Legume Beverages from Chickpea and Lupin, as New Milk Alternatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
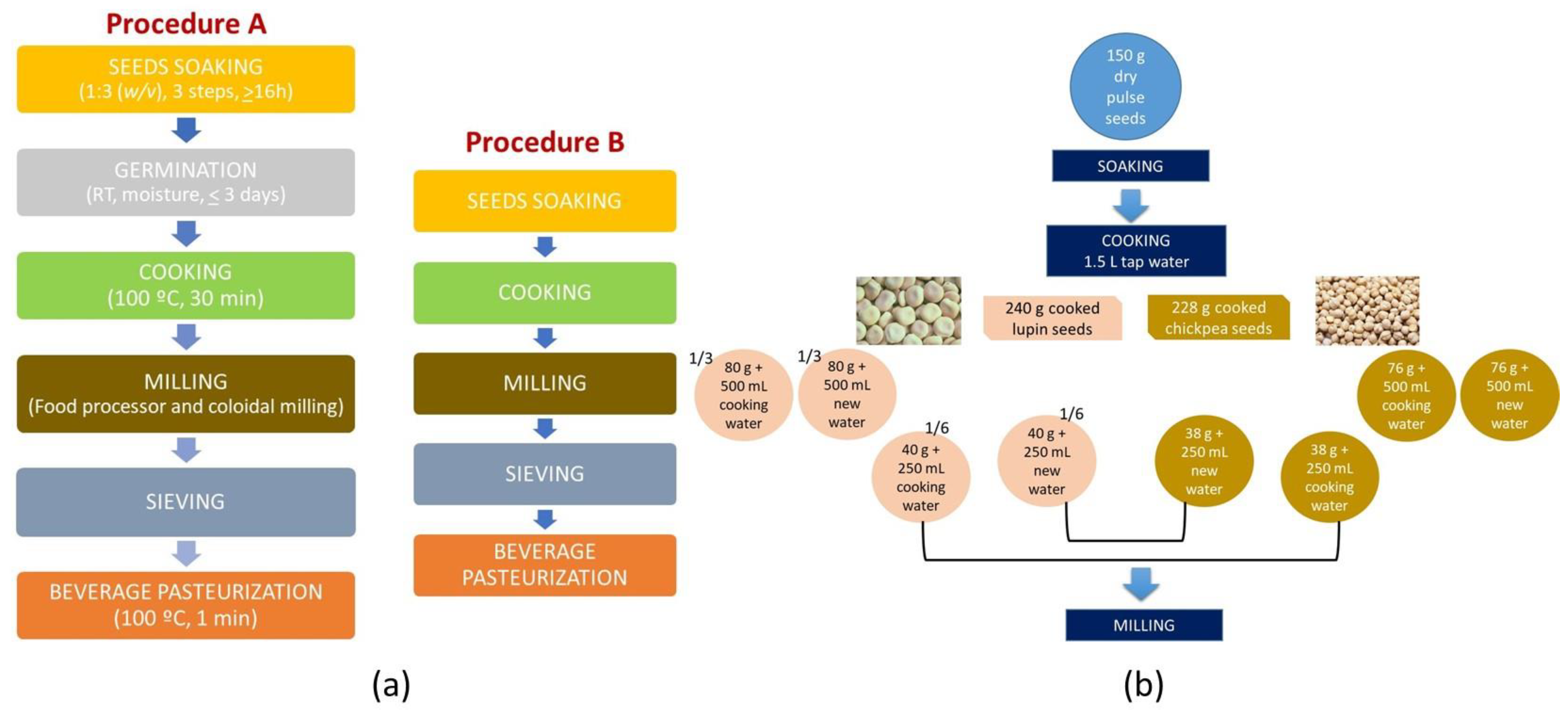
2.2.1. Pulse Beverage Preparation
2.2.2. Color Measurements
2.2.3. Chemical Analysis
2.2.4. High Performance Liquid Chromatography (HPLC) Analysis
2.2.5. Complementary Analyzes
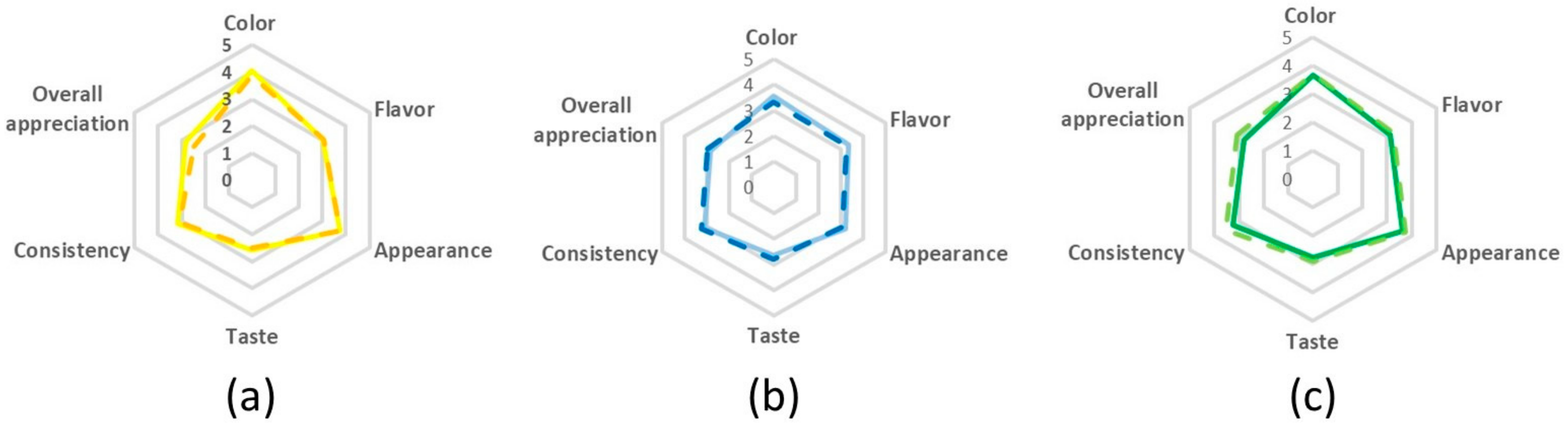
2.2.6. Sensory Evaluation
2.2.7. Characterization of Pulse Beverage Particles
2.2.8. Rheological Measurements
2.2.9. Statistical Analysis
3. Results
3.1. Chronological Progress of Beverage Processing Steps
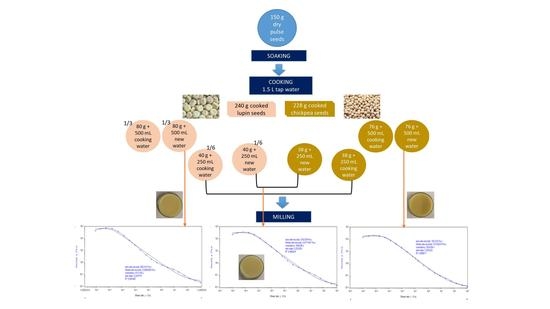
3.2. Achieving the Last Optimization of Beverage Development–Particle Size Reduction
3.2.1. Rheological Behavior of Produced Pulse Beverages
3.2.2. Color Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Market and Markets. Dairy Alternatives Market by Source (Soy, Almond, Coconut, Rice, Oats, Hemp), Application (Milk, Cheese, Yogurt, Ice Creams, Creamers), Distribution Channel (Supermarkets, Health Stores, Pharmacies), Formulation and Region—Global Forecast to 2023. Available online: https://www.marketsandmarkets.com/Market-Reports/dairy-alternative-plant-milk-beverages-market-677.html (accessed on 28 February 2019).
- Mintel Press Office. US Non-Dairy Milk Sales Grow 61% Over the Last Five Years. Available online: https://www.mintel.com/press-centre/food-and-drink/us-non-dairy-milk-sales-grow-61-over-the-last-five-years (accessed on 19 March 2019).
- Scrimshaw, N.S.; Murray, E.B. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am. J. Clin. Nutr. 1988, 48, 1142–1159. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, C.; Szajewska, H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur. J. Pediatr. 2015, 174, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for special dietary needs: Non-dairy plant-based milk substitutes and fermented dairy-type products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Transparency Market Research. Plant-Based Milk Market. Available online: https://www.transparencymarketresearch.com/plant-based-milk-market.html (accessed on 9 April 2019).
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; Available online: http://www.fao.org/3/a-i3437e.pdf (accessed on 22 March 2019).
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Mintel Press Office. Dairy Milk US 2016: Consumer Market Research Report & Non-dairy Milk US 2016: Consumer Market Research Report. Available online: http://store.mintel.com/dairy-milk-us-march-2016; http://store.mintel.com/non-dairy-milk-us-april-2016 (accessed on 19 March 2019).
- Sosulski, F.W.; Chakrabotry, P.; Humbert, E.S. Legume-based imitation and blended milk products. Can. Inst. Food Sci. Technol. J. 1978, 11, 117–123. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Piornos, J.A.; Burgos-Díaz, C.; Ogura, T.; Morales, E.; Rubilar, M.; Maureira-Butler, I.; Salvo-Garrido, H. Functional and physicochemical properties of a protein isolate from AluProt-CGNA: A novel protein-rich lupin variety (Lupinus luteus). Food Res. Int. 2015, 76, 719–724. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, chickpea and lentil protein isolates: Physicochemical characterization and emulsifying properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Cruz, N.; Capellas, M.; Hernandez, M.; Trujillo, A.J.; Guamis, B.; Ferragut, V. Ultra high pressure homogenization of soymilk: Microbiological, physicochemical and microstructural characteristics. Food Res. Int. 2007, 40, 725–732. [Google Scholar] [CrossRef]
- Li, Y.Q.; Tian, W.L.; Mo, H.Z.; Zhang, Y.L.; Zhao, X.Z. Effects of pulsed electric field processing on quality characteristics and microbial inactivation of soymilk. Food Bioprocess. Technol. 2013, 6, 1907–1916. [Google Scholar] [CrossRef]
- Fennema, O.R. Chapter 4: Carbohydrates. In Food Chemistry, 3rd ed.; Fennema, O.R., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 158–221. [Google Scholar]
- Yang, A.; Smyth, H.; Chaliha, M.; James, A. Sensory quality of soymilk and tofu from soybeans lacking lipoxygenases. Food Sci. Nutr. 2016, 4, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Carrão-Panizzi, M.C.; Beléia, A.P.; Prudêncio-Ferreira, S.H.; Oliveira, M.C.N.; Kitamura, K. Effects of isoflavones on beany flavor and astringency of soymilk and cooked whole soybean grains. Pesquisa Agropecuária Brasileira 1999, 34, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Champ, M. Non-nutrient bioactive substances of pulses. Br. J. Nutr. 2010, 88, S307–S319. [Google Scholar] [CrossRef] [PubMed]
- Campos-Veja, R.; Loarca-Piña, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Clemente, A.; Arques, M.C. Bowman-Birk inhibitors from legumes as colorectal chemopreventive agents. World J. Gastroenterol. 2014, 20, 10305–10315. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Chen, Z. Plant protease inhibitors in therapeutics-focus on cancer therapy. Front. Pharmacol. 2016, 7, 470. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.I.G.; Mota, J.; Monteiro, S.A.V.S.; Ferreira, R.M.S.B. Legume seeds and colorectal cancer revisited: Protease inhibitors reduce MMP-9 activity and colon cancer cell migration. Food Chem. 2016, 197, 30–38. [Google Scholar] [CrossRef]
- Khandelwal, S.; Udipi, S.A.; Ghugre, P. Polyphenols and tannins in Indian pulses: Effect of soaking, germination and pressure cooking. Food Res. Int. 2010, 43, 526–530. [Google Scholar] [CrossRef]
- Pan, Z.; Tangratanavalee, W. Characteristics of soybeans as affected by soaking conditions. LWT—Food Sci. Technol. 2003, 36, 143–151. [Google Scholar] [CrossRef]
- Singh, U.; Jambunathan, R. Studies on Desi and Kabull Chickpea (Cicer arietinum L.) Cultivars: Levels of Protease Inhibitors, Levels of Polyphenolic Compounds and In Vitro Protein Digestibility. J. Food Sci. 1981, 46, 1364–1367. [Google Scholar] [CrossRef] [Green Version]
- Savitri, A.; Desikachar, H.S. A comparative study of flatus production in relation to the oligosaccharides content of some legumes. Nutr. Rep. Int. 1985, 31, 337–344. [Google Scholar]
- Subbulakshmi, G.; Ganeshkumar, K.; Venkataraman, L.V. Effect of germination on the carbohydrates, proteins, tripsin inhibitor, amylase inhibitor and hemagglutinin in horse gram. Nutr. Rep. Int. 1976, 12, 19–26. Available online: http://ir.cftri.com/id/eprint/6510 (accessed on 8 July 2020).
- Sampath, S.; Rao, T.M.; Reddy, K.K.; Arun, K.; Reddy, P.V.M. Effect of germination on oligosaccharides in cereals and pulses. J. Food Sci. Technol. Mysore 2008, 45, 196–198. [Google Scholar]
- Kuo, Y.H.; Rozan, P.; Lambein, F.; Frias, J.; Vidal-Valverde, C. Effects of different germination conditions on the contents of free protein and non-proteins amino acids of commercial legumes. Food Chem. 2004, 86, 537–545. [Google Scholar] [CrossRef]
- Murugkar, D.A. Effect of sprouting of soybean on the chemical composition and quality of soymilk and tofu. J. Food Sci. Technol. 2014, 51, 915–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.U.; Belavady, B. Oligosaccharides in Pulses: Varietal differences and effects of cooking and germination. J. Agric. Food Chem. 1978, 26, 316–319. [Google Scholar] [CrossRef]
- Lopes, M.; Duarte, C.M.; Nunes, C.; Raymundo, A.; Sousa, I. Flow Behavior of Vegetable Beverages to Replace Milk. In Proceedings of the Iberian Meeting on Rheology, IBEREO, Porto, Portugal, 4–6 September 2019; Galindo-Rosales, F., Campo-Deaño, L., Afonso, A., Alves, M., Pinho, F., Eds.; Springer Proceedings in Materials. Springer: Cham, Switzerland. [Google Scholar] [CrossRef]
- Nelson, A.I.; Steinberg, M.P.; Wei, L.S. Illinois process for preparation of soymilk. J. Food Sci. 1976, 41, 57–61. [Google Scholar] [CrossRef]
- Francis, F.J.; Clydesdale, F.M. Food Colorimetry: Theory and Applications; AVI Publishing Company Inc.: Westport, CT, USA, 1975; pp. 26–45. [Google Scholar]
- International Organization of Vine and Wine. Type I methods: Total Acidity (OIV-MA-AS313-01: R2015); Volatile Acidity (OIV-MA-AS313-02: R2015). In Compendium of International Methods of Wine and Must Analysis; IOVW: Paris, France, 2019; Volume 1. [Google Scholar]
- International Organization for Standardization. ISO 20483. Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method, 1st ed.; ISO: Geneva, Switzerland, 2006; pp. 1–13. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors’. Crit. Rev. Food Sci. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars and related substances. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Santos, M.V.; Faria, N.T.; Fonseca, C.; Ferreira, F.C. Production of mannosylerythritol lipids from lignocellulose hydrolysates: Tolerance thresholds of Moesziomyces antarcticus to inhibitors. J. Chem. Technol. Biotechnol. 2019, 94, 1064–1107. [Google Scholar] [CrossRef]
- Barnes, H.A. Chapter 9: Shear-thinning liquids. In Handbook of Elementary Rheology, 1st ed.; Institute of Non-Newtonian Fluid Mechanics, Department of Mathematics, University of Wales: Aberystwyth, Wales, UK, 2000. [Google Scholar]
- Sousa, I.M.N.; Morgan, P.J.; Mitchell, J.R.; Harding, S.E.; Hill, S.E. Hydrodynamic characterization of lupin proteins: Solubility, intrinsic viscosity, and molar mass. J. Agric. Food Chem. 1996, 44, 3018–3021. [Google Scholar] [CrossRef]
- PortFIR. Composição de Alimentos. Available online: http://portfir.insa.pt/foodcomp/search (accessed on 21 February 2019).
- Tako, M.; Tamaki, Y.; Teruya, T.; Takeda, Y. The Principles of Starch Gelatinization and Retrogradation. Food Nutr. Sci. 2014, 5, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Table of Typical Grain Bulk Densities and Angles of Repose. Available online: http://www.leoncooksilos.com.au/Typical%20Grain%20Bulk%20Densities%20and%20Angles%20of%20Repose.pdf (accessed on 17 September 2019).
- Wang, S.; Errington, S.; Yap, H.H. Studies on Carotenoids from Lupin Seeds. In Lupins for Health and Wealth, Proceedings of the 12th International Lupin Conference, Fremantle, Australia, 14–18 September 2008; Palta, J.A., Berger, J.B., Eds.; International Lupin Association: Canterbury, New Zealand, 2008; pp. 198–202. [Google Scholar]
- Rezaei, M.K.; Deokar, A.; Tar’an, B. Identification and Expression Analysis of Candidate Genes Involved in Carotenoid Biosynthesis in Chickpea Seeds. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niño-Medina, G.; Muy-Rangel, D.; Garza-Juárez, A.J.; Vázquez-Rodríguez, J.A.; Méndez-Zamora, G.; Urías-Orona, V. Composición nutricional, compuestos fenólicos y capacidad antioxidante de cascarilla de garbanzo (Cicer arietinum). Archivos Latinoamericanos Nutrición 2017, 67, 68–73. Available online: https://www.alanrevista.org/ediciones/2017/1/art-10/ (accessed on 31 January 2020).
- Deswal, A.; Deora, N.S.; Mishra, H.N. Optimization of enzymatic production process of oat milk using response surface methodology. Food Bioprocess. Technol. 2014, 7, 610–618. [Google Scholar] [CrossRef]
- Prakash, V.; Narasinga Rao, M.S. Physicochemical properties of oilseed protein. CRC Crit. Rev. Biochem. Mol. 1986, 20, 265–363. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |


| Lupin Beverage (10% of Lupin) | Chickpea Beverage (10% of Chickpea) | Lupin and Chickpea Beverage (5% of Each) | |
|---|---|---|---|
| Dehulled sprouts (A1) | 5.8 ± 0.1 b,l,u,β,Ψ,τ,µ,λ | 6.9 ± 0.1 a,b,c,d,e,f,g,h,i | 6.3 ± 0.2 f,p,y,δ,Ψ,Ø,ξ,‡ |
| Dehulled sprouts (A2) | 5.9 ± 0.1 c,m,v,Ω,Ø,ηγ | 7.2 ± 0.1 a,j,k,l,m,n,o,p,q,r,s | 6.4 ± 0.1 g,q,z,θ,τ,η,χ,Ƨ,ǁ |
| Dehulled seeds (B1) | 5.8 ± 0.1 d,n,w,π,ξ,χ,σ,ε | 6.7 ± 0.0 j,t,u,v,w,x,y,z,Σ,α | 6.1 ± 0.0 h,r,Σ,&,µ,σ,Ƨ,Ɨ |
| Dehulled seeds (B2) | 5.9 ± 0.1 e,o,x,Δ,‡,ǁ,Ɔ | 7.0 ± 0.1 k,t,β,Ω,π,Δ,δ,θ,&,ω | 6.4 ± 0.0 i,s,α,ω,λ,γ,ε,Ɨ,Ɔ |
| Protein Content (% (w/v)) | ||||
|---|---|---|---|---|
| Sprouts | Seeds | |||
| A1 | A2 | B1 | B2 | |
| Lupin | 2.3 ± 0.1 a,g,o,s,y,Σ,α,β | 2.0 ± 0.1 b,h,p,t,Ω,π,Δ | 2.4 ± 0.1 c,I,q,u,Ω,δ,θ,&,ω,Ψ | 1.8 ± 0.3 d,j,v,y,δ |
| Chickpea | 1.3 ± 0.1 a,b,c,d,e,f | 1.1 ± 0.1 g,h,I,j,k,l,m | 1.5 ±0.1 n,o,p,q,r | 1.0 ± 0.1 n,s,t,u,v,w,x,z |
| Lupin + chickpea | 1.8 ± 0.1 e,k,x,Σ,θ | 1.6 ± 0.1 l,z,α,π,&,τ | 2.0 ± 0.1 f,m,r,w,ω, τ,μ | 1.4 ± 0.1 β,Δ,Ψ,μ |
| Partial Nutritional Composition | |||
|---|---|---|---|
| g/100 mL | Chickpea Beverage | Lupin Beverage | Lupin + Chickpea Beverage |
| Carbohydrates | 9.01 | 3.26 | 5.36 |
| Starch | 0.689 | 0.006 | 0.204 |
| Glucose | 0.45 | 0.06 | 0.28 |
| Group X Beverages | |||
|---|---|---|---|
| ƞ0 (Pa·s) | ƞ∞ (Pa·s) | ||
| Lupin A1 | 524.0 ± 95.0 | 1.9 × 10−2 ± 0.0 | 1.8 × 10−4 ± 0.0 × 10−4 |
| Lupin A2 | 609.5 ± 55.2 | 2.4 × 10−2 ± 0.1 (*) | 2.5 × 10−4 ± 0.2 × 10−4 |
| Chickpea A1 | 456.7 ± 54.7 | 0.9 × 10−2 ± 0.0 | 2.6 × 10−4 ± 0.4 × 10−4 |
| Chickpea A2 | 1243.8 ± 443.4 (*) | 0.9 × 10−2 ± 0.1 | 2.0 × 10−4 ± 0.3 × 10−4 |
| Lupin + chickpea A1 | 439.6 ± 200.1 | 1.4 × 10−2 ± 0.0 | 2.5 × 10−4 ± 0.6 × 10−4 |
| Lupin + chickpea A2 | 233.8 ± 19.2 | 1.3 × 10−2 ± 0.1 | 2.8 × 10−4 ± 0.3 × 10−4 |
| Lupin B1 | 495.3 ± 4.2 | 2.6 × 10−2 ± 0.3 | 2.0 × 10−4 ± 0.0 × 10−4 |
| Lupin B2 | 658.1 ± 34.6 | 2.9 × 10−2 ± 0.1 (*) | 1.9 × 10−4 ± 0.5 × 10−4 |
| Chickpea B1 | 52.1 ± 12.5 | 1.2 × 10−2 ± 0.0 | 12.7 × 10−4 ± 4.3 × 10−4 (*) |
| Chickpea B2 | 176.4 ± 22.4 | 1.3 × 10−2 ± 0.0 | 5.6 × 10−4 ± 1.1 × 10−4 |
| Lupin + chickpea B1 | 149.3 ± 45.2 | 1.5 × 10−2 ± 0.1 | 3.2 × 10−4 ± 0.3 × 10−4 |
| Lupin + chickpea B2 | 354.5 ± 34.6 | 1.5 × 10−2 ± 0.3 | 4.0 × 10−4 ± 1.6 × 10−4 |
| Oat beverage | 52.6 ± 4.2 | 0.7 × 10−2 ± 0.0 | 18.8 × 10−4 ± 1.6 × 10−4 (*) |
| Hazelnut beverage | 125.4 ± 32.8 | 0.8 × 10−2 ± 0.0 | 15.4 × 10−4 ± 2.2 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, M.; Pierrepont, C.; Duarte, C.M.; Filipe, A.; Medronho, B.; Sousa, I. Legume Beverages from Chickpea and Lupin, as New Milk Alternatives. Foods 2020, 9, 1458. https://doi.org/10.3390/foods9101458
Lopes M, Pierrepont C, Duarte CM, Filipe A, Medronho B, Sousa I. Legume Beverages from Chickpea and Lupin, as New Milk Alternatives. Foods. 2020; 9(10):1458. https://doi.org/10.3390/foods9101458
Chicago/Turabian StyleLopes, Mariana, Chloé Pierrepont, Carla Margarida Duarte, Alexandra Filipe, Bruno Medronho, and Isabel Sousa. 2020. "Legume Beverages from Chickpea and Lupin, as New Milk Alternatives" Foods 9, no. 10: 1458. https://doi.org/10.3390/foods9101458
APA StyleLopes, M., Pierrepont, C., Duarte, C. M., Filipe, A., Medronho, B., & Sousa, I. (2020). Legume Beverages from Chickpea and Lupin, as New Milk Alternatives. Foods, 9(10), 1458. https://doi.org/10.3390/foods9101458