1. Introduction
In the last decade, the dairy industry underwent a rapid expansion due to the increasing demand of milk-based products, resulting in high quantity of wastewater and by-products [
1,
2,
3,
4,
5]. Consequently, the sector is facing the waste disposal problem, mainly determined by the related high pollutant load.
Dairy waste treatments include mechanical, physicochemical, and biological methods [
4]. Overall, they are complex, expensive, and time-consuming, and the reason why waste is often disposed illegally in the environment, even if the industries must follow stringent national and community regulations [
6]. A zero-waste approach is urgently required to overcome this issue.
The main dairy derived by-products are cheese whey, ricotta cheese exhausted whey (RCEW), and buttermilk. Whey is a cheese manufacturing-based by-product, derived by precipitation and separation of milk casein and lipid from whole milk. It represents 85–95% of the total processed milk’s volume [
7], resulting in 9 L of whey for each kg of cheese produced [
1]. About 200 million tons of whey per year are globally produced [
8], of which 7–9.5 × 10
6 tons come only from Italy [
9].
Part of the whey is commonly used to produce ricotta cheese, with an alternative eco-friendly (and traditional) food technology. Whey proteins undergo the thermal coagulation (80–90 °C), a very low-efficiency process (about 2–3%) from which however results a large amount of RCEW. This by-product is extensively produced in South Europe, of which 1 million tons for year only in Italy [
10].
RCEW composition is similar to whey, but it is depleted in fats and proteins and enriched in salts, organic acids, and lactose [
11]. Due to the high organic content (4%
w/
v of lactose) [
12], the RCEW is extremely polluting and is characterized by considerable chemical (COD) and biochemical (BOD) oxygen demand values, of about 40 g/L and 66 g/L, respectively [
13].
The high sugar content potentially allows the use of RCEW as a suitable matrix for the biotechnological production of biodiesel [
14], bioethanol [
15], probiotics [
16], lactic acid [
17], lactobionic acid, growth medium for lipid production [
14], and fermented drink [
18].
Although the conversion of whey and RCEW in food, feed, and pharmaceutical and cosmetic ingredients has largely been investigated [
19], more than 50% is still treated as a common waste, generating several environmental and economic problems.
Recently, the implementation of the RCEW processing through the application of filtering membrane technologies that contemporarily allow the recovery of side compounds (e.g., whey proteins and lactose) and the reduction of organic pollutant load, has been proposed [
20,
21,
22]. To date, RCEW fractionation represents one of the most promising and innovative methods to enhance this still under-studied by-product [
23,
24].
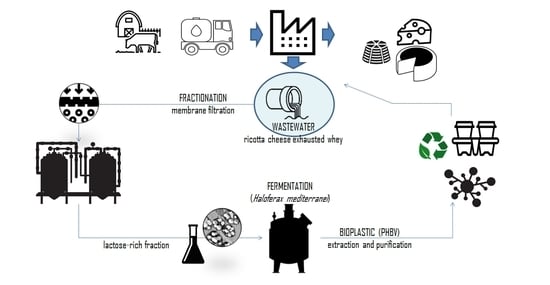
In this work, a new approach to valorize RCEW was proposed. The study describes the set-up and optimization of an integrate biotechnological process based on the combination of RCEW pre-treatment (membrane process) and fermentation (with a halophile microorganism), aiming at producing poly-hydroxyalkanoates (PHA), thermo-polymers considered as an intriguing biodegradable alternative to petroleum-derived plastics [
25,
26].
Despite of its enormous uses and economic benefits, plastics account for significant waste accumulation, which mostly remains undegraded and recalcitrant for decades in the global environment [
27]. PHA are linear polyesters synthesized by a variety of microorganisms, serving as an intracellular reservoir for energy and carbon supply [
28,
29]. Owing to their combined properties of biodegradability, biocompatibility, and thermoplasticity, PHA were identified as promising candidates for bio-based plastics production [
30,
31]. Although it has tremendous market potential, the high production costs still limit the PHA large-scale application [
32].
Recently, halophiles, a diversified group of microorganisms having the ability to survive in hypersaline environments, are attracting the interest of the scientific and industrial research as a cost-effective tool to produce PHA [
31,
33]. The adaptation to extreme conditions confers to halophiles a great potential for PHA production. The foremost advantage is that the high salinity requirements reduces the chances of microbial contamination to a great extent [
34], allowing to perform bioprocesses without expensive sterilization pre-treatments of the substrates. Moreover, microbial cells can be easily lysed in normal water due to the high intracellular osmotic pressure; thus, further reducing PHA recovery cost [
35]. However, the use of halophiles for large-scale PHA production is still associated to several issues [
36,
37]. Among these, the treatment of the saline fermentation effluent and the corrosion of the conventional fermentation equipment due to the high salt concentration of the substrates represent the main difficulties.
Aiming at (i) solving the disposal of a highly polluting agri-food waste and (ii) finding an economic alternative way for the production of bioplastic, a biotechnological process for PHA production, based on multi-step membrane fractionation of RCEW followed by fermentation by Haloferax mediterranei, was here set-up and optimized.
4. Discussion
Among halophiles, the haloarchaeon
Haloferax mediterranei has been extensively studied for efficient PHA production. It has several advantages such as adaptivity, high growth rate, genetic stability, and an efficient synthesis of the polymer [
65]. It was also demonstrated that
Hfx. mediterranei can efficiently use carbon sources from different industrial and household wastes for synthesizing PHA.
Detailed investigations already revealed that the PHA produced by
Hfx. mediterranei is PHBV, a copolymer of 3HB and 3HV from unrelated carbon sources [
66]. Moreover,
Hfx. mediterranei is also capable to synthesize poly(3-hydroxybutyrate-
co-3-hydroxyvalerate-
co-4-hydroxybutyrate) (PHBV4HB) [
31,
67].
PHBV is a polymer technologically and commercially preferred to PHB [
28]. Most organisms require 3HV precursor for PHBV synthesis whereas
Hfx. mediterranei can efficiently synthesize PHBV without any external precursor, thus greatly reducing the production cost [
68].
It was reported that three important factors mainly contribute to the cost of PHA production: substrate usage, fermentation process, and PHA recovery [
31]. Because PHA recovery from halophiles easily carried out by cell lysis using tap-water, the role of substrate and fermentation conditions in the process optimization is of great importance.
A possible strategy to overcome the challenge of an efficient PHA production is the usage of low-cost substrate which can compensate the high production cost resulting from low productivity. Indeed, it was estimated that the substrates account for almost 40–48% of the PHA production cost [
69]. Agri-industrial wastes such as vinasse [
70], olive mill wastewater [
57], chitin waste from seafood industry [
71] have been already tested as carbon substrate for PHA synthesized by
Hfx. mediterranei.
RCEW, one of the by-products of the dairy industry, in an inexpensive and abundant substrate rich in nutrients. Although RCEW is often considered very similar to cheese whey, the concentration of its constituents is unavoidably lower, except for the ash content, which is affected by the acid and salt added to enhance the whey proteins flocculation and aggregation [
72]. Moreover, the RCEW pH resulted higher than that of whey for the same reason (e.g., correction with sodium bicarbonate to improve the whey flocculation), while fat is quite completely retained during ricotta manufacturing [
72].
According to previous findings [
72], the multi-step fractionation described in this work allowed the retaining of the fat in the UF retentate and the separation of a lactose-rich from a protein-rich fractions. The former, containing 12.6% (
w/
v) of lactose, was used as substrate for PHA production; the latter can be easily subjected to the recovery of whey proteins to be used as food and feed supplements.
Moreover, the protein removal lead to the decrease of the nitrogen available during fermentation, hence avoiding the exo-polysaccharides (EPS) synthesis (which synthesis decrease the PHA production efficiency) and moving the microbial metabolism towards the bioplastic synthesis [
73].
Since
Hfx. mediterranei was unable to utilize lactose, enzymatically hydrolyzed R-NF was used as the sole carbon source. The enzymatic hydrolysis was preferred to acid one (that also requires high temperature) since more sustainable from both technical and economic point of views [
74].The enzyme was chosen among commercial and relatively cheap preparations commonly used in dairy processes to hydrolyze lactose in glucose and galactose for lactose-free food production. The ability of
H. mediterranei to metabolize both glucose and galactose was previously reported [
75]. Micronutrients were additionally supplemented to favor sugars utilization for PHBV synthesis [
76].
Optimization of the fermentation conditions (process parameters and substrate formula) is also the key to improve PHA production. Indeed, it is reported that the major drawbacks faced by haloarchaea species, such as
Hfx. mediterranei, are the low PHA productivity resulted from slow growth rate and failing to achieve high cell-density cultivation [
37].
The optimization of the fermentation conditions followed the classical approach of the one-factor-at-a-time (OFAT) experiments, which considered the variation of one factor at a time [
77]. The lactose concentration of the R-NF fraction is the range previously reported as optimal for PHA synthesis through
Hfx. mediterranei [
26,
67]; therefore, it was not changed through optimization process. Overall,
Hfx. mediterranei undergoes temperature-driven fast metabolism which causes nitrogen deficiency and triggers PHA overproduction [
37]. Nevertheless, a significant decrease in polymer production was found at 45 °C compared to 37 °C. Moreover, according to the early study of Fernandez-Castillo et al. [
78], the PHA accumulation was higher when only 10% (
w/
v) NaCl was added to the RCEW fraction.
The addition of yeast extract, rich in free amino acids and minerals, did not improve PHA synthesis, since probably the supplementations altered the C/N ratio of the medium decreasing the polymer productivity by promoting EPS synthesis [
73].
Once formula and temperature were set, the production of PHA in a bioreactor under stable and monitored conditions of pH, temperature, and stirring was tested. Overall, the amount of the polymer recovered at the end of fermentation was higher than that obtained in flasks, reaching amounts higher than 1 g/L of fermented R-NF. A similar experimental design for the optimization of the PHA synthesis through R-NF fermentation was carried out by the authors with a similar approach by using
Azotobacter vinelandii UWD [
79] as producer microorganism; nevertheless, the polymer production in any of the tested conditions, was lower than 127 ± 5 mg/L.
The chemical structure, surface condition of the polymer, and their related physical and thermal properties have also been investigated. Indeed, crystallinity, crystal structures, molecular orientation, melting temperature (T
m), and glass transition temperature (T
g) are known to have crucial effects on the PHAs biodegradation of polyesters [
80]. In this study, the DSC analysis was used to study the thermal properties (T
g, T
m, and
X) of the polymer synthesized by
Hfx. mediterranei in R-NF, in comparison to a commercial PHBV sample. Overall, the polymer thermal behavior observed under this study conditions reflected data previously obtained for PHBV [
50], confirming its identity. Nevertheless, the experimental sample was characterized by a double endothermic melting peak, which can be attributed to the presence of two crystalline phases of different sizes, orders, and thickness [
81,
82].
Previous studies demonstrated that increasing ratio of HV fraction in the PHBV causes the decrease of the melting temperature [
83]. The T
m of the PHBV here synthesized by
Hfx. mediterranei was markedly lower than the commercial sample, this latter previously characterized by the 1–3 mol% HV [
84,
85]. According to the literature [
86], melting point of the experimental PHBV corresponds to an approximately HV content of 16 mol%. Moreover, thermal behavior showed that experimental PHBV was characterized by a low crystallinity, a characteristic associated to improved degradation rate and the processability of the PHBV polymer [
80,
87].
The results of the X-ray photoelectron spectroscopy analysis, providing more quantitative measurement than FTIR, was used to investigate elemental composition of the PHBV synthesized by
Hfx. mediterranei in R-NF. Compared to the commercial sample, a lower contamination of the polymer synthesized in R-NF was observed. Such contaminations are commonly found in different commercial PHA samples as residues of the extraction and purification protocols and reagents [
88].
Overall, the XPS results corroborated the DSC and ATR-IFTR conclusions regarding the abundance of the alkyl region, corresponding to the HV chains. The analysis of the mechanical properties revealed a lower tensile strength than the commercial sample, this latter characterized by a low percentage of HV (1–3 mol%) [
84,
85]. It was previously reported that increasing HV percentages in the PHBV co-polymer cause the decrease of the tensile strength. Nevertheless, a relevant increase of the elongation break was observed in correspondence of high HV incorporation [
85]. Indeed, a, three-fold higher elongation break value was observed for the experimental PHBV sample compared to ENMAT commercial preparation.