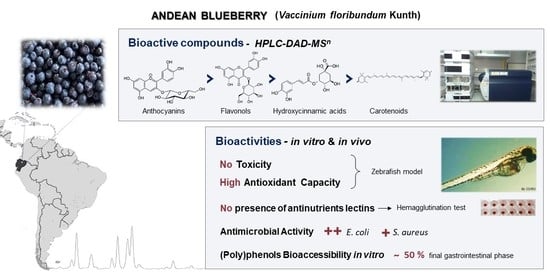
Characterization of Andean Blueberry in Bioactive Compounds, Evaluation of Biological Properties, and In Vitro Bioaccessibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Samples
2.2. Standards, Chemicals, and Solvents
2.3. Physicochemical Analysis
2.4. Identification and Quantification of Phenolic Compounds by HPLC–DAD–ESI/MSn
2.5. Identification and Quantification of Isoprenoids (Carotenoids and α-Tocopherol) by RRLC
2.6. Zebrafish Larvae Collection and Toxicity Test
2.7. Antioxidant Capacity
2.7.1. Antioxidant Capacity In Vitro
2.7.2. Antioxidant Capacity In Vitro: Thiobarbituric Acid Reactive Substances (TBARS) in Zebrafish Larvae Model
2.8. Determination of Antinutritional Factor: Lectins
2.9. Antimicrobial Activity
2.10. In Vitro Gastrointestinal Digestion
2.11. Total Phenolic Content
2.12. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization
3.2. Identification and Quantification of Bioactive Compounds
3.3. Embryo Toxicity Test with Zebrafish
3.4. Antioxidant Capacity In Vitro
3.5. Antioxidant Capacity In Vitro
3.6. Determination of Antinutritional Lectins
3.7. Antimicrobial Activity
3.8. In Vitro Gastrointestinal Digestion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory Effects in Humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015, 6, 2890–2917. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef] [Green Version]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids and Vitamin A in Agro-Food, Nutrition, Health and Disease. Mol. Nutr. Food Res. 2019, 1801045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite profiling of polyphenols in Vaccinium berries and determination of their chemopreventive properties. J. Pharm. Biomed. Anal. 2014, 89, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Chemical Composition and Phenolic Compound Profile of Mortiño ( Vaccinium floribundum Kunth). J. Agric. Food Chem. 2009, 57, 8274–8281. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alarcón-Barrera, K.S.; Armijos-Montesinos, D.S.; García-Tenesaca, M.; Iturralde, G.; Jaramilo-Vivanco, T.; Granda-Albuja, M.G.; Giampieri, F.; Alvarez-Suarez, J.M. Wild Andean blackberry (Rubus glaucus Benth) and Andean blueberry (Vaccinium floribundum Kunth) from the Highlands of Ecuador: Nutritional composition and protective effect on human dermal fibroblasts against cytotoxic oxidative damage. J. Berry Res. 2018, 8, 223–236. [Google Scholar] [CrossRef]
- Llivisaca, S.; Manzano, P.; Ruales, J.; Flores, J.; Mendoza, J.; Peralta, E.; Cevallos-Cevallos, J.M. Chemical, antimicrobial, and molecular characterization of mortiño (Vaccinium floribundum Kunth) fruits and leaves. Food Sci. Nutr. 2018, 6, 934–942. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. The complex carotenoid pattern of orange juices from concentrate. Food Chem. 2008, 109, 546–553. [Google Scholar] [CrossRef]
- NTE INEN 2427: Ecuadorian Technical Standards. Fresh fruit. Mulberries. Requirements. Available online: https://archive.org/stream/ec.nte.2427.2010/ec.nte.2427.2010_djvu.txt (accessed on 18 September 2019).
- AOAC, Association of Official Analytical Chemist. Official Methods of Analysis, 18th ed.; Horwitz, W., Latimer, G., Eds.; AOAC International: North Frederick Avenue, MD, USA, 2005. [Google Scholar]
- Gironés-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; García-Viguera, C.; Moreno, D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Stinco, C.M.; Benítez-González, A.M.; Hernanz, D.; Vicario, I.M.; Meléndez-Martínez, A.J. Development and validation of a rapid resolution liquid chromatography method for the screening of dietary plant isoprenoids: Carotenoids, tocopherols and chlorophylls. J. Chromatogr. A 2014, 1370, 162–170. [Google Scholar] [CrossRef]
- Murphey, R.D.; Zon, L.I. Small molecule screening in the zebraWsh. Methods 2006, 39, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, W.; Gómez-Ruiz, J.A.; Miralles, B.; Ramos, M.; Barrio, D.; Recio, I. Identification of antioxidant peptides of hen egg-white lysozyme and evaluation of inhibition of lipid peroxidation and cytotoxicity in the Zebrafish model. Eur. Food Res. Technol. 2016, 242, 1777–1785. [Google Scholar] [CrossRef] [Green Version]
- Boeri, P.; Piñuel, L.; Sharry, S.; Barrio, D. Caracterización nutricional de la harina integral de algarroba (Prosopis alpataco) de la norpatagonia Argentina. Rev. Fac. Agron. 2017, 116, 129–140. [Google Scholar]
- García-Ruiz, A.; González-Rompinelli, E.M.; Bartolomé, B.; Moreno-Arribas, M.V. Potential of wine-associated lactic acid bacteria to degrade biogenic amines. Int. J. Food Microbiol. 2011, 148, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Villacís-Chiriboga, J.; García-Ruiz, A.; Baenas, N.; Moreno, D.A.; Meléndez-Martínez, A.J.; Stinco, C.M.; Jerves-Andrade, L.; León-Tamariz, F.; Ortiz-Ulloa, J.; Ruales, J. Changes in phytochemical composition, bioactivity and in vitro digestibility of guayusa leaves (Ilex guayusa Loes.) in different ripening stages. J. Sci. Food Agric. 2018, 98, 1927–1934. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- USDA Blueberries Grade and Standards | Agricultural Marketing Service. United States Department of Agriculture. Available online: https://www.ams.usda.gov/grades-standards/blueberries-grade-and-standards (accessed on 23 September 2019).
- Soots, K.; Krikmann, O.; Starast, M.; Olt, J. Determining the dimensional characteristics of blueberries. Agron. Res. 2017, 15, 886–896. [Google Scholar]
- Reque, P.M.; Steffens, R.S.; Da Silva, A.M.; Jablonski, A.; Flôres, S.H.; Rios, A.dO.; De Jong, E.V. Characterization of blueberry fruits (Vaccinium spp.) and derived products. Food Sci. Technol. 2014, 34, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Matiacevich, S.; Celis Cofré, D.; Silva, P.; Enrione, J.; Osorio, F. Quality Parameters of Six Cultivars of Blueberry Using Computer Vision. Int. J. Food Sci. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.M.; Calvo, D.; Medina, J.J.; Barrau, C.; Romero, F. Fruit quality parameters of some southern highbush blueberries (Vaccinium xcorymbosum L.) grown in Andalusia (Spain). Span. J. Agric. Res. 2008, 6, 671. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, J.; Marín-Arroyo, M.R.; Noriega-Domínguez, M.J.; Navarro, M.; Arozarena, I. Color, phenolics, and antioxidant activity of blackberry (Rubus glaucus Benth.), blueberry (Vaccinium floribundum Kunth.), and apple wines from ecuador. J. Food Sci. 2013, 78. [Google Scholar] [CrossRef]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Alakolanga, A.G.A.W.; Siriwardane, A.M.D.A.; Savitri Kumar, N.; Jayasinghe, L.; Jaiswal, R.; Kuhnert, N. LC-MSn identification and characterization of the phenolic compounds from the fruits of Flacourtia indica (Burm. F.) Merr. and Flacourtia inermis Roxb. Food Res. Int. 2014, 62, 388–396. [Google Scholar] [CrossRef]
- Elsadig Karar, M.G.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of Phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) Leaves, Fruits and their Herbal Derived Drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2016, 01. [Google Scholar] [CrossRef] [Green Version]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Wang, J.; Yousef, G.; Lila, M.A.; De Mejia, E.G. Antioxidant capacity and in vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vaccinium floribundum and Aristotelia chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Vallejo, W.; Camargo, G.; Muñoz-Acevedo, A.; Quiñones, C.; Schott, E.; Zarate, X. Potential use of an anthocyanin-rich extract from berries of Vaccinium meridionale Swartz as sensitizer for TiO2 thin films—An experimental and theoretical study. J. Photochem. Photobiol. A Chem. 2019, 384, 112050. [Google Scholar] [CrossRef]
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011, 125, 201–208. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD 2002 OECD iLibrary. Available online: https://www.oecd-ilibrary.org/environment/guidance-document-on-the-use-of-the-harmonised-system-for-the-classification-of-chemicals-which-are-hazardous-for-the-aquatic-environment_9789264078444-en (accessed on 20 May 2020).
- Wibowo, I.; Permadi, K.; Hartati, R.; Damayanti, S. Ethanolic extract of pomegranate (Punica granatum L) peel: Acute toxicity tests on zebrafish (Danio rerio) embryos and its toxicity prediction by in silico ARTICLE INFO ABSTRACT. J. Appl. Pharm. Sci. 2018, 8, 82–086. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A. Rapid Assays to Evaluate the Antioxidant Capacity of Phenols in Virgin Olive Oil. In Olives and Olive Oil in Health and Disease Prevention; Elsevier Academic Press: Amsterdam, The Netherlands, 2010; pp. 625–635. ISBN 9780123744203. [Google Scholar]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonol glycosides and antioxidant capacity of various blackberry and blueberry genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2005, 85, 2149–2158. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; Mcewen, J.; O’brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity As Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46, 7–2686. [Google Scholar] [CrossRef]
- Gaviria Montoya, C.; David, J.; Arredondo, H.; Arias, M.L.; Inés, C.; Cano, M.; Benjamín, Y.; Rojano, A. Changes in the Antioxidant Activity in Mortiño Fruits (Vaccinium meridionale Sw.) during Development and Ripening. Rev. Fac. Nac. Agron. Medellín 2012, 65, 87-6495. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0304-28472012000100019 (accessed on 4 June 2020).
- Timme-Laragy, A.R.; Karchner, S.I.; Franks, D.G.; Jenny, M.J.; Harbeitner, R.C.; Goldstone, J.V.; McArthur, A.G.; Hahn, M.E. Nrf2b, novel zebrafish paralog of oxidant-responsive transcription factor NF-E2-related factor 2 (NRF2). J. Biol. Chem. 2012, 287, 4609–4627. [Google Scholar] [CrossRef] [Green Version]
- Vallverdú-Queralt, A.; Boix, N.; Piqué, E.; Gómez-Catalan, J.; Medina-Remon, A.; Sasot, G.; Mercader-Martí, M.; Llobet, J.M.; Lamuela-Raventos, R.M. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-Orbitrap-MS. Food Chem. 2015, 181, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.; Zimmermann, B.F.; Jungfer, E.; Chrubasik-Hausmann, S. Prevention of Urinary Tract Infections with Vaccinium Products. Phyther. Res. 2014, 28, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Ştefănescu, R.E.; Eșianu, S.; Laczkó-Zöld, E.; Mare, A.; Tudor, B.; Dogaru, M.T. Short Period Storage Impact on Bioactive Constituents from Bilberries and Blueberries. Acta Med. Marisiensis 2017, 63, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Zhao, Y.Y.; Luo, C.X.; Li, J.; Gao, Y.Q. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crops Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Barik, S.K.; Russell, W.R.; Moar, K.M.; Cruickshank, M.; Scobbie, L.; Duncan, G.; Hoggard, N. The anthocyanins in black currants regulate postprandial hyperglycaemia primarily by inhibiting α-glucosidase while other phenolics modulate salivary α-amylase, glucose uptake and sugar transporters. J. Nutr. Biochem. 2020, 78, 108325. [Google Scholar] [CrossRef]
- McDougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 2005, 23, 189–195. [Google Scholar] [CrossRef]


| Parameters | Content |
|---|---|
| Weight (g unit −1) | 3.5 ± 0.051 |
| Length (cm unit−1) | 1.75 ± 0.04 |
| Diameter (mm unit−1) | 8.5 ± 0.75 |
| pH | 2.61 ± 0.05 |
| Moisture (%) | 88.69 ± 0.08 |
| °Brix | 11.17 ± 0.03 |
| Titratable acidity (% citric acid) | 1.62 ± 0.00 |
| Peak Number | Rt (min) | DAD λ (nm) | [M−H]− | Fragment Ions (MSn) | Phenolic Compounds 1 |
|---|---|---|---|---|---|
| 1 | 6.0 | 330 | 707 (2[M−H]−) 353 | 191, 179 | 3-O-Caffeoylquinic acid * |
| 2 | 10.8 | 330 | 353 | 191 | 5-O-Caffeoylquinic acid |
| 3 | 16.7 | 280, 520 | 465 | 303 | Delphinidin-3-O-hexoside I |
| 4 | 18.5 | 280, 520 | 465 | 303 | Delphinidin-3-O-hexoside II |
| 5 | 19.6 | 280, 520 | 449 | 287 | Cyanidin-3-O-hexoside I |
| 6 | 20.8 | 280, 520 | 435 | 303 | Delphinidin-3-O-pentoside |
| 7 | 21.8 | 280, 520 | 449 | 287 | Cyanidin-3-O-hexoside II |
| 8 | 23.9 | 280, 520 | 419 | 287 | Cyanidin-3-O-pentoside |
| 9 | 26.6 | 320 | 335 | 179, 161, 131 | Caffeoylshikimic acid |
| 10 | 28.7 | 360 | 433 | 323, 179, 161 | Caffeic acid derivative |
| 11 | 33.6 | 360 | 463 | 301 | Quercetin-3-O-hexoside I |
| 12 | 35.2 | 360 | 463 | 301 | Quercetin-3-O-hexoside II |
| 13 | 37.5 | 360 | 433 | 301 | Quercetin-3-O-pentoside I |
| 14 | 39.6 | 360 | 433 | 301 | Quercetin-3-O-pentoside II |
| 15 | 41.2 | 360 | 433 | 301 | Quercetin-3-O-pentoside III |
| 16 | 42.8 | 360 | 447 | 301 | Quercetin-3-O-rhamnoside |
| Concentration | ||
|---|---|---|
| Phenolic Compounds | (µg/g DW) | |
| Hydroxycinnamic acids | ||
| 3-O-Caffeoylquinic acid | 236.1 | ± 37.7 1 |
| 5-O-Caffeoylquinic acid | 845.5 | ± 1.25 |
| Caffeoylshikimic acid | 35.8 | ± 1.58 |
| Caffeic acid derivative | 273.0 | ± 40.0 |
| Total | 1390.3 | ± 78.9 |
| Anthocyanins | ||
| Delphinidin-3-O-hexoside I | 395.7 | ± 58.5 |
| Delphinidin-3-O-hexoside II | 274.0 | ± 50.0 |
| Cyanidin-3-O-hexoside I | 1963.9 | ± 140 |
| Delphinidin-3-O-pentoside | 392.1 | ± 29.5 |
| Cyanidin-3-O-hexoside II | 71.1 | ± 22.3 |
| Cyanidin-3-O-pentoside | 2289.8 | ± 327 |
| Total | 5386.4 | ± 567 |
| Flavonols | ||
| Quercetin-3-O-hexoside I | 849.7 | ± 25.9 |
| Quercetin-3-O-hexoside II | 70.0 | ± 13.9 |
| Quercetin-3-O-pentoside I | 186.0 | ± 23.1 |
| Quercetin-3-O-pentoside II | 45.4 | ± 2.47 |
| Quercetin-3-O-pentoside III | 683.5 | ± 23.5 |
| Quercetin-3-O-rhamnoside | 219.0 | ± 25.9 |
| Total | 2095.5 | ± 184 |
| Total phenolic compounds | 8875.3 | ± 787 |
| Carotenoids | ||
| Lutein | 5.94 | ± 1.34 |
| Antioxidant Capacity (µmol Trolox g−1 DW) | |||
|---|---|---|---|
| ABTS· | DPPH− | ORAC | |
| Andean blueberry | 278.2 ± 59 1 | 85.1 ± 27 | 402.2 ± 17 |
| Gastrointestinal (GI) Phase | Total Phenolic Content (mg GAE g−1) | % Loss | % Bioaccessibility | Antioxidant Capacity (µmol Trolox g−1) |
|---|---|---|---|---|
| Initial | 11.27 ± 0.20 1 a | 40.53 ± 2.40 b | ||
| Oral | 10.10 ± 0.94 a | 10 | 90 | 41.67 ± 1.73 b |
| Gastric | 9.54 ± 1.09 a | 15 | 85 | 25.94 ± 2.14 c |
| Intestinal | 6.36 ± 0.36 b | 43 | 56 | 68.67 ± 2.98 a |
| Final | 5.74 ± 0.62 b | 49 | 51 | 63.97 ± 4.79 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baenas, N.; Ruales, J.; Moreno, D.A.; Barrio, D.A.; Stinco, C.M.; Martínez-Cifuentes, G.; Meléndez-Martínez, A.J.; García-Ruiz, A. Characterization of Andean Blueberry in Bioactive Compounds, Evaluation of Biological Properties, and In Vitro Bioaccessibility. Foods 2020, 9, 1483. https://doi.org/10.3390/foods9101483
Baenas N, Ruales J, Moreno DA, Barrio DA, Stinco CM, Martínez-Cifuentes G, Meléndez-Martínez AJ, García-Ruiz A. Characterization of Andean Blueberry in Bioactive Compounds, Evaluation of Biological Properties, and In Vitro Bioaccessibility. Foods. 2020; 9(10):1483. https://doi.org/10.3390/foods9101483
Chicago/Turabian StyleBaenas, Nieves, Jenny Ruales, Diego A. Moreno, Daniel Alejandro Barrio, Carla M. Stinco, Gabriela Martínez-Cifuentes, Antonio J. Meléndez-Martínez, and Almudena García-Ruiz. 2020. "Characterization of Andean Blueberry in Bioactive Compounds, Evaluation of Biological Properties, and In Vitro Bioaccessibility" Foods 9, no. 10: 1483. https://doi.org/10.3390/foods9101483
APA StyleBaenas, N., Ruales, J., Moreno, D. A., Barrio, D. A., Stinco, C. M., Martínez-Cifuentes, G., Meléndez-Martínez, A. J., & García-Ruiz, A. (2020). Characterization of Andean Blueberry in Bioactive Compounds, Evaluation of Biological Properties, and In Vitro Bioaccessibility. Foods, 9(10), 1483. https://doi.org/10.3390/foods9101483