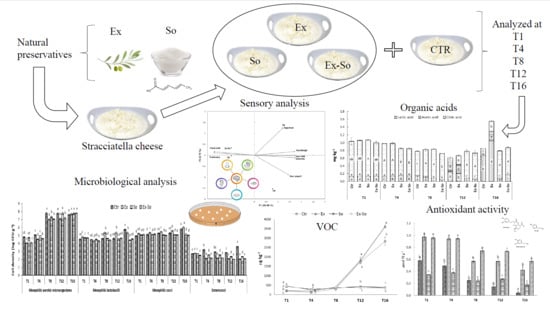
Evolution of VOC and Sensory Characteristics of Stracciatella Cheese as Affected by Different Preservatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preservatives Concentration and Sample Preparation
2.2. Chemical, Sensory and Microbiological Analyses
2.2.1. Volatile Organic Compounds (VOCs)
2.2.2. Antioxidant Activity and Oxidative Stability
2.2.3. Color
2.2.4. Organic Acids and pH
2.2.5. Sensory Analysis
2.2.6. Cultivable Microbiota
2.3. Statistical Analysis
3. Results and Discussion
3.1. VOC
3.2. Sensory Analysis and Possible Connections with VOC
3.3. Chemical and Microbial Analyses
3.3.1. Antioxidant Activity and Color
3.3.2. Organic Acids and pH
3.3.3. Cultivable Microbiota
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghosh, P.R.; Fawcett, D.; Sharma, S.B.; Poinern, G.E.J. Progress towards sustainable utilisation and management of food wastes in the global economy. Int. J. Food Sci. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djekic, I.; Miloradovic, Z.; Djekic, S.; Tomasevic, I. Household food waste in Serbia—Attitudes, quantities and global warming potential. J. Clean. Prod. 2019, 229, 44–52. [Google Scholar] [CrossRef]
- Tylewicz, U.; Inchingolo, R.; Rodriguez-Estrada, M.T. Food Aroma Compounds; Elsevier Inc.: Cambridge, MA, USA, 2017; ISBN 9780128052570. [Google Scholar]
- Shahidi, F. Indicators for evaluation of lipid oxidation and off-flavor development in food. Dev. Food Sci. 1998, 40, 55–68. [Google Scholar] [CrossRef]
- Wilkes, J.G.; Conte, E.D.; Kim, Y.; Holcomb, M.; Sutherland, J.B.; Miller, D.W. Sample preparation for the analysis of flavors and off-flavors in foods. J. Chromatogr. A 2000, 880, 3–33. [Google Scholar] [CrossRef]
- Wilson, A.D.; Oberle, C.S.; Oberle, D.F. Detection of off-flavor in catfish using a conducting polymer electronic-nose technology. Sensors 2013, 13, 15968–15984. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Velazquez, G.; Torres, J.A.; Qian, M.C. Quantitative determination of thermally derived off-flavor compounds in milk using solid-phase microextraction and gas chromatography. J. Dairy Sci. 2005, 88, 3764–3772. [Google Scholar] [CrossRef]
- Natrella, G.; Faccia, M.; Lorenzo, J.M.; De Palo, P.; Gambacorta, G. Short communication: Sensory characteristics and volatile organic compound profile of high-moisture mozzarella made by traditional and direct acidification technology. J. Dairy Sci. 2020, 103. [Google Scholar] [CrossRef]
- Ercan, D.; Korel, F.; Karagül Yüceer, Y.; Kınık, Ö. Physicochemical, textural, volatile, and sensory profiles of traditional Sepet cheese. J. Dairy Sci. 2011, 94, 4300–4312. [Google Scholar] [CrossRef] [Green Version]
- Wolf, I.V.; Perotti, M.C.; Zalazar, C.A. Composition and volatile profiles of commercial Argentinean blue cheeses. J. Sci. Food Agric. 2011, 91, 385–393. [Google Scholar] [CrossRef]
- Cheng, H. Volatile flavor compounds in yogurt: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Karatapanis, A.E.; Badeka, A.V.; Riganakos, K.A.; Savvaidis, I.N.; Kontominas, M.G. Changes in flavour volatiles of whole pasteurized milk as affected by packaging material and storage time. Int. Dairy J. 2006, 16, 750–761. [Google Scholar] [CrossRef]
- D’Incecco, P.; Limbo, S.; Hogenboom, J.; Rosi, V.; Gobbi, S.; Pellegrino, L. Impact of extending hard-cheese ripening: A multiparameter characterization of Parmigiano reggiano cheese ripened up to 50 months. Foods 2020, 9, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natrella, G.; Gambacorta, G.; Faccia, M. Volatile organic compounds throughout the manufacturing process of Mozzarella di Gioia del Colle PDO cheese. Czech J. Food Sci. 2020, 38, 215–222. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Franco, D.; Bermúdez, R.; Trindade, M.A.; Lorenzo, J.M. Effect of natural antioxidants in Spanish salchichón elaborated with encapsulated n-3 long chain fatty acids in konjac glucomannan matrix. Meat Sci. 2017, 124, 54–60. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Carballo, J.; Franco, D. Effect of the inclusion of chestnut in the finishing diet on volatile compounds of dry-cured ham from celta pig breed. J. Integr. Agric. 2013, 12, 2002–2012. [Google Scholar] [CrossRef]
- Tian, H.; Xu, X.; Chen, C.; Yu, H. Flavoromics approach to identifying the key aroma compounds in traditional Chinese milk fan. J. Dairy Sci. 2019. [Google Scholar] [CrossRef]
- Gracka, A.; Jeleń, H.H.; Majcher, M.; Siger, A.; Kaczmarek, A. Flavoromics approach in monitoring changes in volatile compounds of virgin rapeseed oil caused by seed roasting. J. Chromatogr. A 2016, 1428, 292–304. [Google Scholar] [CrossRef]
- CLAL. EU-28: Dairy Sector. Available online: https://www.clal.it/en/?section=quadro_europa (accessed on 30 September 2020).
- Altieri, C.; Scrocco, C.; Sinigaglia, M.; Del Nobile, M.A. Use of chitosan to prolong mozzarella cheese shelf life. J. Dairy Sci. 2005, 88, 2683–2688. [Google Scholar] [CrossRef]
- Evert-Arriagada, K.; Hernández-Herrero, M.M.; Juan, B.; Guamis, B.; Trujillo, A.J. Effect of high pressure on fresh cheese shelf-life. J. Food Eng. 2012, 110, 248–253. [Google Scholar] [CrossRef]
- Ricciardi, E.F.; Lacivita, V.; Conte, A.; Chiaravalle, E.; Zambrini, A.V.; Del Nobile, M.A. X-ray irradiation as a valid technique to prolong food shelf life: The case of ricotta cheese. Int. Dairy J. 2019, 99, 104547. [Google Scholar] [CrossRef]
- Di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. LWT Food Sci. Technol. 2011, 44, 2324–2327. [Google Scholar] [CrossRef]
- Gammariello, D.; Conte, A.; Attanasio, M.; Del Nobile, M.A. A study on the synergy of modified atmosphere packaging and chitosan on stracciatella shelf life. J. Food Process Eng. 2011, 34, 1394–1407. [Google Scholar] [CrossRef]
- Trani, A.; Gambacorta, G.; Gomes, T.F.; Loizzo, P.; Cassone, A.; Faccia, M. Production and characterisation of reduced-fat and PUFA-enriched Burrata cheese. J. Dairy Res. 2016, 83, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Gammariello, D.; Conte, A.; Di Giulio, S.; Attanasio, M.; Del Nobile, M.A. Shelf life of Stracciatella cheese under modified-atmosphere packaging. J. Dairy Sci. 2009, 92, 483–490. [Google Scholar] [CrossRef]
- Conte, A.; Brescia, I.; Del Nobile, M.A. Lysozyme/EDTA disodium salt and modified-atmosphere packaging to prolong the shelf life of burrata cheese. J. Dairy Sci. 2011, 94, 5289–5297. [Google Scholar] [CrossRef]
- Dambrosio, A.; Quaglia, N.C.; Saracino, M.; Malcangi, M.; Montagna, C.; Quinto, M.; Lorusso, V.; Normanno, G. Microbiological quality of burrata cheese produced in Puglia region: Southern Italy. J. Food Prot. 2013, 76, 1981–1984. [Google Scholar] [CrossRef]
- Rea, S.; Marino, L.; Stocchi, R.; Branciari, R.; Loschi, A.R.; Miraglia, D.; Ranucci, D. Differences in chemical, physical and microbiological characteristics of Italian Burrata cheeses made in artisanal and industrial plants of the Apulia region. Ital. J. Food Saf. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, H.; Hosseini, O.; Jafari, S.M.; Katouzian, I. Evaluation of physicochemical and antioxidant properties of yogurt enriched by olive leaf phenolics within nanoliposomes. J. Agric. Food Chem. 2018, 66, 9231–9240. [Google Scholar] [CrossRef]
- Palmeri, R.; Parafati, L.; Trippa, D.; Siracusa, L.; Arena, E.; Restuccia, C.; Fallico, B. Addition of olive leaf extract (OLE) for producing fortified fresh pasteurized milk with an extended shelf life. Antioxidants 2019, 8, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roila, R.; Valiani, A.; Ranucci, D.; Ortenzi, R.; Servili, M.; Veneziani, G.; Branciari, R. Antimicrobial efficacy of a polyphenolic extract from olive oil by-product against “Fior di latte” cheese spoilage bacteria. Int. J. Food Microbiol. 2019, 295, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, M.; Di Mise, A.; Difonzo, G.; Centrone, M.; Venneri, M.; Pellegrino, T.; Russo, A.; Mastrodonato, M.; Caponio, F.; Valenti, G.; et al. Green olive leaf extract (OLE) provides cytoprotection in renal cells exposed to low doses of cadmium. PLoS ONE 2019, 14, e0214159. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Difonzo, G.; Calasso, M.; Cosmai, L.; De Angelis, M. Effects of olive leaf extract addition on fermentative and oxidative processes of table olives and their nutritional properties. Food Res. Int. 2019, 116, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Squeo, G.; Calasso, M.; Pasqualone, A.; Caponio, F. Physico-chemical, microbiological and sensory evaluation of ready-to-use vegetable pâté added with olive leaf extract. Foods 2019, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing Union list of food additives. Off. J. Eur. Union 2011, 295, 1–177. [Google Scholar]
- Difonzo, G.; Pasqualone, A.; Silletti, R.; Cosmai, L.; Summo, C.; Paradiso, V.M.; Caponio, F. Use of olive leaf extract to reduce lipid oxidation of baked snacks. Food Res. Int. 2018, 108, 48–56. [Google Scholar] [CrossRef]
- Faccia, M.; Trani, A.; Natrella, G.; Gambacorta, G. Short communication: Chemical-sensory and volatile compound characterization of ricotta forte, a traditional fermented whey cheese. J. Dairy Sci. 2018, 101, 5751–5757. [Google Scholar] [CrossRef]
- CIE, International Commission on Illumination. Survey of Reference Materials for Testing the Performance of Spectrophotometers and Colorimeters; Publication CIE No. 114.1; Central Bureau of the CIE: Vienna, Austria, 1994. [Google Scholar]
- Buffa, M.; Guamis, B.; Saldo, J.; Trujillo, A.J. Changes in organic acids during ripening of cheeses made from raw, pasteurized or high-pressure-treated goats’ milk. LWT Food Sci. Technol. 2004, 37, 247–253. [Google Scholar] [CrossRef]
- Gambera, A. Metodica di assaggio dei formaggi. In L’assaggio dei Formaggi, 2nd ed.; Gambera, A., Ed.; Comunecazione Snc: Bra, Italy, 2017; pp. 9–23. [Google Scholar]
- Minervini, F.; Conte, A.; Del Nobile, M.A.; Gobbetti, M.; De Angelis, M. Dietary fibers and protective lactobacilli drive burrata cheese microbiome. Appl. Environ. Microbiol. 2017, 83, e01494-17. [Google Scholar] [CrossRef] [Green Version]
- McSweeney, P.L.; Fox, P.F. Advanced Dairy Chemistry, 4th ed.; Springer: Boston, MA, USA, 2003. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.T.; Bisig, W.; Guggisberg, D.; Jakob, E.; Turgay, M.; Wechsler, D. Cheeses with propionic acid fermentation. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2017; Volume 2, pp. 889–910. [Google Scholar] [CrossRef]
- Natrella, G.; Gambacorta, G.; De Palo, P.; Lorenzo, J.M.; Faccia, M. Evolution of volatile compounds from milk to curd during manufacturing of mozzarella. Mljekarstvo 2020, 70. [Google Scholar] [CrossRef]
- Ziino, M.; Condurso, C.; Romeo, V.; Giuffrida, D.; Verzera, A. Characterization of “Provola dei Nebrodi”, a typical Sicilian cheese, by volatiles analysis using SPME-GC/MS. Int. Dairy J. 2005, 15, 585–593. [Google Scholar] [CrossRef]
- Morales, P.; Feliu, I.; Fernández-García, E.; Nuñez, M. Volatile compounds produced in cheese by Enterobacteriaceae strains of dairy origin. J. Food Prot. 2004, 67, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Reineccius, G.A. Quantification of aroma compounds in parmigiano reggiano cheese by a dynamic headspace gas chromatography-mass spectrometry technique and calculation of odor activity value. J. Dairy Sci. 2003, 86, 770–776. [Google Scholar] [CrossRef] [Green Version]
- Wolf, I.V.; Perotti, M.C.; Bernal, S.M.; Zalazar, C.A. Study of the chemical composition, proteolysis, lipolysis and volatile compounds profile of commercial Reggianito Argentino cheese: Characterization of Reggianito Argentino cheese. Food Res. Int. 2010, 43, 1204–1211. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Peker, H.; Arslan, S. Effect of olive leaf extract on the quality of low fat apricot yogurt. J. Food Process. Preserv. 2017, 41, e13107. [Google Scholar] [CrossRef]
- Cho, W.Y.; Kim, D.H.; Lee, H.J.; Yeon, S.J.; Lee, C.H. Quality characteristic and antioxidant activity of yogurt containing olive leaf hot water extract. CyTA J. Food 2020, 18, 43–50. [Google Scholar] [CrossRef] [Green Version]
- García-Pérez, F.J.; Lario, Y.; Fernández-López, J.; Sayas, E.; Pérez-Alvarez, J.A.; Sendra, E. Effect of orange fiber addition on yogurt color during fermentation and cold storage. Color Res. Appl. 2005, 30, 457–463. [Google Scholar] [CrossRef]
- Farrag, A.F.; Bayoumi, H.M.; Ibrahim, W.A.; El-Sheikh, M.M.; Eissa, H.A. Characteristics of white soft cheese fortified with hibiscus soft drink as antimicrobial and hypertension treatment. Science 2017, 12, 122–129. [Google Scholar] [CrossRef]
- Zeppa, G.; Rolle, L. A study on organic acid, sugar and ketone contents in typical piedmont cheeses. Ital. J. Food Sci. 2008, 20, 127–139. [Google Scholar]
- Akalin, A.S.; Gönç, S.; Akbaş, Y. Variation in organic acids content during ripening of pickled white cheese. J. Dairy Sci. 2002, 85, 1670–1676. [Google Scholar] [CrossRef] [Green Version]
- Manolaki, P.; Katsiari, M.C.; Alichanidis, E. Effect of a commercial adjunct culture on organic acid contents of low-fat Feta-type cheese. Food Chem. 2006, 98, 658–663. [Google Scholar] [CrossRef]
- Adda, J.; Gripon, J.C.; Vassal, L. The chemistry of flavour and texture generation in cheese. Food Chem. 1982, 9, 115–129. [Google Scholar] [CrossRef]
- Roila, R.; Branciari, R.; Ranucci, D.; Ortenzi, R.; Urbani, S.; Servili, M.; Valiani, A. Antimicrobial activity of olive mill wastewater extract against Pseudomonas fluorescens isolated from mozzarella cheese. Ital. J. Food Saf. 2016, 5, 111–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, I.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. Int. J. Food Microbiol. 2011, 147, 45–52. [Google Scholar] [CrossRef]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozès, N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012, 31, 1–8. [Google Scholar] [CrossRef]
- Von Staszewski, M.; Pilosof, A.M.R.; Jagus, R.J. Antioxidant and antimicrobial performance of different Argentinean green tea varieties as affected by whey proteins. Food Chem. 2011, 125, 186–192. [Google Scholar] [CrossRef]
- Liao, C.H. Pseudomonas and related genera. In Food Spoilage Microorganisms, 1st ed.; Blackburn, C.W., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2006; pp. 507–540. [Google Scholar] [CrossRef]
- Faccia, M.; Gambacorta, G.; Natrella, G.; Caponio, F. Shelf life extension of Italian mozzarella by use of calcium lactate buffered brine. Food Control 2019, 100, 287–291. [Google Scholar] [CrossRef]
- Markín, D.; Duek, L.; Berdícevsky, I. In vitro antimicrobial activity of olive leaves. Mycoses 2003, 46, 132–136. [Google Scholar] [CrossRef]
- Brocklehurst, T.F.; Lund, B.M. Microbiological changes in cottage cheese varieties during storage at +7 °C. Food Microbiol. 1985, 2, 207–233. [Google Scholar] [CrossRef]
- Lucera, A.; Mastromatteo, M.; Conte, A.; Zambrini, A.V.; Faccia, M.; Del Nobile, M.A. Effect of active coating on microbiological and sensory properties of fresh mozzarella cheese. Food Packag. Shelf Life 2014, 1, 25–29. [Google Scholar] [CrossRef]







| CTR | Ex | So | Ex-So | |
|---|---|---|---|---|
| Cream/mozzarella strands (w/w as g) | 500/500 | 500/500 | 500/500 | 500/500 |
| OLE (mg kg−1) | - | 400 | - | 400 |
| Sorbic acid (mg kg−1) | - | - | 1000 | 1000 |
| Parameter | T1 | T4 | T8 | T12 | T16 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | Ex | So | Ex-So | Ctr | Ex | So | Ex-So | Ctr | Ex | So | Ex-So | Ctr | Ex | So | Ex-So | Ctr | Ex | So | Ex-So | |
| Aldehydes | 9.65 a ± 1.55 | 8.00 a ± 0.12 | 10.02 a ± 1.99 | 0.78 b ± 0.06 | 5.01 b ± 2.42 | 5.19 b ± 0.34 | 12.03 a ± 2.15 | 5.82 b ± 1.26 | 46.09 a ± 20.77 | 39.94 a ± 4.84 | 0.46 b ± 0.02 | 0.52 b ± 0.08 | 9.46 b ± 1.05 | 36.58 a ± 1.43 | 0.88 c ± 0.25 | 1.03 c ± 0.17 | 4.98 b ± 0.54 | 20.89 a ± 2.74 | 0.72 c ± 0.3 | 0.83 c ± 0.11 |
| Ketones | 57.04 a ± 11.9 | 62.83 a ± 0.92 | 67.84 a ± 11.34 | 58.57 a ± 4.4 | 58.36 b ± 10.68 | 78.65 ab ± 3.5 | 65.94 ab ± 10.59 | 81.26 a ± 2.19 | 105.58 a ± 32.84 | 96.70 a ± 1.86 | 80.20 a ± 15.25 | 93.16 a ± 2.97 | 1251.03 a ± 64.69 | 865.58 b ± 93.71 | 83.64 c ± 7.9 | 85.64 c ± 1.06 | 1894.70 a ± 111.37 | 1603.86 b ± 10.74 | 105.02 c ± 7.19 | 82.11 d ± 6.34 |
| Esters | 46.28 a ± 18.4 | 62.21 a ± 0.6 | 64.75 a ± 18.6 | 61.29 a ± 5.76 | 24.32 b ± 7.23 | 58.56 a ± 0.05 | 52.25 a ± 7.23 | 57.46 a ± 5.88 | 47.88 c ± 11.75 | 77.24 b ± 2.28 | 83.05 a ± 1.55 | 86.36 a ± 2.89 | 41.33d ± 3.68 | 178.18 a ± 4.07 | 80.11 b ± 0.06 | 75.04 c ± 0.99 | 134.08 b ± 7.24 | 187.41 a ± 7.08 | 77.62 c ± 14.99 | 103.54 c ± 11.02 |
| Alcohols | 22.16 ab ± 7.89 | 18.82 b ± 4.0 | 27.75 a ± 3.74 | 23.58 ab ± 1.98 | 27.00 a ± 2.92 | 13.68 c ± 0.42 | 17.55 b ± 1.53 | 17.36 b ± 1.14 | 76.38 a ± 25.79 | 115.65 a ± 15.62 | 18.08 b ± 0.52 | 18.16 b ± 2.96 | 372.65 b ± 21.01 | 629.53 a ± 32.08 | 20.50 c ± 5.29 | 30.49 c ± 5.51 | 659.30 b ± 60.15 | 1685.94 a ± 76.51 | 17.51 d ± 1.70 | 39.64 c ± 8.6 |
| Sulfur compounds | 0.43 ab ± 0.09 | 0.19 c ± 0.14 | 0.52 a ± 0.08 | 0.28 bc ± 0.06 | 0.31 b ± 0.03 | 0.41 a ± 0.04 | 0.40 ab ± 0.10 | 0.35 ab ± 0.04 | 4.88 a ± 1.13 | 0.51 b ± 0.09 | 0.58 b ± 0.02 | 0.64 b ± 0.04 | 11.35 a ± 3.38 | 0.54 b ± 0.12 | 0.73 b ± 0.07 | 0.80 b ± 0.19 | 3.82 b ± 0.03 | 3.90 b ± 0.62 | 2.18 c ± 0.15 | 7.51 a ± 0.75 |
| Acids | 5.53 b ± 1.89 | 3.29 b ± 0.35 | 212.66 a ± 33.12 | 275.92 a ± 35.46 | 2.66 c ± 0.76 | 4.42 b ± 0.21 | 89.75 a ± 16.42 | 85.54 a ± 7.65 | 26.68 b ± 19.02 | 17.95 b ± 2.74 | 89.99 a ± 8.93 | 114.94 a ± 33.11 | 47.55 c ± 10.53 | 80.32 bc ± 26.1 | 164.21 a ± 22.03 | 103.14 b ± 4.07 | 126.86 a ± 30.43 | 93.17 a ± 6.01 | 139.67 a ± 78.84 | 95.47 a ± 8.14 |
| Aliphatic hydrocarbons | 59.35 a ± 5.63 | 62.64 a ± 4.3 | 61.53 a ± 22.44 | 54.06 a ± 5.32 | 0.00 c ± 0.00 | 33.00 a ± 0.63 | 43.37 a ± 10.75 | 2.62 b ± 0.17 | 1.35 b ± 0.53 | 54.31 a ± 15.58 | 82.17 a ± 15.05 | 73.20 a ± 10.46 | 17.59 b ± 6.69 | 27.31 b ± 4.39 | 80.26 a ± 6.97 | 67.47 a ± 7.15 | 14.20 a ± 7.05 | 24.16 a ± 6.65 | 33.80 a ± 12.32 | 2.37 b ± 0.03 |
| Ctr | Ex | So | Ex-So | R | |
|---|---|---|---|---|---|
| Aldehydes | |||||
| Acetaldehyde | 3.7 b ± 0.5 | 12.0 a ± 2.3 | - | - | Ms,Std |
| 3-methylbutanal | 0.9 b ± 0.1 | 8.4 a ± 0.3 | - | - | Ms,Std |
| Ketones | |||||
| acetone | 4.5 b ± 0.3 | 5.1 b ± 0.2 | 21.6 a ± 5.0 | 15.5 a ± 1.0 | Ms,Std |
| 2-butanone | 15.9 b ± 0.9 | 55.0 a ± 2.4 | 19.4 b ± 2.8 | 19.0 b ± 0.8 | Ms,Std |
| 2-heptanone | 19.7 c ± 0.6 | 22.4 c ± 0.8 | 49.9 a ± 4.1 | 32.9 b ± 2.5 | Ms,Std |
| 2-hydroxy-3-pentanone | 11.8 a ± 1.6 | 5.1 b ± 0.6 | - | - | Ms,Std |
| 2-nonanone | 2.4 b ± 0.2 | 2.8 b ± 0.2 | 5.0 ab ± 0.2 | 5.9 a ± 1.7 | Ms,Std |
| acetoin | 1839.5 a ± 109.0 | 1512.5 b ± 10.0 | 7.8 c ± 2.9 | 7.4 c ± 1.8 | Ms,Std |
| Esters | |||||
| ethyl acetate | 37.5 c ± 0.8 | 107.5 a ± 5.8 | 68.3 b ± 13.8 | 81.8 ab ± 0.3 | Ms,Std |
| butanoic acid, ethyl ester | 93.5 a ± 7.7 | 75.0 b ± 1.3 | 2.8 c ± 0.6 | 13.0 c ± 1.3 | Ms,Std |
| 2-butenoic acid, ethyl ester (E) | 0.5 b ± 0.1 | 1.4 a ± 0.1 | 0.4 b ± 0.0 | - | Ms |
| hexanoic acid, ethyl ester | 2.0 a ± 0.3 | 3.2 a ± 0.1 | 0.1 b ± 0.0 | 2.2 a ± 0.8 | Ms,Std |
| sorbic acid, ethyl ester | - | - | 6.0 a ± 0.5 | 6.5 a ± 1.9 | Ms |
| Alcohols | |||||
| ethanol | 556.4 b ± 48.0 | 713.6 a ± 49.5 | 4.2 c ± 0.4 | 7.8 c ± 0.1 | Ms,Std |
| 1-propanol, 2 methyl- | 9.7 b ± 0.6 | 76.3 a ± 1.7 | 3.2 c ± 0.3 | - | Ms |
| 1-butanol, 3-methyl | 89.1 b ± 11.2 | 888.3 a ± 25.2 | 5.0 c ± 0.3 | 7.9 c ± 1.3 | Ms,Std |
| 1-pentanol | 0.5 b ± 0.1 | 0.8 b ± 0.1 | 1.2 b ± 0.4 | 3.9 a ± 0.1 | Ms,Std |
| 4-methyl-2-hexanol | 2.9 b ± 0.1 | 3.2 b ± 0.0 | 2.4 b ± 1.2 | 18.3 a ± 7.1 | Ms |
| 1-hexanol, 2-ethyl | 0.4 b ± 0.1 | 0.9 ab ± 0.0 | 1.4 a ± 0.3 | 1.6 a ± 0.3 | Ms |
| phenilethyl alcohol | 0.1 b ± 0.0 | 2.7 a ± 0.1 | 0.1 b ± 0.0 | 0.1 b ± 0.0 | Ms,Std |
| Sulfur Compounds | |||||
| dimethyl sulfide | 3.5 b ± 0.0 | 3.6 b ± 0.5 | 1.4 c ± 0.2 | 6.8 a ± 0.7 | Ms,Std |
| Acids | |||||
| acetic acid | 19.6 b ± 8.4 | 52.2 a ± 5.4 | 3.4 c ± 0.2 | 10.1 b ± 3.1 | Ms,Std |
| butanoic acid | 43.9 a ± 12.2 | 14.6 b ± 0.3 | 5.8 c ± 3.4 | 5.9 c ± 1.5 | Ms,Std |
| hexanoic acid | 51.3 a ± 8.0 | 18.0 b ± 0.2 | 10.8 c ± 6.0 | 9.2 c ± 3.3 | Ms,Std |
| octanoic acid | 9.7 a ± 1.6 | 4.4 b ± 0.2 | 3.3 b ± 1.4 | 3.8 b ± 0.9 | Ms,Std |
| propanoic acid, 2 methyl | 0.1 b ± 0.0 | 1.4 a ± 0.0 | - | - | Ms,Std |
| butanoic acid, 3-methyl | 0.6 b ± 0.1 | 1.5 a ± 0.2 | 0.2 bc ± 0.0 | - | Ms,Std |
| sorbic acid | - | - | 115.9 a ± 68.3 | 66.3 a ± 0.7 | Ms |
| Aliphatic hydrocarbons | |||||
| 1-heptene, 2,4-dimethyl- | 14.2 ab ± 2.0 | 24.1 ab ± 6.7 | 38.8 a ± 12.3 | 2.3 c ± 0.1 | Ms |
| Volatile Compound | Ctr | Ex | So | Ex-So | Odor Description | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T4 | T8 | T12 | T16 | T1 | T4 | T8 | T12 | T16 | T1 | T4 | T8 | T12 | T16 | T1 | T4 | T8 | T12 | T16 | ||
| 3-Methylbutanal | - | 4.5 | 228.2 | 14.2 | 4.5 | - | - | 197.2 | 180.5 | 42.3 | - | - | - | - | - | - | - | - | - | - | Powerful penetrating, fusty, acrid, apple-like |
| Hexanal | 2.0 | - | - | - | - | 1.6 | 1.0 | - | - | - | 2.0 | 2.5 | - | - | - | - | 1.2 | - | - | - | Green, fatty, fruity |
| Nonanal | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.0 | - | Sweet, orange, orange peel |
| 2-Nonanone | - | - | 1.2 | - | - | - | - | - | 1.1 | - | - | - | - | - | 1.0 | - | - | - | - | 1.2 | Fresh sweet, weedy, earthy, herbal |
| Acetoin | - | - | - | 1.5 | 2.3 | - | - | - | 1.0 | 1.9 | - | - | - | - | - | - | - | - | - | - | Intense buttery, creamy |
| Ethyl acetate | 9.3 | 4.9 | 8.3 | 5.1 | 7.5 | 12.4 | 11.7 | 12.0 | 27.1 | 21.5 | 12.1 | 10.1 | 15.2 | 14.3 | 13.7 | 11.5 | 10.8 | 15.5 | 13.2 | 16.4 | Ethereal, fruity, green |
| Butanoic acid, ethyl ester | - | - | 45.4 | 149.8 | 935.0 | - | - | 131.2 | 302.1 | 749.8 | - | - | 28.7 | 31.9 | 27.9 | - | - | 45.2 | 38.9 | 130.1 | Pineapple |
| Hexanoic acid, ethyl ester | - | - | 3.8 | 1.3 | 6.8 | - | - | 7.6 | 36.6 | 10.8 | - | - | 1.3 | 1.4 | - | - | - | 2.1 | 5.5 | 7.2 | Floral, fruity, apple, banana, pineapple |
| Ethanol | - | 1.0 | 2.4 | 35.1 | 69.6 | - | - | 5.6 | 29.5 | 89.2 | 1.3 | - | - | - | - | 1.3 | - | - | - | 1.0 | Pleasant, weak, ethereal, vinous |
| 1-Butanol, 3-methyl | - | - | - | 1.2 | 1.3 | - | - | - | 5.3 | 12.5 | - | - | - | - | - | - | - | - | - | - | Banana, alcohol, fruity |
| Phenilethyl alcohol | 1.4 | - | - | - | - | - | - | - | - | 2.3 | 1.0 | - | - | - | - | - | - | - | - | - | Characteristic rose-like |
| Dimethyl sulfide | - | - | 14.3 | 36.6 | 11.8 | - | - | - | - | 11.9 | - | - | - | - | 4.7 | - | - | - | - | 22.7 | Unpleasant odor of wild radish, cabbage-like |
| Parameter | T1 | T4 | T8 | T12 | T16 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | Ex | So | Ex-So | Ctr | Ex | So | Ex-So | Ctr | Ex | So | Ex-So | Ctr | Ex | So | Ex-So | Ctr | Ex | So | Ex-So | |
| L* | 86.54 a ± 11.84 | 92.34 a ± 7.21 | 93.29 a ± 2.00 | 95.35 a ± 4.89 | 95.27 a ± 3.87 | 94.12 a ± 4.95 | 95.28 a ± 3.00 | 97.9 a ± 1.28 | 95.3 a ± 1.67 | 95.16 a ± 1.12 | 98.32 a ± 1.32 | 93.84 a ± 1.98 | 93.28 a ± 0.15 | 90.64 a ± 0.86 | 93.58 a ± 2.77 | 92.96 a ± 3.87 | 90.17 a ± 4.41 | 88.70 a ± 4.91 | 90.77 a ± 1.31 | 77.27 b ± 2.55 |
| a* | −0.21 a ± 0.13 | −0.38 a ± 0.12 | −0.16 a ± 0.03 | −0.3 a ± 0.03 | −0.08 a ± 0.17 | −0.23 a ± 0.10 | −0.14 a ± 0.01 | −0.38 a ± 0.08 | −0.06 a ± 0.20 | −0.28 a ± 0.12 | −0.12 a ± 0.01 | −0.25 a ± 0.18 | 0.10 a ± 0.05 | 0.06 a ± 0.09 | 0.02 a ± 0.04 | −0.05 a ± 0.10 | 0.21 a ± 0.05 | 0.10 b ± 0.10 | 0.02 b ± 0.01 | 0.26 a ± 0.20 |
| b* | 8.70 a ± 0.49 | 8.67 a ± 0.10 | 8.36 a ± 0.21 | 8.93 a ± 0.57 | 7.52 a ± 0.12 | 8.51 a ± 0.39 | 9.08 a ± 0.91 | 8.16 a ± 0.69 | 8.84 a ± 0.16 | 10.0 a ± 0.17 | 8.31 b ± 0.46 | 8.79 b ± 0.21 | 8.79 b ± 0.06 | 9.97 a ± 0.43 | 9.08 a ± 0.99 | 9.28 a ± 0.52 | 8.67 a ± 0.10 | 9.17 a ± 0.50 | 9.17 a ± 0.25 | 7.61 b ± 0.76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natrella, G.; Difonzo, G.; Calasso, M.; Costantino, G.; Caponio, F.; Faccia, M. Evolution of VOC and Sensory Characteristics of Stracciatella Cheese as Affected by Different Preservatives. Foods 2020, 9, 1446. https://doi.org/10.3390/foods9101446
Natrella G, Difonzo G, Calasso M, Costantino G, Caponio F, Faccia M. Evolution of VOC and Sensory Characteristics of Stracciatella Cheese as Affected by Different Preservatives. Foods. 2020; 9(10):1446. https://doi.org/10.3390/foods9101446
Chicago/Turabian StyleNatrella, Giuseppe, Graziana Difonzo, Maria Calasso, Giuseppe Costantino, Francesco Caponio, and Michele Faccia. 2020. "Evolution of VOC and Sensory Characteristics of Stracciatella Cheese as Affected by Different Preservatives" Foods 9, no. 10: 1446. https://doi.org/10.3390/foods9101446
APA StyleNatrella, G., Difonzo, G., Calasso, M., Costantino, G., Caponio, F., & Faccia, M. (2020). Evolution of VOC and Sensory Characteristics of Stracciatella Cheese as Affected by Different Preservatives. Foods, 9(10), 1446. https://doi.org/10.3390/foods9101446