Determination of Biochemical Composition in Peach (Prunus persica L. Batsch) Accessions Characterized by Different Flesh Color and Textural Typologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peach Cultivars and Breeding Selections
2.2. Fruit Quality and Sensorial Analysis
2.3. Polyphenol Oxidase (PPO) Activity
2.4. Ascorbic Acid Extraction and High-Performance Liquid Chromatography (HPLC) Quantification
2.5. Extraction of Peach Phenolics and Analysis by HPLC
2.6. Statistical Analysis
3. Results
3.1. Impact of Flesh Color and Textural Typology on Firmness at Harvest
3.2. Impact of Flesh Characteristics on Peach Polyphenol Oxidase (PPO)
3.3. Impact of Flesh Characteristics on Peach Ascorbic Acid Concentration
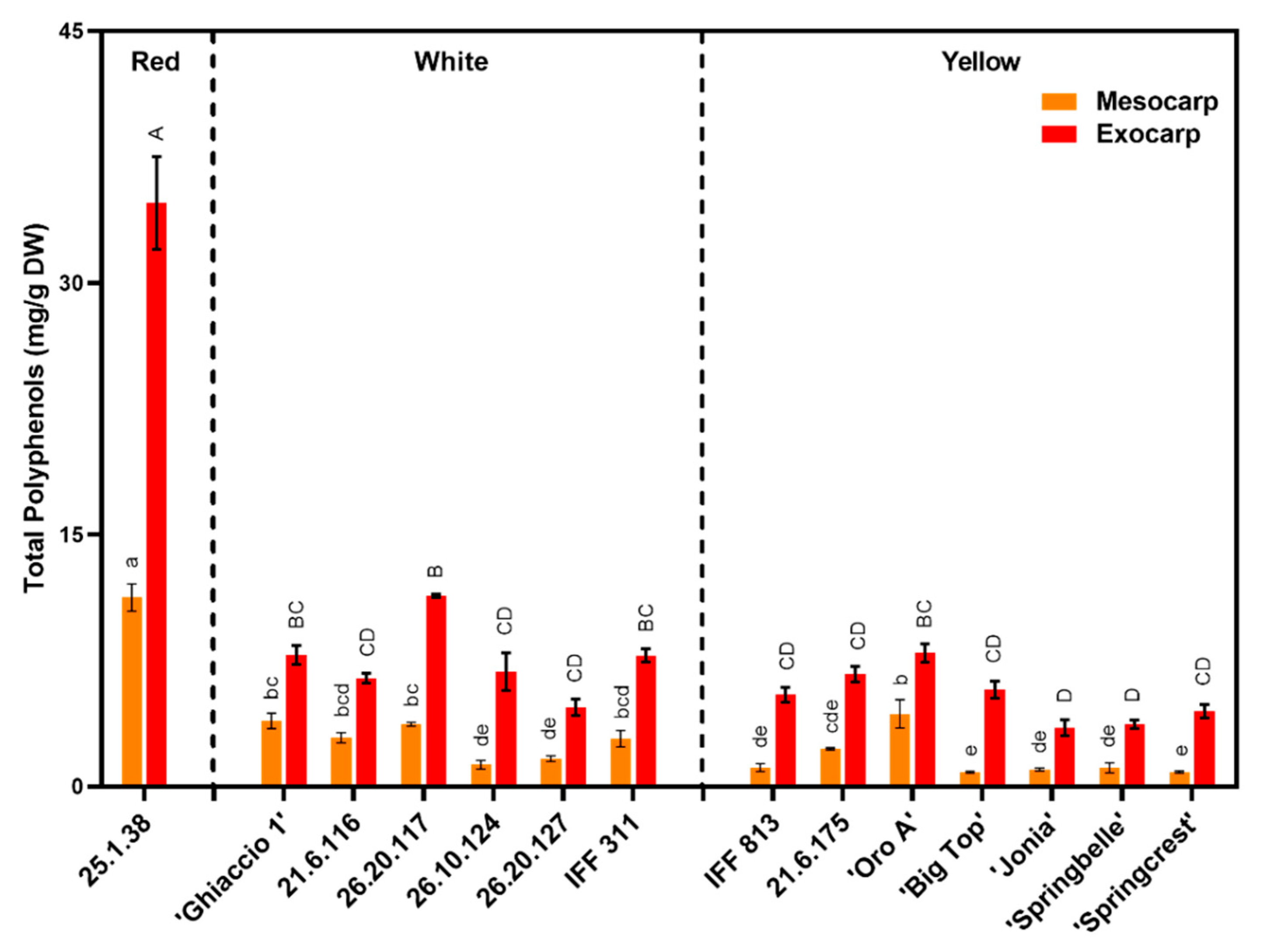
3.4. Phenolic Concentration and Composition Determined by Flesh Characteristics in Peach Varieties
3.5. Principal Component Analysis of Biochemical Composition Across Peach Varieties Characterized by Different Flesh Color
3.6. Sensorial and Fruit Quality Analyses for Commercial Cultivars of Varying Textural Typologies
4. Discussion
4.1. Firmness at Harvest Appeared to Follow Textural Typology Characteristics
4.2. Polyphenol Oxidase, Phenolic Concentration and Browning Potential Across Accessions
4.3. Breeding Selections Demonstrated a High Level of Ascorbic Acid Compared to Commercial Cultivars
4.4. Phenolic Concentration Was Superior in Peach Exocarp and Varied by Flesh Color
4.5. Sensory Panel Illustrates a Consumer Preference for Cultivars with a High SSC/TA Ratio and Low Firmness
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wojdyło, A.; Oszmiański, J. Antioxidant Activity Modulated by Polyphenol Contents in Apple and Leaves during Fruit Development and Ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, F.C.; Shukitt-Hale, B.; Joseph, J.A. The beneficial effects of fruit polyphenols on brain aging. Neurobiol. Aging 2005, 26, 128–132. [Google Scholar] [CrossRef]
- González, G.J.; Mediavilla, M.G.; Campos, S.S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammmation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [Green Version]
- Chong, M.F.F.; Macdonald, R.; Lovegrove, J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010, 104, S28–S39. [Google Scholar] [CrossRef] [Green Version]
- Byrne, D.H.; Noratto, G.; Cisneros-Zevallos, L.; Porter, W.; Vizzotto, M. Health benefits of peach, nectarine and plums. Acta Hortic. 2009, 841, 267–274. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Byrne, D.; Okie, W.R.; Cisneros-Zevallos, L. Selecting new peach and plum accessions rich in phenolic compounds and enhanced functional properties. Food Chem. 2006, 96, 273–280. [Google Scholar] [CrossRef]
- Cantin, C.M.; Moreno, M.A.; Gogorcena, Y. Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine [Prunus persica (L.) Batsch] breeding progenies. J. Agric. Food Chem. 2009, 57, 4586–4592. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Crisosto, C. How do we increase peach consumption? Acta Hort. 2002, 592, 601–605. [Google Scholar] [CrossRef]
- Liverani, A.; Brandi, F.; Quacquarelli, I.; Sirri, S.; Giovannini, D. Superior taste and keeping quality are steady goals of the peach breeding activity at CRA-FRF, Italy. Acta Hort. 2015, 1084, 179–186. [Google Scholar] [CrossRef]
- Lester, D.R.; Sherman, W.B.; Atwell, B.J. Endopolygalacturonase and the melting flesh (M) locus in peach. J. Am. Soc. Hortic. Sci. 1996, 121, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M. Genetical studies on the fruit quality of peach varieties. Texture and keeping quality. Bull. Fruit Tree Res. Sta. 1976, A3, 1–16. [Google Scholar]
- Mignani, I.; Ortugno, C.; Bassi, D. Biochemical Parameters for the Evaluation of Different Peach Flesh Types. Acta Hort. 2006, 713, 441–448. [Google Scholar] [CrossRef]
- Giovannini, D.; Liverani, A.; Merli, M.; Brandi, F. Breeding strategies to improve peach fruit quality. Acta Hortic. 2006, 713, 107–112. [Google Scholar] [CrossRef]
- Bassi, D.; Monet, R. Botany and Taxonomy. The Peach: Botany, Production and Uses; CABI: Oxfordshire, UK, 2008; pp. 1–36. [Google Scholar]
- Ghiani, A.; Negrini, N.; Morgutti, S.; Baldin, F.; Nocito, F.F.; Spinardi, A.; Cocucci, M. Melting of ‘Big Top’ nectarine fruit: Some physiological, biochemical, and molecular aspects. J. Am. Soc. Hort. Sci. 2011, 136, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Ciacciulli, A.; Chiozzotto, R.; Attanasio, G.; Cirilli, M.; Bassi, D. Identification of a melting type variant among peach (P. persica L. Batsch) fruit textures by a digital penetrometer. J. Texture Stud. 2018, 49, 370–377. [Google Scholar] [CrossRef]
- Hayama, H.; Tatsuki, M.; Ito, A.; Kashimura, Y. Ethylene and fruit softening in the stony hard mutation in peach. Postharvest Biol. Technol. 2006, 41, 16–21. [Google Scholar]
- Ghiani, A.; Onelli, E.; Aina, R.; Cocucci, M.; Citterio, S. A comparative study of melting and non-melting flesh peach cultivars reveals that during fruit ripening endo-polygalacturonase (endo-PG) is mainly involved in pericarp textural changes, not in firmness reduction. J. Expt. Bot. 2011, 62, 4043–4054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liverani, A.; Brandi, F.; Quacquarelli, I.; Sirri, S.; Giovannini, D. Advanced stony-hard peach and nectarine selections from CREA-FRF breeding program. Acta Hort. 2017, 1172, 2019–2024. [Google Scholar] [CrossRef]
- Cirilli, M.; Giovannini, D.; Ciacciulli, A.; Chiozzotto, R.; Gattolin, S.; Rossini, L.; Liverani, A.; Bassi, D. Integrative genomics approaches validate PpYUC11-like as candidate gene for the stony hard trait in peach (P. persica L. Batsch). BMC Plant Biol. 2018, 18, 88. [Google Scholar] [CrossRef]
- Scorza, R.; Sherman, W.B. Peaches. Fruit Breed. Trees Trop. Fruit 1996, 1, 325–440. [Google Scholar]
- Haji, T.; Yaegaki, H.; Yamaguchi, M. Inheritance and expression of fruit texture melting, non-melting and stony hard in peach. Sci. Hortic. 2005, 105, 241–248. [Google Scholar] [CrossRef]
- Giovannoni, J. Molecular biology of fruit maturation and ripening. Ann. Rev. Plant Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic Regulation of Fruit Development and Ripening. Plant Cell. 2004, 16, 170–180. [Google Scholar] [CrossRef] [Green Version]
- Trainotti, L.; Tadiello, A.; Casadoro, G. The involvement of auxin in the ripening of climacteric fruits comes of age: The hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J. Exp. Bot. 2007, 58, 3299–3308. [Google Scholar] [CrossRef] [Green Version]
- Ceccarelli, D.; Simeone, A.M.; Nota, P.; Piazza, M.G.; Fideghelli, C.; Caboni, E. Phenolic compounds (hydroxycinnamic acids, flavan-3-ols, flavonols) profile in fruit of Italian peach varieties. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2016, 150, 1370–1375. [Google Scholar] [CrossRef]
- Vizzotto, M.; Cisneros-Zevallos, L.; Byrne, D.H.; Ramming, D.W.; Okie, W.R. Large variation found in the phytochemical and antioxidant activity of peach and plum germplasm. J. Am. Soc. Hortic. Sci. 2007, 132, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Aubert, C.; Chalot, G. Physicochemical characteristics, vitamin C, and polyphenolic composition of four European commercial blood-flesh peach cultivars (Prunus persica L. Batsch). J. Food Comp. Anal. 2020, 86, 103337. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof/ms characterization of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Careri, M.; Elviri, L.; Mangia, A.; Musci, M. Spectrophotometric and coulometric detection in the high performance liquid chromatography of flavonoids and optimization of sample treatment for the determination of quercetin in orange juice. J. Chromatogr. A 2000, 881, 449–460. [Google Scholar] [CrossRef]
- Mattivi, F.; Tonon, D.; Sanchez, C. Gli antiossidanti polifenolici naturali. Laboratorio 2002, 3, 46–56. [Google Scholar]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Delicado, E.; Serrano-Megías, M.; Pérez-López, A.J.; López-Nicolás, J.M. Polyphenol oxidase from Dominga table grape. J. Agric. Food Chem. 2005, 53, 6087–6093. [Google Scholar] [CrossRef]
- Alcobendas, R.; Mirás-Avalos, J.M.; Alarcón, J.J.; Pedrero, F.; Nicolás, E. Combined effects of irrigation, crop load and fruit position on size, color and firmness of fruits in an extra-early cultivar of peach. Sci. Hortic. 2012, 142, 128–135. [Google Scholar] [CrossRef]
- Bonora, E.; Noferini, M.; Vidoni, S.; Costa, G. Modeling fruit ripening for improving peach homogeneity in planta. Sci. Hortic. 2013, 159, 166–171. [Google Scholar] [CrossRef]
- Stone, H.; Joel, L.; Bloomquist, S.; Bloomquist, J. Quantitative descriptive analysis. In Descriptive Sensory Analysis in Practice; Gacula, M.C., Ed.; Food & Nutrition Press Inc.: Trimbull, CT, USA, 1997; pp. 63–69. ISBN 0-917678-37-0. [Google Scholar]
- Cañal, M.J.; Tamés, R.S.; Fernández, B. Peroxidase and polyphenol oxidase activities in Cyperus esculentus leaves following glyphosate applications. Phys. Plant. 1988, 74, 125–130. [Google Scholar] [CrossRef]
- Murata, M.; Tsurutani, M.; Tomita, M.; Homma, S.; Kaneko, K. Relationship between apple ripening and browning: Changes in polyphenol content and polyphenol oxidase. J. Agric. Food Chem. 1995, 43, 1115–1121. [Google Scholar] [CrossRef]
- Masia, A.; Ventura, M.; Gemma, H.; Sansavini, S. Effect of some plant growth regulator treatments on apple fruit ripening. Plant Growth Reg. 1998, 25, 127–134. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Antioxidant defenses in olive trees during drought stress: Changes in activity of some antioxidant enzymes. Func. Plant Biol. 2005, 32, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Hernandez-Jover, T.; Martin-Belloso, O. Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Analytical, nutritional and clinical Methods. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- Dumville, J.C.; Fry, S.C. Solubilisation of tomato fruit pectins by ascorbate: A possible non-enzymic mechanism of fruit softening. Planta 2003, 217, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, C.; Ravaglia, D.; Ragaini, A.; Costa, G. Phenolic compounds in peach (Prunus persica) cultivars at harvest and during fruit maturation. Ann. Appl. Biol. 2008, 153, 11–23. [Google Scholar] [CrossRef]
- Valero, C.; Crisosto, C.H.; Slaughter, D. Relationship between nondestructive firmness measurements and commercially important ripening fruit stages for peaches, nectarines and plums. Postharvest Biol. Technol. 2007, 44, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Bassi, D.; Liverani, A.; Giovannini, D.; Mignani, I.; Spinardi, A.; Ghiani, A.; Negrini, N.; Morghutti, S. La nettarina ‘Big Top’: Un caso di studio? In Proceedings of the VI Convegno Nazionale sulla Peschicoltura Meridionale, Caserta, Italy, 6–7 March 2009; pp. 20–27. [Google Scholar]
- Crisosto, C.H. Stone fruit maturity indices: A descriptive. Postharvest News Inf. 1994, 5, 65–68. [Google Scholar]
- Brovelli, E.A.; Brecht, J.K.; Sherman, W.B.; Sims, C.A. Potential maturity indices and developmental aspects of melting-flesh and nonmelting-flesh peach accessions for the fresh market. J. Am. Soc. Hortic. Sci. 1998, 123, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Chaparro, J.X.; Werner, D.J.; Whetten, R.W.; O’Malley, D.M. Inheritance, genetic interaction and biochemical characterization of anthocyanin phenotypes in peach. J. Hered. 1995, 86, 32–38. [Google Scholar] [CrossRef]
- Chang, S.; Tan, C.; Frankel, E.N.; Barrett, D.M. Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars. J. Agric. Food Chem. 2000, 48, 147–151. [Google Scholar] [CrossRef]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. (Review). J. Chromatogr. 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- Lee, C.Y.; Kagan, V.; Jaworski, A.W.; Brown, S.K. Enzymic browning in relation to phenolic compounds and polyphenoloxidase activity among various peach cultivars. J. Agric. Food Chem. 1990, 38, 99–101. [Google Scholar] [CrossRef]
- Saidani, F.; Giménez, R.; Aubert, C.; Chalot, G.; Betrán, J.A.; Gogorcena, Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J. Food Comp. Anal. 2017, 62, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Mapson, L.W. Vitamins in Fruits. In The Biochemistry of Fruits and Their Products; Hulme, A.C., Ed.; Academic Press: New York, NY, USA; London, UK, 1970; Volume I, pp. 369–383. [Google Scholar]
- Ceccarelli, D.; Talento, C.; Sartori, A.; Terlizzi, M.; Caboni, E.; Carbone, K. Comparative characterization of fruit quality, phenols and antioxidant activity of de-pigmented “Ghiaccio” and white flesh peaches. Adv. Hortic. Sci. 2016, 30, 175–182. [Google Scholar]
- Liverani, A.; d’Alessandro, D. La qualità gustativa dei frutti nell’attività di miglioramento genetico del pesco presso l’ISF di Forlì. Riv. Fruttic. Ortofloric. 1999, 2, 30–37. [Google Scholar]
- Viola, R.; Brennan, R.; Davies, H.; Sommerville, L. L-ascorbic acid accumulation in berries of Ribes nigrum L. J. Hort. Sci. Biotechnol. 2000, 75, 409–412. [Google Scholar] [CrossRef]
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of storage temperature, storage duration, and subsequent ripening on the physicochemical characteristics, volatile compounds, and phytochemicals of western red nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Byrne, D.H.; Ramming, D.W.; Okie, W.R.; Vizzotto, M. Total phenolic, carotenoid, and anthocyanin content and antioxidant activity of peach and plum accessions. Acta Hortic. 2006, 713, 453–456. [Google Scholar]
- Manzoor, M.; Anwar, F.; Mahmood, Z.; Rashid, U.; Ashraf, M. Variation in minerals, phenolics and antioxidant activity of peel and pulp of different varieties of peach (Prunus persica L.) fruit from Pakistan. Molecules 2012, 17, 6491–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remorini, D.; Tavarini, S.; Degl’innocenti, E.; Loreti, F.; Massai, R.; Guidi, L. Effect of rootstocks and harvesting time on the nutritional quality of peel and flesh of peach fruits. Food Chem. 2008, 110, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic composition and antioxidant properties of different peach [Prunus persica (L.) Batsch] cultivars in China. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monet, R. Transmission génétique du caractère “fruit doux” chez le pêcher. Incidence sur la sélection pour la qualité. In Proceedings of the Eucarpia Fruit Section Symposium, Tree Fruit Breeding, INRA, Angers, France, 3–7 September 1979; pp. 273–276. [Google Scholar]
- Moing, A.; Svanella, L.; Monet, R.; Rothan, C.; Just, D.; Diakou, P.; Gaudillère, J.P.; Rolin, D. Organic acid metabolism during the fruit development of two peach cultivars. Acta Hortic. 1998, 465, 425–432. [Google Scholar] [CrossRef]
- Harker, F.R.; Marsh, K.B.; Young, H.; Murray, S.H.; Gunson, F.A.; Walker, S.B. Sensory interpretation of instrumental measurements 2: Sweet and acid taste of apple fruit. Postharvest Biol. Technol. 2002, 24, 241–250. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M. Relationship between ripe soluble solids concentration (RSSC) and consumer acceptance of high and low acid melting flesh peach and nectarine (Prunus persica (L.) Batsch) cultivars. Postharvest Biol. Technol. 2005, 38, 239–246. [Google Scholar] [CrossRef]
- Gamberini, A. Molecular Markers and Controlling Genes of Peach Flesh Texture. Ph.D. Thesis, Bologna University, Bologna, Italy, 2007. [Google Scholar]
- Farina, V.; Lo Bianco, R.; Mazzaglia, A. Evaluation of late-maturing peach and nectarine fruit quality by chemical, physical, and sensory determinations. Agriculture 2019, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Infante, R.; Meneses, C.; Predieri, S. Sensory quality performance of two nectarine flesh typologies exposed to distant market conditions. J. Food Qual. 2008, 31, 526–535. [Google Scholar] [CrossRef]








| Cultivars | Flesh Color | Flesh Typology 1 | Peach/Nectarine | Harvest Date 2 |
|---|---|---|---|---|
| 25.1.38 | Red | M | Nectarine | 09/07/07 |
| ‘Ghiaccio 1’ | White | SH | Peach | 14/07/07 |
| 21.6.116 | White | M | Peach | 25/07/07 |
| 26.20.117 | White | M | Peach | 09/07/07 |
| 26.20.124 | White | M | Peach | 09/07/07 |
| 26.20.127 | White | M | Peach | 25/07/07 |
| IFF 311 | White | M | Peach | 25/07/07 |
| IFF 813 | Yellow | NM | Nectarine | 09/07/07 |
| 21.6.175 | Yellow | M | Peach | 25/07/07 |
| ‘Oro A’ | Yellow | NM | Peach | 12/06/07 |
| ‘Jonia’ | Yellow | NM | Peach | 29/06/07 |
| ‘Big Top’ | Yellow | SS | Nectarine | 12/07/07 |
| ‘Springbelle’ | Yellow | M | Peach | 13/06/07 |
| ‘Springcrest’ | Yellow | M | Peach | 19/06/07 |
| Flesh Characteristics | Firmness (kg) | PPO 2 (uPPO/g FW) | Ascorbic Acid (mg/100 g FW) | Total Phenolics (Mesocarp) (mg/g DW) | Total Phenolics (Exocarp) (mg/g DW) | |
|---|---|---|---|---|---|---|
| Flesh color | Red | 0.41 b | 8.73 | 44.91 a | 11.25 a | 34.75 a |
| White | 2.05 a | 11.57 | 14.74 b | 2.88 b | 7.55 b | |
| Yellow | 2.64 a | 9.76 | 10.99 b | 1.43 c | 5.15 b | |
| Significance | *** | ns | *** | *** | *** | |
| Flesh typology 1 | M | 1.60 b | 10.53 | 14.32 a,b | 2.71 a,b | 8.63 a |
| NM | 2.42 b | 9.39 | 20.00 a | 2.15 b,c | 5.64 b | |
| SH | 3.86 a | 11.63 | 9.25 b,c | 3.90 a | 7.83 a,b | |
| SS | 3.60 a | 9.96 | 5.88 c | 0.85 c | 5.76 b | |
| Significance | *** | ns | *** | ** | ns | |
| Tissue Type | Genotype | Flesh Color | Flesh Type 1 | Flavan-3-ols (mg/g DW) | Cinnamic Acids (mg/g DW) | Anthocyanins (mg/g DW) | Flavanols (mg/g DW) | Unknowns (mg/g DW) |
|---|---|---|---|---|---|---|---|---|
| Mesocarp | 25.1.38 | Red | M | - | 5.82 a | 1.87 a | 3.46 a | 0.10 e |
| ‘Ghiaccio 1’ | White | SH | 0.66 b | 2.99 b | - | - | 0.22 b,c,d,e | |
| 21.6.116 | M | 0.77 b | 1.56 c | 0.04 b,c | 0.06 b | 0.47 a | ||
| 26.20.117 | M | 0.34 b,c | 3.04 b | 0.02 c | - | 0.25 b,c,d,e | ||
| 26.10.124 | M | 0.20 b,c | 0.81 c | 0.09 b,c | 0.06 b | 0.12 d,e | ||
| 26.20.127 | M | 0.53 b,c | 0.90 c | 0.10 b,c | - | 0.12 d,e | ||
| IFF 311 | M | 1.48 a | 1.05 c | 0.15 b | 0.07 b | 0.10 e | ||
| IFF 813 | Yellow | NM | 0.50 b,c | 0.38 c | - | 0.10 b | 0.15 c,d,e | |
| 21.6.175 | M | 0.72 b | 0.86 c | - | 0.26 b | 0.40 a,b,c | ||
| ‘Oro A’ | NM | 0.36 b,c | 3.28 b | - | 0.24 b | 0.42 a,b | ||
| ‘Big Top’ | SS | - | 0.54 c | 0.05 b,c | - | 0.25 b,c,d,e | ||
| ‘Jonia’ | NM | - | 0.63 c | - | - | 0.38 a,b,c | ||
| ‘Springbelle’ | M | 0.13 c | 0.42 c | - | 0.23 b | 0.33 a,b,c,d | ||
| ‘Springcrest’ | M | 0.01 c | 0.50 c | - | - | 0.35 a,b,c | ||
| Significance | *** | *** | *** | *** | *** | |||
| Exocarp | 25.1.38 | Red | M | 0.26 g | 9.96 a | 6.01 a | 17.76 a | 0.66 b |
| ‘Ghiaccio 1’ | White | SH | 2.36 a | 4.45 b | - | 0.54 b | 0.47 b | |
| 21.6.116 | M | 1.70 b,c,d,e | 2.51 c | 0.18 e,f | 1.17 b | 0.88 a,b | ||
| 26.20.117 | M | 1.46 c,d,e | 4.74 b | 2.48 b | 1.93 b | 0.75 a,b | ||
| 26.10.124 | M | 1.30 d,e | 2.47 c | 1.08 c,d,e,f | 1.41 b | 0.55 b | ||
| 26.20.127 | M | 1.79 b,c,d,e | 1.50 c,d | 0.58 c,d,e,f | 0.44 b | 0.41 b | ||
| IFF 311 | M | 2.23 a,b | 1.67 c,d | 1.52 b,c,d | 1.79 b | 0.59 b | ||
| IFF 813 | Yellow | NM | 2.00 a,b,c,d | 1.11 c,d | - | 2.01 b | 0.81 a,b | |
| 21.6.175 | M | 2.02 a,b,c | 2.26 c | 0.20 d,e,f | 0.99 b | 1.22 a | ||
| ‘Oro A’ | NM | 1.13 e,f | 5.35 b | - | 1.02 b | 0.43 b | ||
| ‘Big Top’ | SS | 0.14 g | 1.47 c,d | 2.27 b | 1.33 b | 0.53 b | ||
| ‘Jonia’ | NM | 0.46 f,g | 1.04 c,d | 0.34 d,e,f | 0.87 b | 0.77 a,b | ||
| ‘Springbelle’ | M | 0.18 g | 0.76 d | 1.62 b,c | 0.66 b | 0.48 b | ||
| ‘Springcrest’ | M | 0.27 g | 1.23 c,d | 1.18 c,d,e | 1.19 b | 0.60 b | ||
| Significance | *** | *** | *** | *** | ** | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, S.; Anthony, B.; Masia, A.; Giovannini, D.; Musacchi, S. Determination of Biochemical Composition in Peach (Prunus persica L. Batsch) Accessions Characterized by Different Flesh Color and Textural Typologies. Foods 2020, 9, 1452. https://doi.org/10.3390/foods9101452
Serra S, Anthony B, Masia A, Giovannini D, Musacchi S. Determination of Biochemical Composition in Peach (Prunus persica L. Batsch) Accessions Characterized by Different Flesh Color and Textural Typologies. Foods. 2020; 9(10):1452. https://doi.org/10.3390/foods9101452
Chicago/Turabian StyleSerra, Sara, Brendon Anthony, Andrea Masia, Daniela Giovannini, and Stefano Musacchi. 2020. "Determination of Biochemical Composition in Peach (Prunus persica L. Batsch) Accessions Characterized by Different Flesh Color and Textural Typologies" Foods 9, no. 10: 1452. https://doi.org/10.3390/foods9101452
APA StyleSerra, S., Anthony, B., Masia, A., Giovannini, D., & Musacchi, S. (2020). Determination of Biochemical Composition in Peach (Prunus persica L. Batsch) Accessions Characterized by Different Flesh Color and Textural Typologies. Foods, 9(10), 1452. https://doi.org/10.3390/foods9101452







