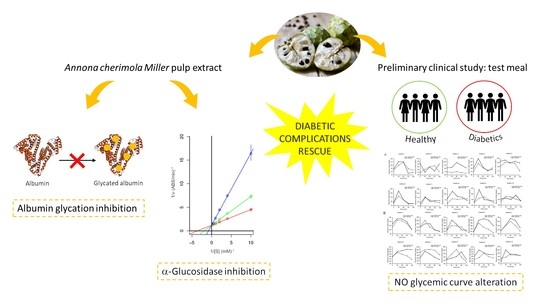
Annona cherimola Miller Fruit as a Promising Candidate against Diabetic Complications: An In Vitro Study and Preliminary Clinical Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Annona cherimola Miller Pulp Extract
2.3. Determination of Total Content of Polyphenols and Carbohydrates
2.4. Evaluation of Antioxidant and Radical Scavenging Activities
2.5. In Vitro Non-Enzymatic Glycation of Human Serum Albumin (aAGE)
2.6. The In Vitro Inhibition of aAGE Formation
2.7. Native Polyacrylamide Gel Electrophoresis (N-PAGE)
2.8. α-Glucosidase Activity Assay
2.9. In Vivo Preliminary Clinical Study
2.9.1. Enrollment of Participants
2.9.2. Cross-Over Design Intervention Study
2.9.3. Clinical Testing Schedule
2.10. Statistical Analysis for In Vitro Experiments and Cross-Over Design Intervention Study
3. Results and Discussion
3.1. Biochemical Characterization of Annona cherimola Miller Pulp Extract
3.2. In Vitro Annona cherimola Miller Pulp Extract Anti-Glycation Activity
3.3. Annona cherimola Mill Pulp Extract Competitively Inhibits α-Glucosidase Enzymatic Activity
3.4. Impact of Annona cherimola Miller Pulp Fruit on Changes in Postprandial Glycemia in Healthy Subjects and DM2 Patients
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36, S67–S74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Diagnosis and Management of Type 2 Diabetes (HEARTS-D); World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Vasarri, M.; Barletta, E.; Ramazzotti, M.; Degl’Innocenti, D. In Vitro Anti-Glycation Activity of the Marine Plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 259, 112960. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, P.; Bourdon, E. The Glycation of Albumin: Structural and Functional Impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, S.; Sánchez, S.S.; Yousuf, S.; Honoré, S.M.; Choudhary, M.I. Drug repurposing: In-vitro anti-glycation properties of 18 common drugs. PLoS ONE 2018, 13, e0190509. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Well 2014, 3, 136–174. [Google Scholar] [CrossRef] [Green Version]
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.P. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef]
- Devalaraja, S.; Jain, S.; Yadav, H. Exotic Fruits as Therapeutic Complements for Diabetes, Obesity and Metabolic Syndrome. Food Res. Int. 2011, 44, 1856–1865. [Google Scholar] [CrossRef] [Green Version]
- Bonavia, D.; Ochoa, C.M.; Óscar Tovar, S.; Cerrón Palomino, R. Archaeological evidence of cherimoya(Annona cherimolia Mill.) and Guanabana (Annona muricata L.) in ancient Peru. Econ. Bot. 2004, 58, 509–522. [Google Scholar] [CrossRef]
- Gullo, G.; Dattola, A.; Zappia, R. Comparative study of some fruit quality characteristics of two of Annona cherimola Mill. grown in southern Italy. AIMS Agric. Food 2019, 4, 658–671. [Google Scholar] [CrossRef]
- Amoo, I.A.; Emenike, A.E.; Akpambang, V.O.E. Compositional evaluation of Annona cherimoya (custard apple) fruit. Trends Appl. Sci. Res. 2008, 2, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Solares-Pascasio, J.I.; Ordoñez-Razo, R.M.; Velazquez, C.; Barbosa, E.; García-Hernández, N.; Mendez-Luna, D.; Correa-Basurto, J. Antihyperglycemic Activity of the Leaves from Annona cherimola Miller and Rutin on Alloxan-induced Diabetic Rats. Pharmacogn. Res. 2017, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Falé, P.L.; Ferreira, C.; Maruzzella, F.; Helena Florêncio, M.; Frazão, F.N.; Serralheiro, M.L. Evaluation of cholesterol absorption and biosynthesis by decoctions of Annona cherimola leaves. J. Ethnopharmacol. 2013, 150, 718–723. [Google Scholar] [CrossRef]
- Verma, A.M.; Kumar, A.P.; Shekar, R.K.; Kumar, K.A.; Chakrapani Rani, R.A. Pharmacological Screening of Annona cherimola for Antihyperlipidemic Potential. J. Basic Clin. Pharm. 2011, 2, 63–69. [Google Scholar]
- Martínez-Vázquez, M.; Estrada-Reyes, R.; Araujo Escalona, A.G.; Ledesma Velázquez, I.; Martínez-Mota, L.; Moreno, J.; Heinze, G. Antidepressant-like effects of an alkaloid extract of the aerial parts of Annona cherimolia in mice. J. Ethnopharmacol. 2012, 139, 164–170. [Google Scholar] [CrossRef]
- Velázquez, C.; Calzada, F.; Torres, J.; González, F.; Ceballos, G. Antisecretory activity of plants used to treat gastrointestinal disorders in Mexico. J. Ethnopharmacol. 2006, 103, 66–70. [Google Scholar] [CrossRef]
- Albuquerque, T.G.; Santos, F.; Sanches-Silva, A.; Beatriz Oliveira, M.; Bento, A.C.; Costa, H.S. Nutritional and phytochemical composition of Annona cherimola Mill. fruits and by-products: Potential health benefits. Food Chem. 2016, 193, 187–195. [Google Scholar] [CrossRef]
- Gupta-Elera, G.; Garrett, A.R.; Martinez, A.; Robison, R.A.; O’Neill, K.L. The antioxidant properties of the cherimoya (Annona cherimola) fruit. Int. Food Res. J. 2011, 44, 2205–2209. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Avallone, L.; Menichini, F. Radical scavenging, antioxidant and metal chelating activities of Annona cherimola Mill. (cherimoya) peel and pulp in relation to their total phenolic and total flavonoid contents. J. Food Compos. Anal. 2012, 25, 179–184. [Google Scholar] [CrossRef]
- Barletta, E.; Ramazzotti, M.; Fratianni, F.; Pessani, D.; Degl’Innocenti, D. Hydrophilic extract from Posidonia oceanica inhibits activity and expression of gelatinases and prevents HT1080 human fibrosarcoma cell line invasion. Cell Adh. Migr. 2015, 9, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leri, M.; Ramazzotti, M.; Vasarri, M.; Peri, S.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. Bioactive Compounds from Posidonia oceanica (L.) Delile Impair Malignant Cell Migration through Autophagy Modulation. Mar. Drugs 2018, 16, 137. [Google Scholar] [CrossRef] [Green Version]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl’Innocenti, D. Anti-inflammatory properties of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 247, 112252. [Google Scholar] [CrossRef]
- Sharma, S.D.; Pandey, B.N.; Mishra, K.P.; Sivakami, S. Amadori product and age formation during nonenzymatic glycosylation of bovine serum albumin in vitro. J. Biochem. Mol. Biol. Biophys. 2002, 6, 233–242. [Google Scholar] [CrossRef]
- Moradi-Afrapoli, F.; Asghari, B.; Saeidnia, S.; Ajani, Y.; Mirjani, M.; Malmir, M.; Dolatabadi Bazaz, R.; Hadjiakhoondi, A.; Salehi, P.; Hamburger, M.; et al. In vitro α-glucosidase inhibitory activity of phenolic constituents from aerial parts of Polygonum hyrcanicum. Daru 2012, 20, 37. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://ndb.nal.usda.gov/fdc-app.html#/food-details/173953/nutrients (accessed on 13 January 2020).
- Ghatule, R.R.; Gautam, M.K.; Goel, S.; Singh, A.; Joshi, V.K.; Goel, R.K. Protective effects of Aegle marmelos fruit pulp on 2,4,6-trinitrobenzene sulfonic acid-induced experimental colitis. Pharmacogn. Mag. 2014, 10, S147–S152. [Google Scholar] [CrossRef]
- Kebebew, Z.; Shibeshi, W. Evaluation of anxiolytic and sedative effects of 80% ethanolic Carica papaya L. (Caricaceae) pulp extract in mice. J. Ethnopharmacol. 2013, 150, 665–671. [Google Scholar] [CrossRef]
- Belhekar, S.N.; Chaudhari, P.D.; Saryawanshi, J.S.; Mali, K.K.; Pandhare, R.B. Antidiabetic and antihyperlipidemic effects of Thespesia populnea fruit pulp extracts on alloxan-induced diabetic rats. Indian J. Pharm. Sci. 2013, 75, 217–221. [Google Scholar]
- Giomaro, G.; Karioti, A.; Bilia, A.R.; Bucchini, A.; Giamperi, L.; Ricci, D.; Fraternale, D. Polyphenols profile and antioxidant activity of skin and pulp of a rare apple from Marche region (Italy). Chem. Cent. J. 2014, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Salas, P.; Gómez-Caravaca, A.M.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification and quantification of phenolic and other polar compounds in the edible part of Annona cherimola and its by-products by HPLC-DAD-ESI-QTOF-MS. Int. Food Res. J. 2015, 78, 246–257. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Ficarra, S.; Tellone, E.; Leuzzi, U.; Galtieri, A.; Bellocco, E. Evaluation of the antioxidant and cytoprotective properties of the exotic fruit Annona cherimola Mill. (Annonaceae). Int. Food Res. J. 2011, 44, 2302–2310. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Santos, M.C.; Moro, T.M.; Basto, G.J.; Andrade, R.M.; Gonçalves, É.C. Formulation and characterization of functional foods based on fruit and vegetable residue flour. J. Food Sci. Technol. 2015, 52, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Bonnefont-Rousselot, D. Thérapeutiques anti-oxydantes et anti-AGE: Bilans et perspectives [Antioxidant and anti-AGE therapeutics: Evaluation and perspectives]. J. Soc. Biol. 2001, 195, 391–398. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Alpha-glucosidase inhibitors. Endocrinol. Metab. Clin. N. Am. 1997, 26, 539–551. [Google Scholar] [CrossRef]
- Van de Laar, F.A. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef] [Green Version]
- Derosa, G.; Maffioli, P. α-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. 2012, 8, 899–906. [Google Scholar] [CrossRef]
- Ceriello, A. Postprandial Hyperglycemia and Diabetes Complications. Diabetes 2005, 54, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, H.K.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [Green Version]





| Cappuccino (120 g of Milk) | Brioche (45 g) | |
|---|---|---|
| Proteins (g) | 4 | 3.6 |
| Fats (g) | 4 | 9 |
| Carbohydrates (g) | 6 | 29 |
| Calories (kcal) | 77 | 205.5 |
| Nutrient | Unit | Amount |
|---|---|---|
| Water | g | 79.66 |
| Proteins | g | 1.58 |
| Lipids (total) | g | 0.68 |
| Sugars (total) | g | 12.91 |
| Carbohydrates | g | 17.77 |
| Fibers (total) | g | 3 |
| Energy | kcal | 75 |
| Minerals | ||
| Calcium | mg | 10 |
| Iron | mg | 0.27 |
| Sodium | mg | 7 |
| Potassium | mg | 288 |
| Zinc | mg | 0.16 |
| Magnesium | mg | 17 |
| Phosphorus | mg | 26 |
| Vitamins | ||
| Thiamine | mg | 0.101 |
| Vitamin C | mg | 12.6 |
| Niacin | mg | 0.646 |
| Riboflavin | mg | 0.131 |
| Folate | μg | 23 |
| Vitamin B-6 | mg | 0.258 |
| Vitamin B-12 | μg | 0.000 |
| Vitamin A | μg | 0.000 |
| Vitamin E | mg | 0.27 |
| Vitamin (IU) | IU | 5 |
| Lipids | ||
| Fatty acids, total polyunsaturated | g | 0.189 |
| Fatty acids (total saturated) | g | 0.234 |
| Fatty acids (total monounsaturated) | g | 0.055 |
| Fatty acids, total trans | g | 0.000 |
| Cholesterol | mg | 0.000 |
| TC | TP | Antioxidant Power | Radical Scavenging Activity | |
|---|---|---|---|---|
| Method | Phenol-sulfuric acid | Folin–Ciocalteau | FRAP | DPPH |
| Reference | Glucose | Gallic acid | Ascorbic acid | Ascorbic acid |
| CE (mg/g) * | 3306 ± 154.64 | 9.249 ± 0.41 | 1.134 ± 0.082 | 7.212 ± 0.034 |
| CE (mg/mL) ** | 480.23 ± 22.46 | 1.343 ± 0.03 | 0.165 ± 0.012 | 1.047 ± 0.005 |
| Healthy Subjects | DM2 Patients | |
|---|---|---|
| Sex | N = 10 (F = 8; M = 2) | N = 10 (F = 7; M = 3) |
| Age (years) | 29.3 ± 8.6 | 63.90 ± 9.35 |
| Body weight (kg) | 58.20 ± 10.34 | 65.12 ± 9.1 |
| Height (cm) | 163.4 ± 7.1 | 161.2 ± 4.3 |
| Body Mass Index (kg/m2) | 21.73 ± 2.62 | 25.06 ± 2.45 |
| Fasting blood glucose (mg/dL) | 74.22 ± 8.71 | 135.20 ± 28.91 |
| Healthy Subjects | DM2 Patients | |||
|---|---|---|---|---|
| Time (min) | Standard Meal | Test Meal | Standard Meal | Test Meal |
| 0 | 74.0 ± 2.90 | 83.1 ± 2.08 | 134.9 ± 8.80 | 130.2 ± 8.21 |
| 30 | 91.6 ± 9.27 | 104 ± 6.10 | 167.3 ± 8.71 | 182.2 ± 10.78 |
| 60 | 88.5 ± 3.98 | 90.5 ± 2.95 | 173.2 ± 9.91 | 176.3 ± 7.27 |
| 90 | 81.1 ± 2.62 | 84.3 ± 2.79 | 152.8 ± 8.50 | 167.8 ± 10.08 |
| 120 | 76.1 ± 2.40 | 81.7 ± 2.35 | 125.8 ± 9.23 | 144.1 ± 11.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasarri, M.; Barletta, E.; Vinci, S.; Ramazzotti, M.; Francesconi, A.; Manetti, F.; Degl’Innocenti, D. Annona cherimola Miller Fruit as a Promising Candidate against Diabetic Complications: An In Vitro Study and Preliminary Clinical Results. Foods 2020, 9, 1350. https://doi.org/10.3390/foods9101350
Vasarri M, Barletta E, Vinci S, Ramazzotti M, Francesconi A, Manetti F, Degl’Innocenti D. Annona cherimola Miller Fruit as a Promising Candidate against Diabetic Complications: An In Vitro Study and Preliminary Clinical Results. Foods. 2020; 9(10):1350. https://doi.org/10.3390/foods9101350
Chicago/Turabian StyleVasarri, Marzia, Emanuela Barletta, Santina Vinci, Matteo Ramazzotti, Andrea Francesconi, Francesco Manetti, and Donatella Degl’Innocenti. 2020. "Annona cherimola Miller Fruit as a Promising Candidate against Diabetic Complications: An In Vitro Study and Preliminary Clinical Results" Foods 9, no. 10: 1350. https://doi.org/10.3390/foods9101350
APA StyleVasarri, M., Barletta, E., Vinci, S., Ramazzotti, M., Francesconi, A., Manetti, F., & Degl’Innocenti, D. (2020). Annona cherimola Miller Fruit as a Promising Candidate against Diabetic Complications: An In Vitro Study and Preliminary Clinical Results. Foods, 9(10), 1350. https://doi.org/10.3390/foods9101350