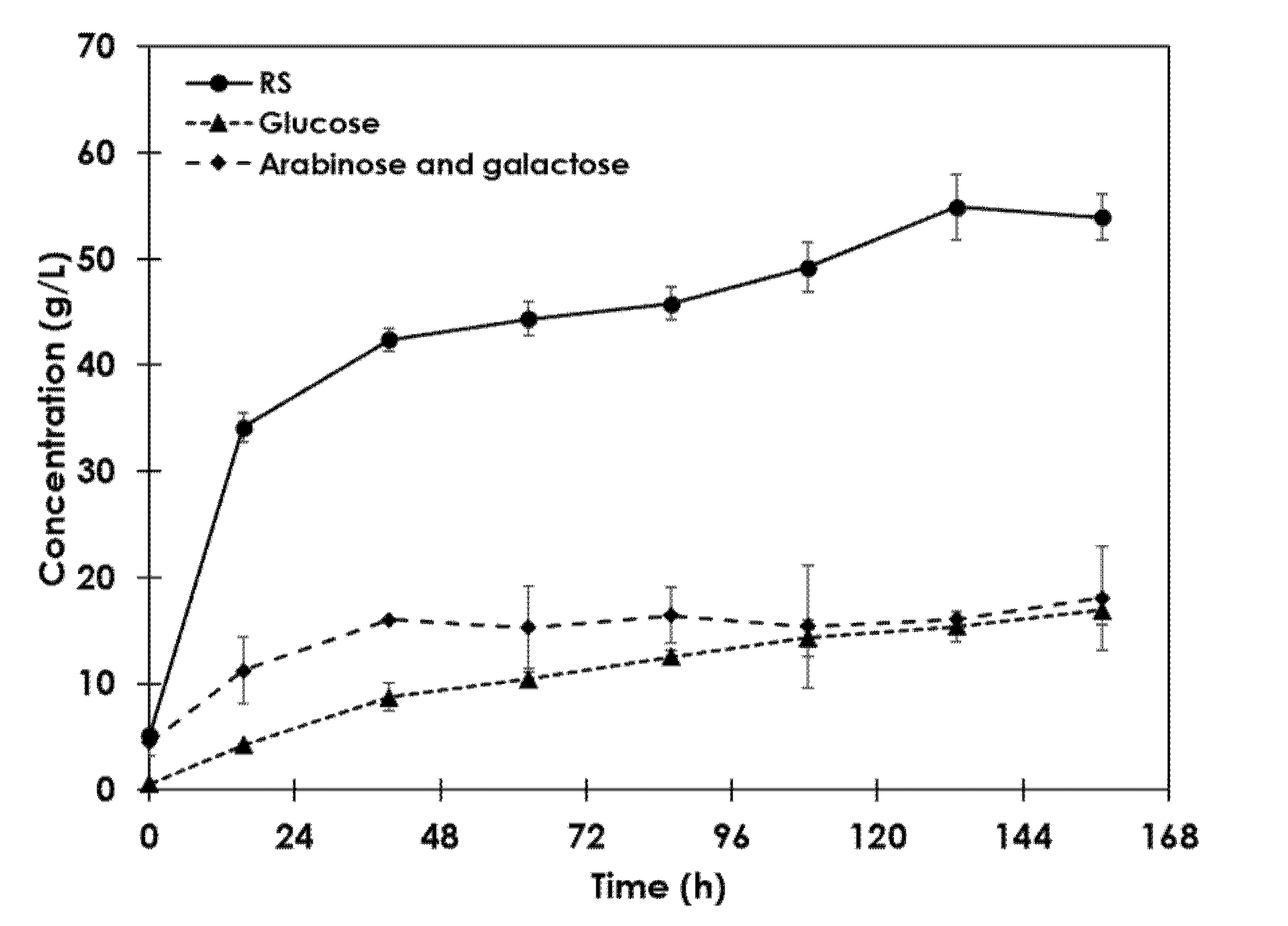
3.2. Evaluation of the Proposed Methodology for ESBPP Hydrolysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diaz, A.B.; Blandino, A.; Caro, I. Value added products from fermentation of sugars derived from agro-food residues. Trends Food Sci. Technol. 2018, 71, 52–64. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Biotransformation of lignocellulosic materials into value-added products-A review. Int. J. Biol. Macromol. 2017, 98, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Cárdenas, P.; López-López, A.; Moeller-Chávez, G.E.; León-Becerril, E. Current Pretreatments of Lignocellulosic Residues in the Production of Bioethanol. Waste Biomass Valorization 2017, 8, 161–181. [Google Scholar] [CrossRef]
- Cooray, S.T.; Chen, W.N. Valorization of brewer’s spent grain using fungi solid-state fermentation to enhance nutritional value. J. Funct. Foods 2018, 42, 85–94. [Google Scholar] [CrossRef]
- Manisha, S.K.Y. Technological advances and applications of hydrolytic enzymes for valorization of lignocellulosic biomass. Bioresour. Technol. 2017, 245, 1727–1739. [Google Scholar] [CrossRef]
- Xu, X.; Lin, M.; Zang, Q.; Shi, S. Solid state bioconversion of lignocellulosic residues by Inonotus obliquus for production of cellulolytic enzymes and saccharification. Bioresour. Technol. 2018, 247, 88–95. [Google Scholar] [CrossRef]
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic hydrolysis of lignocellulosic biomass: Converting food waste in valuable products. Curr. Opin. Food Sci. 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Anand, G.; Yadav, S.; Yadav, D. Production, purification and biochemical characterization of an exo-polygalacturonase from Aspergillus niger MTCC 478 suitable for clarification of orange juice. 3 Biotech 2017, 7, 122. [Google Scholar] [CrossRef] [Green Version]
- Díaz, A.B.; De Ory, I.; Caro, I.; Blandino, A. Enhance hydrolytic enzymes production by Aspergillus awamori on supplemented grape pomace. Food Bioprod. Process. 2012, 90, 72–78. [Google Scholar] [CrossRef]
- Brijwani, K.; Oberoi, H.S.; Vadlani, P.V. Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochem. 2010, 45, 120–128. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Yanniotis, S.; Kookos, I.; Koutinas, A.A. Utilisation of by-products from sunflower-based biodiesel production processes for the production of fermentation feedstock. Waste Biomass Valorization 2013, 4, 529–537. [Google Scholar] [CrossRef]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef]
- Chang, C.W.; Webb, C. Production of a generic microbial feedstock for lignocellulose biorefineries through sequential bioprocessing. Bioresour. Technol. 2016, 227, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Valorisation of food waste via fungal hydrolysis and lactic acid fermentation with Lactobacillus casei Shirota. Bioresour. Technol. 2016, 217, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Papapostolou, H.; Alexandri, M.; Kopsahelis, N.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.G.; Koutinas, A.A. Fumaric acid production using renewable resources from biodiesel and cane sugar production processes. Environ. Sci. Pollut. Res. 2018, 25, 35960–35970. [Google Scholar] [CrossRef] [PubMed]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Valorization of agro-industrial wastes to produce hydrolytic enzymes by fungal solid-state fermentation. Waste Manag. Res. 2018, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Gonçalves, C.; Rodriguez-Jasso, R.M.; Gomes, N.; Teixeira, J.A.; Belo, I. Adaptation of dinitrosalicylic acid method to microtiter plates. Anal. Methods 2010, 2, 2046–2048. [Google Scholar] [CrossRef] [Green Version]
- APHA. Standards Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Caro, I.; Blandino, A.; Díaz, A.B.; Marzo, C. A kinetic model considering the heterogeneous nature of the enzyme hydrolysis of lignocellulosic materials. Biofuels, Bioprod. Biorefining 2019, 13, 1044–1056. [Google Scholar] [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Kumar Prasad, R.; Chatterjee, S.; Behari Mazumder, P.; Kumar Gupta, S.; Sharma, S.; Gunvant Vairale, M.; Datta, S.; Kumar Dwivedi, S.; Kumar Gupta, D. Bioethanol production from waste lignocelluloses: A review on microbial degradation potential. Chemosphere 2019, 231, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Chaturvedi, R.; Singh, S.K. To study the variation in growth of Aspergillus and Trichoderma strains under the effect of metal ions, pH, temperature and solvents. Res. Biotechnol. 2014, 5, 14–20. [Google Scholar]
- Darah, I.; Haritharan, W.; Lim, S.H. Involvement of physicochemical parameters on pectinase production by Aspergillus niger HFD5A-1. J. Pure Appl. Microbiol. 2013, 7, 2541–2549. [Google Scholar]
- Botella, C.; Diaz, A.; de Ory, I.; Webb, C.; Blandino, A. Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochem. 2007, 42, 98–101. [Google Scholar] [CrossRef]
- Umsza-Guez, M.A.; Díaz, A.B.; Ory, I.D.; Blandino, A.; Gomes, E.; Caro, I. Xylanase Production By Aspergillus Awamori Under Solid State Fermentation Conditions on Tomato Pomace. Brazilian J. Microbiol. 2011, 42, 1585–1597. [Google Scholar] [CrossRef] [Green Version]
- Lemos, J.L.S.; Bon, E.P.S.; Santana, M.d.F.E.; Pereira Junior, N. Thermal stability of xylanases produced by Aspergillus awamori. Brazilian J. Microbiol. 2000, 31, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Díaz, A.B.; Marzo, C.; Caro, I.; de Ory, I.; Blandino, A. Valorization of exhausted sugar beet cossettes by successive hydrolysis and two fermentations for the production of bio-products. Bioresour. Technol. 2017, 225, 225–233. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef] [Green Version]
- Hodge, D.B.; Karim, M.N.; Schell, D.J.; McMillan, J.D. Model-Based Fed-Batch for High-Solids Enzymatic Cellulose Hydrolysis. Appl. Biochem. Biotechnol. 2009, 152, 88–107. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Zhang, Y.; Yuan, Z.; Xie, J. Optimization of high solids fed-batch saccharification of sugarcane bagasse based on system viscosity changes. J. Biotechnol. 2015, 211, 5–9. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Yuan, Z.; Zhang, Y.; Liu, Y.; Liang, C. Optimization of fed-batch enzymatic hydrolysis from alkali-pretreated sugarcane bagasse for high-concentration sugar production. Bioresour. Technol. 2014, 167, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Park, H.M.; Kim, D.H.; Yang, J.; Kim, K.H. Fed-Batch Enzymatic Saccharification of High Solids Pretreated Lignocellulose for Obtaining High Titers and High Yields of Glucose. Appl. Biochem. Biotechnol. 2017, 182, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Al-Shorgani, N.K.N.; Shukor, H.; Abdeshahian, P.; Kalil, M.S.; Yusoff, W.M.W.; Hamid, A.A. Enhanced butanol production by optimization of medium parameters using Clostridium acetobutylicum YM1. Saudi J. Biol. Sci. 2018, 25, 1308–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Banerjee, R.; Sinha, P.K. Enzymatic delignification and saccharification of Bambusa bambos for biobutanol production. Ind. Crop. Prod. 2018, 125, 386–394. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Kim, M.; Yang, Y.; Morikawa-Sakura, M.S.; Wang, Q.; Lee, M.V.; Lee, D.-Y.; Feng, C.; Zhou, Y.; Zhang, Z. Hydrogen production by anaerobic co-digestion of rice straw and sewage sludge. Int. J. Hydrog. Energy 2012, 37, 3142–3149. [Google Scholar] [CrossRef]



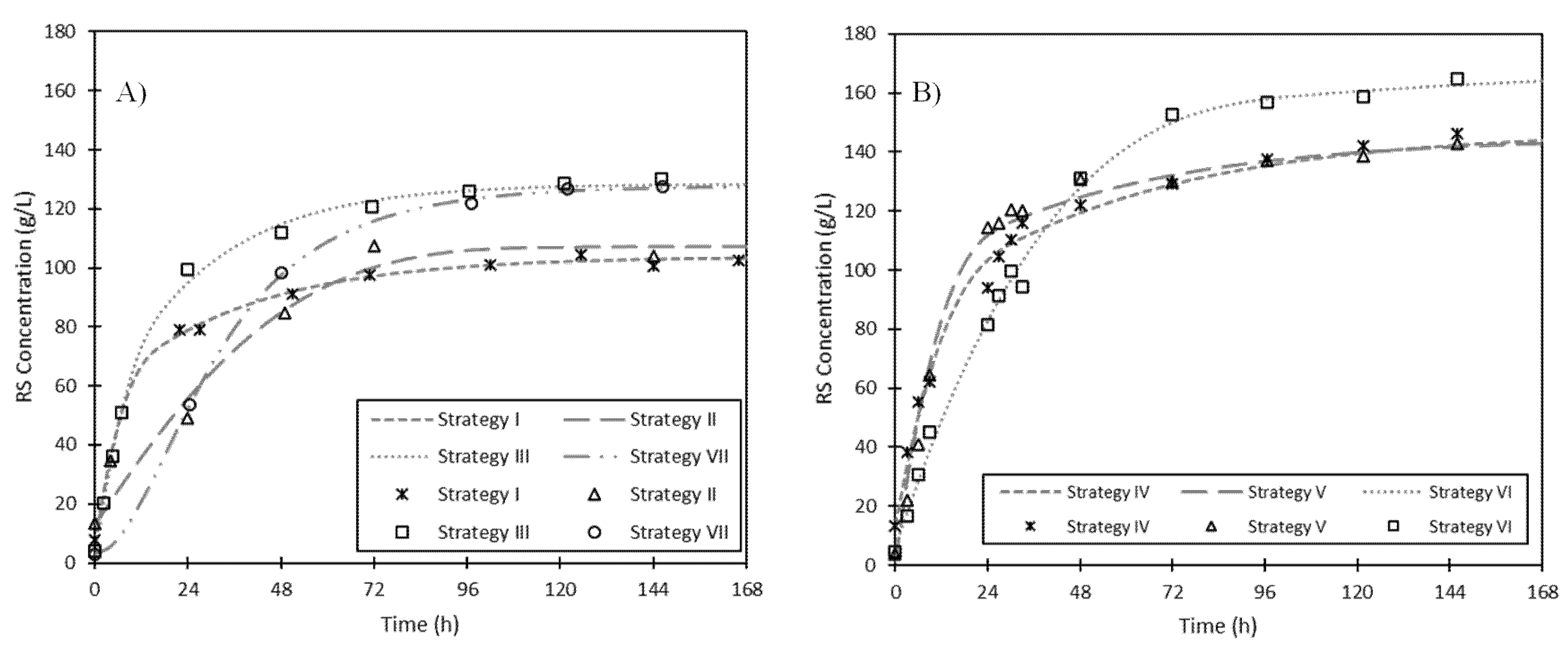
| Strategy Code | Total FFR | Addition Strategy | |||
|---|---|---|---|---|---|
| Fresh ESBPPs | Fermented ESBPPs | ||||
| I | 15:55 | 55 g | t = 0 h | 15 g | t = 0 h |
| II | 15:55 | 30 g; 25 g | t = 0; 24 h | 15 g | t = 0 h |
| III | 15:55 | 13.75 g (×4) | t = 0; 2.5; 5; 7.5 h | 15 g | t = 0 h |
| IV | 15:90 | 90 g | t = 0 h | 15 g | t = 0 h |
| V | 15:90 | 22.5 g (×4) | t = 0; 3; 6; 9 h | 15 g | t = 0 h |
| VI | 15:90 | 11.25 g (×8) | t = 0; 3; 6; 9; 24; 27; 30; 33 h | 15 g | t = 0 h |
| VII | 15:55 | 55 g | t = 0 h | 5 g (×3) | t = 0; 24; 48 h |
| FFR | T (°C) | G0 (g·L−1) | Gf (g·L−1) | kh (h−1) | P (g·L−1·h−1) | r2 | Yield (%) |
|---|---|---|---|---|---|---|---|
| 5:30 | 50 | 5.3 | 36.8 | 0.100 | 3.1 | 0.991 | 36.1 |
| 5:30 | 55 | 3.1 | 28.4 | 0.136 | 3.5 | 0.999 | 27.9 |
| 15:15 | 50 | 2.7 | 17.0 | 0.125 | 1.8 | 0.987 | 21.8 |
| 15:15 | 55 | 1.9 | 15.4 | 0.169 | 2.2 | 0.967 | 19.8 |
| 30:0 | 50 | 2.2 | 8.2 | 0.051 | 0.3 | 0.999 | 12.7 |
| 30:0 | 55 | 1.5 | 14.5 | 0.072 | 0.9 | 0.993 | 22.5 |
| FFR | G0 (g·L−1) | Gf (g·L−1) | kh (h−1) | P (g·L−1·h−1) | r2 | Yield (%) |
|---|---|---|---|---|---|---|
| 5:30 | 3.1 | 28.4 | 0.136 | 3.5 | 0.999 | 27.9 |
| 10:30 | 3.3 | 36.5 | 0.141 | 4.7 | 0.997 | 32.4 |
| 15:30 | 6.1 | 36.7 | 0.200 | 6.1 | 0.997 | 29.7 |
| 15:35 | 4.3 | 37.3 | 0.195 | 6.4 | 0.999 | 26.9 |
| 15:40 | 4.1 | 40.7 | 0.179 | 6.5 | 0.994 | 26.5 |
| 15:45 | 5.3 | 42.9 | 0.170 | 6.4 | 0.995 | 25.4 |
| 15:50 | 4.7 | 61.9 | 0.137 | 7.8 | 0.992 | 33.6 |
| 15:55 | 5.1 | 66.0 | 0.133 | 8.1 | 0.987 | 33.1 |
| U/g | G0 (g·L−1) | Gf (g·L−1) | kh (h−1) | P (g·L−1·h−1) | r2 | Yield (%) |
|---|---|---|---|---|---|---|
| 0 | 5.1 | 66.0 | 0.133 | 8.1 | 0.987 | 33.1 |
| 0.72 | 8.2 | 80.0 | 0.136 | 9.8 | 0.996 | 40.1 |
| 1.44 | 10.1 | 94.7 | 0.146 | 12.4 | 0.999 | 47.5 |
| 2.17 | 8.2 | 106.8 | 0.183 | 18.1 | 0.995 | 53.6 |
| 2.89 | 6.6 | 104.0 | 0.193 | 18.8 | 0.997 | 52.1 |
| Strategy | G0 (g·L−1) | Gf (g·L−1) | kh (h−1) | P (g·L−1·h−1) | r2 | Yield (%) |
|---|---|---|---|---|---|---|
| I | 6.6 | 104.0 | 0.193 | 18.8 | 0.997 | 52.1 |
| II | 13.4 | 107.7 | 0.087 | 8.2 | 0.962 | 54.0 |
| III | 2.4 | 127.3 | 0.195 | 24.3 | 0.998 | 63.8 |
| IV | 13.3 | 147.1 | 0.113 | 15.1 | 0.991 | 48.1 |
| V | 1.3 | 143.6 | 0.119 | 16.9 | 0.991 | 47.0 |
| VI | 6.8 | 168.0 | 0.040 | 6.4 | 0.996 | 54.9 |
| VII | 2.9 | 126.7 | 0.112 | 13.9 | 0.999 | 63.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Conversion of Exhausted Sugar Beet Pulp into Fermentable Sugars from a Biorefinery Approach. Foods 2020, 9, 1351. https://doi.org/10.3390/foods9101351
Marzo C, Díaz AB, Caro I, Blandino A. Conversion of Exhausted Sugar Beet Pulp into Fermentable Sugars from a Biorefinery Approach. Foods. 2020; 9(10):1351. https://doi.org/10.3390/foods9101351
Chicago/Turabian StyleMarzo, Cristina, Ana Belén Díaz, Ildefonso Caro, and Ana Blandino. 2020. "Conversion of Exhausted Sugar Beet Pulp into Fermentable Sugars from a Biorefinery Approach" Foods 9, no. 10: 1351. https://doi.org/10.3390/foods9101351








