Optimization of Osmotic Dehydration of Tomatoes in Solutions of Non-Conventional Sweeteners by Response Surface Methodology and Desirability Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Pretreatments and Osmotic Dehydration (OD) Process
2.2. Physico-Chemical and Quality Parameters
2.2.1. Mass Transfer
2.2.2. Color
2.2.3. Texture
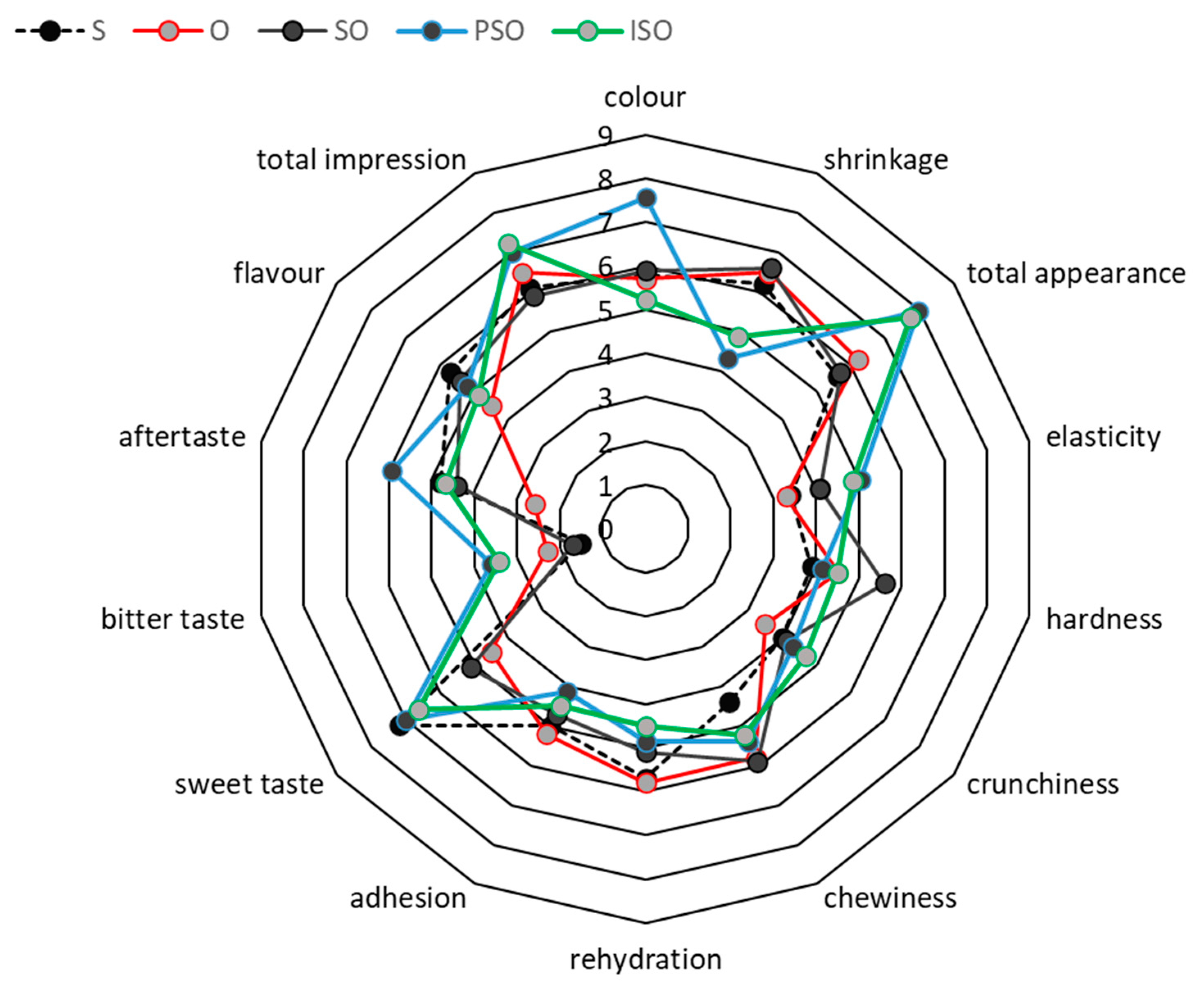
2.2.4. Sensory Analysis
2.3. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Process Parameters’ Effect on Color Characteristics during Osmotic Dehydration (OD)
3.2. Process Parameters’ Effect on Texture Characteristics during Osmotic Dehydration (OD)
3.3. Determination of Quality Factor Interactions during Osmotic Dehydration (OD)
3.4. Optimization of Process Conditions, Based on Mass Transfer and Quality Requirements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blum, A.; Monir, M.; Wirsansky, I.; Ben-Arzi, S. The beneficial effects of tomatoes. Eur. J. Intern. Med. 2005, 16, 402–404. [Google Scholar] [CrossRef]
- Telis, V.R.N.; Murari, R.C.B.D.L.; Yamashita, F. Diffusion coefficients during osmotic dehydration of tomatoes in ternary solutions. J. Food Eng. 2004, 61, 253–259. [Google Scholar] [CrossRef]
- Katsoufi, S.; Lazou, A.E.; Giannakourou, M.C.; Krokida, M.K. Mass transfer kinetics and quality attributes of osmo-dehydrated candied pumpkins using nutritious sweeteners. J. Food Sci. Technol. 2017, 54, 3338–3348. [Google Scholar] [CrossRef]
- Krasnova, I.; Seglina, D.; Pole, V. The effect of pre-treatment methods on the quality of dehydrated candied Japanese quince fruits during storage. J. Food Sci. Technol. 2018, 55, 4468–4476. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Tappi, S.; Wang, C.; Yu, Y.; Zhu, S.; Rocculi, P. Study and optimization of high hydrostatic pressure (HHP) to improve mass transfer and quality characteristics of candied green plums (Prunus mume). J. Food Process. Preserv. 2018, 42, e13769. [Google Scholar] [CrossRef]
- Fito, P.; Chiralt, A.; Barat, J.M.; Andrés, A.; Martínez-Monzó, J.; Martínez-Navarrete, N. Vacuum impregnation for development of new dehydrated products. J. Food Eng. 2001, 49, 297–302. [Google Scholar] [CrossRef]
- Vilela, A.; Sobreira, C.; Abraão, A.S.; Lemos, A.M.; Nunes, F.M. Texture Quality of Candied Fruits as Influenced by Osmotic Dehydration Agents. J. Texture Stud. 2016, 47, 239–252. [Google Scholar] [CrossRef]
- Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Effect of candying on cell wall polysaccharides of plums (Prunus domestica L.) and influence of cell wall enzymes. Food Chem. 2008, 111, 538–548. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Boulekou, S.; Giannakourou, M.; Taoukis, P. Osmodehydrofreezing of tomato: From production to consumption. Acta Hortic. 2007, 758, 159–164. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Boulekou, S.; Taoukis, P.S. Mass transfer kinetics during osmotic dehydration of cherry tomatoes pre-treated by high hydrostatic pressure. Acta Hortic. 2008, 802, 127–132. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Andreou, V.; Alexandrakis, Z.; Katsaros, G.J.; Giannakourou, M.C.; Taoukis, P.S. The hurdle effect of osmotic pretreatment and high-pressure cold pasteurisation on the shelf-life extension of fresh-cut tomatoes. Int. J. Food Sci. Technol. 2017, 52, 916–926. [Google Scholar] [CrossRef]
- Derossi, A.; Severini, C.; Del Mastro, A.; De Pilli, T. Study and optimization of osmotic dehydration of cherry tomatoes in complex solution by response surface methodology and desirability approach. LWT—Food Sci. Technol. 2015, 60 Pt 1, 641–648. [Google Scholar] [CrossRef]
- Torreggiani, D. Osmotic dehydration in fruit and vegetable processing. Food Res. Int. 1993, 26, 59–68. [Google Scholar] [CrossRef]
- Torreggiani, D.; Bertolo, G. Osmotic pre-treatments in fruit processing: Chemical, physical and structural effects. J. Food Eng. 2001, 49, 247–253. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Cichowska, J.; Kowalska, H.; Czajkowska, K.; Lenart, A. Osmotic dehydration of Braeburn variety apples in the production of sustainable food products. Int. Agrophys. 2018, 32, 141–146. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Kowalska, H.; Czajkowska, K.; Lenart, A. Osmotic dehydration in production of sustainable and healthy food. Trends Food Sci. Technol. 2016, 50, 186–192. [Google Scholar] [CrossRef]
- Kowalska, H.; Marzec, A.; Kowalska, J.; Ciurzyńska, A.; Czajkowska, K.; Cichowska, J.; Rybak, K.; Lenart, A. Osmotic dehydration of Honeoye strawberries in solutions enriched with natural bioactive molecules. LWT—Food Sci. Technol. 2017, 85, 500–505. [Google Scholar] [CrossRef]
- Brochier, B.; Marczak, L.D.F.; Noreña, C.P.Z. Osmotic Dehydration of Yacon Using Glycerol and Sorbitol as Solutes: Water Effective Diffusivity Evaluation. Food Bioprocess. Technol. 2014, 8, 623–636. [Google Scholar] [CrossRef]
- Brochier, B.; Marczak, L.D.F.; Noreña, C.P.Z. Use of different kinds of solutes alternative to sucrose in osmotic dehydration of yacon. Braz. Arch. Biol. Technol. 2015, 58, 34–40. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Lech, K.; Figiel, A. Influence of Osmodehydration Pretreatment and Combined Drying Method on the Bioactive Potential of Sour Cherry Fruits. Food Bioprocess. Technol. 2015, 8, 824–836. [Google Scholar] [CrossRef]
- Sabetghadam, M.; Tavakolipour, H. Osmo-coating and ultrasonic dehydration as pre-treatment for hot air-drying of flavoured apple. Eng. Agric. Environ. Food 2015, 8, 318–327. [Google Scholar] [CrossRef]
- Gong, C.; Liao, M.; Zhang, H.; Xu, Y.; Miao, Y.; Jiao, S. Investigation of Hot Air–Assisted Radio Frequency as a Final-Stage Drying of Pre-Dried Carrot Cubes. Food Bioprocess. Technol. 2020, 13, 419–429. [Google Scholar] [CrossRef]
- Kumari, V.; Yadav, B.S.; Yadav, R.B.; Nema, P.K. Effect of osmotic agents and ultasonication on osmo-convective drying of sweet lime (Citrus limetta) peel. J. Food Process. Eng. 2020, 43, e13371. [Google Scholar] [CrossRef]
- Rodriguez, A.; Sancho, A.M.; Barrio, Y.; Rosito, P.; Gozzi, M.S. Combined drying of Nopal pads (Opuntia ficus-indica): Optimization of osmotic dehydration as a pretreatment before hot air drying. J. Food Process. Preserv. 2019, 43, e14183. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Giannakourou, M.C.; Taoukis, P. Stability of dehydrofrozen tomatoes pretreated with alternative osmotic solutes. J. Food Eng. 2007, 78, 272–280. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Giannakourou, M.C.; Taoukis, P.S. Kinetic modelling of the degradation of quality of osmo-dehydrofrozen tomatoes during storage. Food Chem. 2007, 103, 985–993. [Google Scholar] [CrossRef]
- Ozen, B.F.; Dock, L.L.; Ozdemir, M.; Floros, J.D. Processing factors affecting the osmotic dehydration of diced green peppers. Int. J. Food Sci. Technol. 2002, 37, 492–502. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, Y. Use of vacuum impregnation to develop high quality and nutritionally fortified frozen strawberries. J. Food Process. Preserv. 2004, 28, 117–132. [Google Scholar] [CrossRef]
- Jalali, V.R.R.; Narain, N.; Silva, G.F. Effect of osmotic predehydration on drying characteristics of banana fruits. Food Sci. Technol. 2008, 28, 269–273. [Google Scholar] [CrossRef][Green Version]
- Nowacka, M.; Tylewicz, U.; Laghi, L.; Dalla Rosa, M.; Witrowa-Rajchert, D. Effect of ultrasound treatment on the water state in kiwifruit during osmotic dehydration. Food Chem. 2014, 144, 18–25. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. Freezing Characteristics and Storage Stability of Broccoli (Brassica oleracea L. var. botrytis L.) under Osmodehydrofreezing and Ultrasound-Assisted Osmodehydrofreezing Treatments. Food Bioprocess. Technol. 2014, 7, 1736–1744. [Google Scholar]
- Parniakov, O.; Bals, O.; Lebovka, N.; Vorobiev, E. Effects of pulsed electric fields assisted osmotic dehydration on freezing-thawing and texture of apple tissue. J. Food Eng. 2016, 183, 32–38. [Google Scholar] [CrossRef]
- Parniakov, O.; Lebovka, N.I.; Bals, O.; Vorobiev, E. Effect of electric field and osmotic pre-treatments on quality of apples after freezing-thawing. Innov. Food Sci. Emerg. Technol. 2015, 29, 23–30. [Google Scholar] [CrossRef]
- Ahmed, I.; Qazi, I.M.; Jama, S. Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov. Food Sci. Emerg. Technol. 2016, 34, 39–43. [Google Scholar] [CrossRef]
- Samuel, P.; Ayoob, K.T.; Magnuson, B.A.; Wölwer-Rieck, U.; Jeppesen, P.B.; Rogers, P.J.; Rowland, I.; Mathews, R. Stevia Leaf to Stevia Sweetener: Exploring Its Science, Benefits, and Future Potential. J. Nutr. 2018, 148, 1186S–1205S. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Arraez, S.; Capella, J.V.; Ortolá, M.D.; Castelló, M.L. Modelling osmotic dehydration of lemon slices using new sweeteners. Int. J. Food Sci. Technol. 2015, 50, 2046–2051. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Giannakourou, M.C. Modelling dehydration of apricot in a non-conventional multi-component osmotic solution: Effect on mass transfer kinetics and quality characteristics. J. Food Sci. Technol. 2018, 55, 4079–4089. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Giannakourou, M.C. Evaluation and modelling of osmotic pre-treatment of peach using alternative agents in a multiple-component solution. J. Sci. Food Agric. 2019, 99, 1240–1249. [Google Scholar] [CrossRef]
- Peinado, I.; Rosa, E.; Heredia, A.; Escriche, I.; Andrés, A. Influence of processing on the volatile profile of strawberry spreads made with isomaltulose. Food Chem. 2013, 138, 621–629. [Google Scholar] [CrossRef]
- Tan, V.W.K.; Wee, M.S.M.; Tomic, O.; Forde, C.G. Temporal sweetness and side tastes profiles of 16 sweeteners using temporal check-all-that-apply (TCATA). Food Res. Int. 2019, 121, 39–47. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Zachariou, I.; Andreou, V.; Taoukis, P. Effect of pulsed electric fields on mass transfer and quality of osmotically dehydrated kiwifruit. Food Bioprod. Process. 2016, 100, 535–544. [Google Scholar] [CrossRef]
- Nambiar, S.S.; Basu, A.; Shetty, N.P.; Rastogi, N.K.; Prapulla, S.G. Infusion of fructooligosaccharide in Indian gooseberry (Emblica officinalis) fruit using osmotic treatment and its effect on the antioxidant activity of the fruit. J. Food Eng. 2016, 190, 139–146. [Google Scholar] [CrossRef]
- Azarpazhooh, E.; Ramaswamy, H.S. Modeling and Optimization of Microwave Osmotic Dehydration of Apple Cylinders under Continuous-Flow Spray Mode Processing Conditions. Food Bioprocess. Technol. 2012, 5, 1486–1501. [Google Scholar] [CrossRef]
- Eren, İ.; Kaymak-Ertekin, F. Optimization of osmotic dehydration of potato using response surface methodology. J. Food Eng. 2007, 79, 344–352. [Google Scholar] [CrossRef]
- Lazou, A.E.; Dermesonlouoglou, E.K.; Giannakourou, M.C. Modeling and Evaluation of the Osmotic Pretreatment of Tomatoes (S. lycopersicum) with Alternative Sweeteners for the Production of Candied Products. Food Bioprocess. Technol. 2020, 13, 948–961. [Google Scholar] [CrossRef]
- Rao, S.N.; Barringer, S.A. Timing of calcium treatment on resistance of raw and canned diced tomatoes to mechanical abuse. J. Food Process. Preserv. 2005, 29, 1–7. [Google Scholar] [CrossRef]
- Roueita, G.; Hojjati, M.; Noshad, M. Study of Physicochemical Properties of Dried Kiwifruits Using the Natural Hypertonic Solution in Ultrasound-Assisted Osmotic Dehydration as Pretreatment. Int. J. Fruit Sci. 2020. [Google Scholar] [CrossRef]
- Nishinari, K.; Kohyama, K.; Kumagai, H.; Funami, T.; Bourne, M.C. Parameters of Texture Profile Analysis. Food Sci. Technol. Res. 2013, 19, 519–521. [Google Scholar] [CrossRef]
- Bourne, M.C. Food Texture and Viscosity: Concept and Measurements, 2nd ed.; Academic Press: New York, NY, USA, 2002. [Google Scholar]
- Terkmane, N.; Krea, M.; Moulai-Mostefa, N. Optimisation of inulin extraction from globe artichoke (Cynara cardunculus L. subsp. scolymus (L.) Hegi) by electromagnetic induction heating process. Int. J. Food Sci. Technol. 2016, 51, 1997–2008. [Google Scholar]
- Mayers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 415–420. [Google Scholar]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Costa, N.; Lourenço, J. A comparative study of multiresponse optimization criteria working ability. Chemometr. Intell. Lab. Syst. 2014, 138, 171–177. [Google Scholar] [CrossRef]
- Costa, N.R.; Lourenço, J.; Pereira, Z.L. Desirability function approach: A review and performance evaluation in adverse conditions. Chemometr. Intell. Lab. Syst. 2011, 107, 234–244. [Google Scholar] [CrossRef]
- Piombino, P.; Sinesio, F.; Moneta, E.; Cammareri, M.; Genovese, A.; Lisanti, M.T.; Mogno, M.; Peparaio, M.; termolino, P.; Moio, L.; et al. Investigating physicochemical volatile and sensory parameters playing a positive or a negative role on tomato liking. Food Res. Int. 2013, 50, 409–419. [Google Scholar] [CrossRef]
- Heredia, A.; Andres, A. Mathematical Equations to Predict Mass Fluxes and Compositional Changes during Osmotic Dehydration of Cherry Tomato Halves. Dry. Technol. 2008, 26, 873–883. [Google Scholar] [CrossRef]
- Contreras, C.; Martın-Esparza, M.E.; Chiralt, A.; Martınez-Navarrete, N. Influence of microwave application on convective drying: Effects on drying kinetics and optical and mechanical properties of apple and strawberry. J. Food Eng. 2008, 88, 55–64. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; Al-Harahsheh, M.; Hararah, M.; Magee, T.R.A. Drying characteristics and quality change of unutilized-protein rich-tomato pomace with and without osmotic pre-treatment. Ind. Crops Prod. 2010, 31, 171–177. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Wang, Y.; Li, J.; Yang, Y.; Liu, X. The Effects of Storage Conditions on Lycopene Content and Color of Tomato Hot Pot Sauce Open Access. Int. J. Anal. Chem. 2018, 2018, 1273907. [Google Scholar] [CrossRef]
- Jorge, A.; Almeida, D.M.; Giovanetti Canteri, M.H.; Sequinel, T.; Toniolo Kubaski, E.; Mazurek Tebcherani, S. Evaluation of the chemical composition and colour in long-life tomatoes (Lycopersicon esculentum Mill) dehydrated by combined drying methods. Int. J. Food Sci. Technol. 2014, 49, 2001–2007. [Google Scholar] [CrossRef]
- Wu, G.-F.; Jinag, W.; Wang, C.-G.; Li, L. Quality changes during tomato shelf-life based on texture profile analysis and visible/near-infrared spectroscopy. Mod. Food Sci. Technol. 2015, 31, 290–294. [Google Scholar]
- Barragán-Iglesias, J.; Rodríguez-Ramírez, J.; Sablani, S.S.; Méndez-Lagunas, L.L. Texture analysis of dried papaya (Carica papaya L., cv. Maradol) pretreated with calcium and osmotic dehydration. Dry. Technol. 2019, 37, 906–919. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.A.; Kumari, A.; Rafiq, M.; Siddeeg, A.; Manzoor, M.F.; Baloch, Z.; Ahmed, Z. Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochem. 2019, 58, 104643. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Simpson, R.; Pizarro, N.; Parada, K.; Pinilla, N.; Reyes, J.E.; Almonacid, S. Effect of ohmic heating and vacuum impregnation on the quality and microbial stability of osmotically dehydrated strawberries (cv. Camarosa). J. Food Eng. 2012, 110, 310–316. [Google Scholar] [CrossRef]
- Martynenko, A.; Janaszek, M.A. Texture Changes during Drying of Apple Slices. Dry. Technol. 2014, 32, 567–577. [Google Scholar] [CrossRef]
- Grenby, T.H. Intense sweeteners for the food industry: An overview. Trends Food Sci. Technol. 1991, 2, 2–6. [Google Scholar] [CrossRef]
- Lina, B.A.R.; Jonker, D.; Kozianowski, G. Isomaltulose (Palatinose®): A review of biological and toxicological studies. Food Chem. Toxicol. 2002, 40, 1375–1381. [Google Scholar] [CrossRef]
- Konopacka, D.; Jesionkowska, K.; Klewicki, R.; Bonazzi, C. The effect of different osmotic agents on the sensory perception of osmo-treated dried fruit. J. Hortic. Sci. Biotechnol. 2009, 84, 80–84. [Google Scholar] [CrossRef]

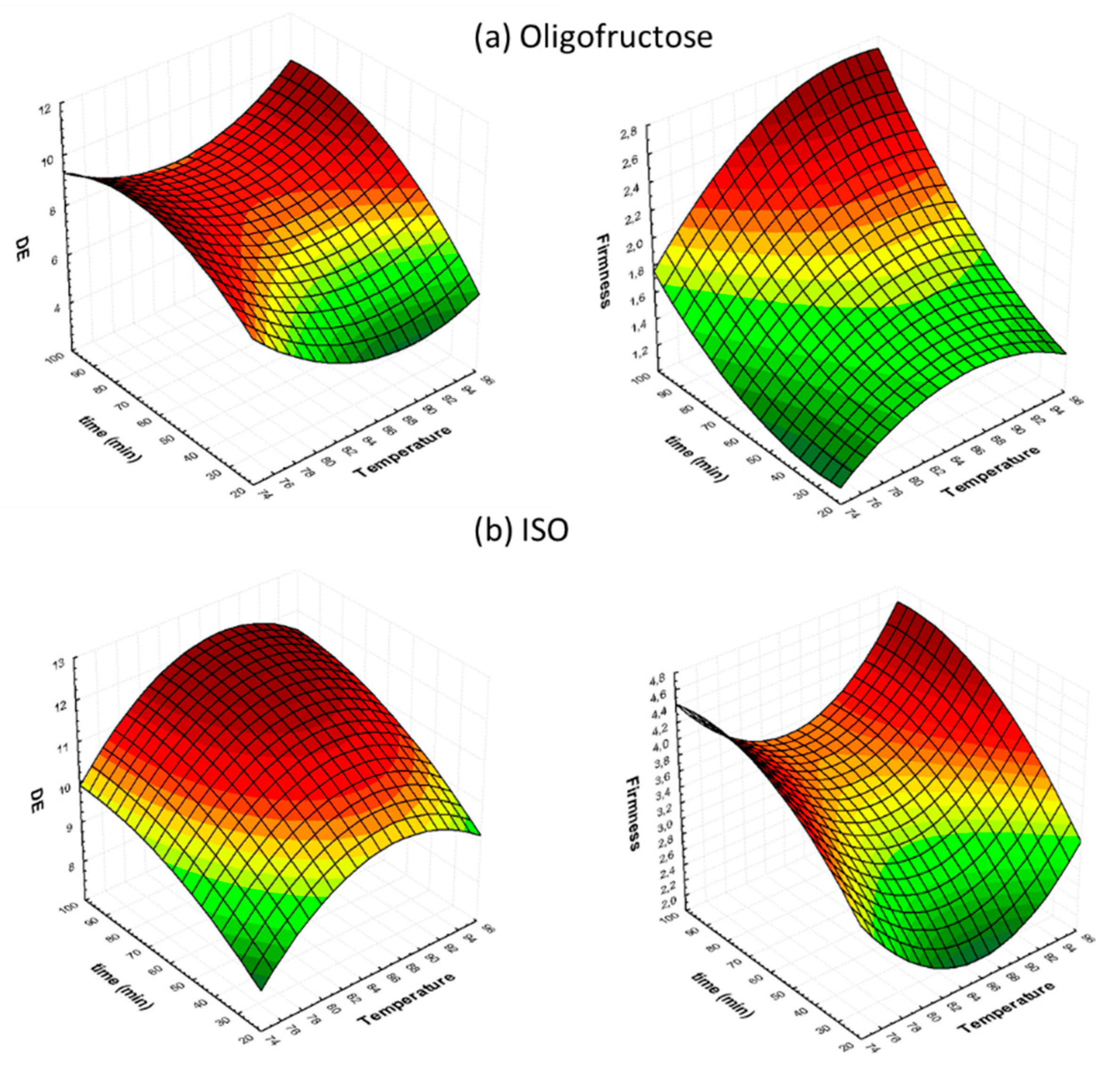

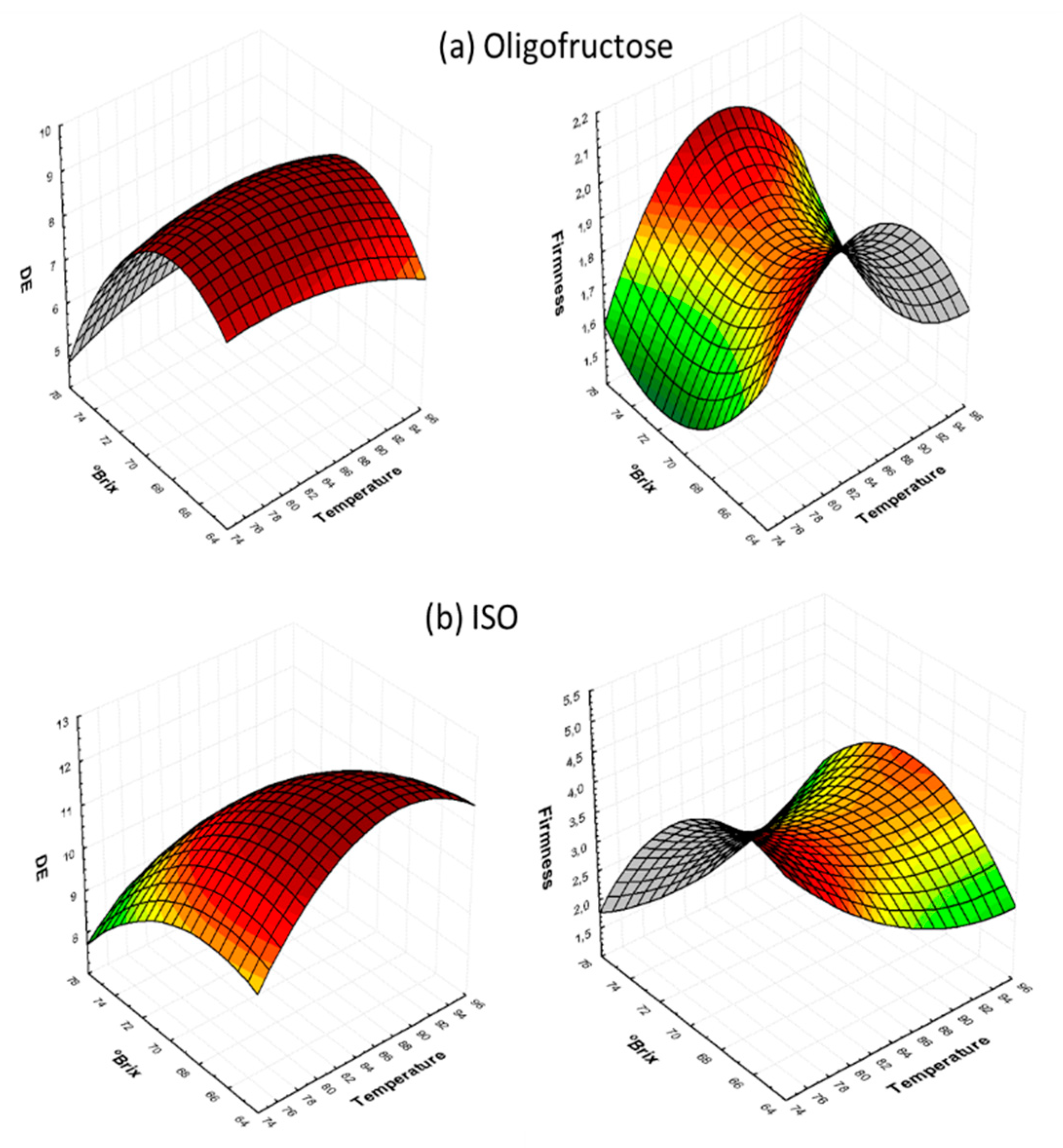

| OD Treatment Variables | Temperature (°C) | Concentration (°Brix) | OD Time (min) | X1 | X2 | X3 |
|---|---|---|---|---|---|---|
| High | 95 | 75 | 90 | +1 | +1 | +1 |
| Center | 85 | 70 | 60 | 0 | 0 | 0 |
| Low | 75 | 65 | 30 | −1 | −1 | −1 |
| Standard order | X1 | X2 | X3 | |||
| 1 | −1 | −1 | 0 | |||
| 2 | +1 | −1 | 0 | |||
| 3 | −1 | +1 | 0 | |||
| 4 | +1 | +1 | 0 | |||
| 5 | −1 | 0 | −1 | |||
| 6 | +1 | 0 | −1 | |||
| 7 | −1 | 0 | +1 | |||
| 8 | +1 | 0 | +1 | |||
| 9 | 0 | −1 | −1 | |||
| 10 | 0 | +1 | −1 | |||
| 11 | 0 | −1 | +1 | |||
| 12 | 0 | +1 | +1 | |||
| 13 | 0 | 0 | 0 | |||
| 14 | 0 | 0 | 0 | |||
| 15 | 0 | 0 | 0 | |||
| Coefficient 1 | S | O | SO | PSO | ISO |
|---|---|---|---|---|---|
| Constant a0 | 197.021 | −191.318 | −301.734 * | 59.033 | −260.059 * |
| Linear | |||||
| a1 | 2.823 | −0.003 | 3.975 * | 0.347 | 1.842 * |
| a2 | −9.366 | 5.810 | 4.069 * | −1.856 | 5.626 * |
| a3 | 0.376 | 0.094 | −0.047 | 0.151 | 0.128 |
| Quadratic | |||||
| a11 | −0.0178 * | −0.003 | −0.012 * | 0.0015 | −0.007 * |
| a22 | 0.065 | −0.046 * | −0.011 | 0.018 | −0.0036 * |
| a33 | −0.0011 | −0.001 | −0.001* | −0.0005 | −0.001 |
| Interaction | |||||
| a12 | 0.005 | 0.006 | −0.030 * | −0.009 | −0.010 |
| a23 | −0.002 | 0.001 | 0.001 | 0.0002 | 0.001 |
| a13 | −0.0002 | −0.001 | 0.002 | −0.0009 | −0.001 |
| R2 | 0.799 | 0.881 | 0.957 | 0.906 | 0.941 |
| Coefficient 1 | S | O | SO | PSO | ISO |
|---|---|---|---|---|---|
| Constant a0 | −54.187 | 87.773 * | −35.2925 | 56.895 | −8.9811 |
| Linear | |||||
| a1 | −0.5259 | −0.4163 | 0.4087 * | −2.3113 * | −1.82015 * |
| a2 | 2.2739 | −2.0168 * | 0.5165 | 1.2697 | 2.655 * |
| a3 | 0.1519 | 0.0052 | 0.0078 | 0.119 | 0.00596 |
| Quadratic | |||||
| a11 | 0.0007 | −0.00039 | −0.0014 | 0.00804 * | 0.00534 |
| a22 | −0.0198 * | 0.0104 * | −0.0017 | −0.0171 | −0.02688 * |
| a33 | −0.0001 | 0.00009 | −0.0001 | −0.00019 | −0.0008 |
| Interaction | |||||
| a12 | 0.0057 | 0.00696 * | −0.0027 | 0.0131 * | 0.01229 * |
| a23 | −0.0002 | 0.00015 | 0.0006 * | 0.00033 | 0.00025 |
| a13 | −0.0016 | −0.00024 | −0.0005 | −0.0015 | 0.0009 |
| R2 | 0.867 | 0.904 | 0.937 | 0.868 | 0.938 |
| OD Process Conditions | Predictions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OD Solute Type | OD Temperature (°C) | OD Solute Concentration (° Brix) | OD Time (min) | aw | WL | SG | ΔE | Firmness (N) | Desirability |
| S | 95 | 73.6 | 30 | 0.8343 | 11.0584 | 4.31 | 8.91 | 2.81 | 0.439 |
| O | 75 | 75 | 30 | 0.982 | 7.84 | 1.01 | 4.41 | 1.29 | 0.634 |
| SO | 95 | 65 | 30 | 0.955 | 11.79 | 3.31 | 6.87 | 1.77 | 0.472 |
| PSO | 95 | 75 | 30 | 0.974 | 9.01 | 3.09 | 7.37 | 2.86 | 0.625 |
| ISO | 75 | 75 | 90 | 0.942 | 7.29 | 4.53 | 8.98 | 2.50 | 0.657 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakourou, M.C.; Lazou, A.E.; Dermesonlouoglou, E.K. Optimization of Osmotic Dehydration of Tomatoes in Solutions of Non-Conventional Sweeteners by Response Surface Methodology and Desirability Approach. Foods 2020, 9, 1393. https://doi.org/10.3390/foods9101393
Giannakourou MC, Lazou AE, Dermesonlouoglou EK. Optimization of Osmotic Dehydration of Tomatoes in Solutions of Non-Conventional Sweeteners by Response Surface Methodology and Desirability Approach. Foods. 2020; 9(10):1393. https://doi.org/10.3390/foods9101393
Chicago/Turabian StyleGiannakourou, Maria C., Andriana E. Lazou, and Efimia K. Dermesonlouoglou. 2020. "Optimization of Osmotic Dehydration of Tomatoes in Solutions of Non-Conventional Sweeteners by Response Surface Methodology and Desirability Approach" Foods 9, no. 10: 1393. https://doi.org/10.3390/foods9101393
APA StyleGiannakourou, M. C., Lazou, A. E., & Dermesonlouoglou, E. K. (2020). Optimization of Osmotic Dehydration of Tomatoes in Solutions of Non-Conventional Sweeteners by Response Surface Methodology and Desirability Approach. Foods, 9(10), 1393. https://doi.org/10.3390/foods9101393





