Enteropathogenic Potential of Bacillus thuringiensis Isolates from Soil, Animals, Food and Biopesticides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Cell Lines and Culture Conditions
2.3. PCR Analyses
2.4. PanC Sequence Typing
2.5. Spore Preparation
2.6. Germination
2.7. Motility Assay
2.8. Enzyme Immunoassays (EIAs)
2.9. WST-1 Bioassay
2.10. Haemolysis Assays
2.11. Disk Diffusion Assays
2.12. Antimicrobial Broth Dilution Assays
2.13. Statistical Analyses
3. Results
3.1. Genetic Prerequisites of the Tested Strains
3.2. Strain-Specific Germination and Motility
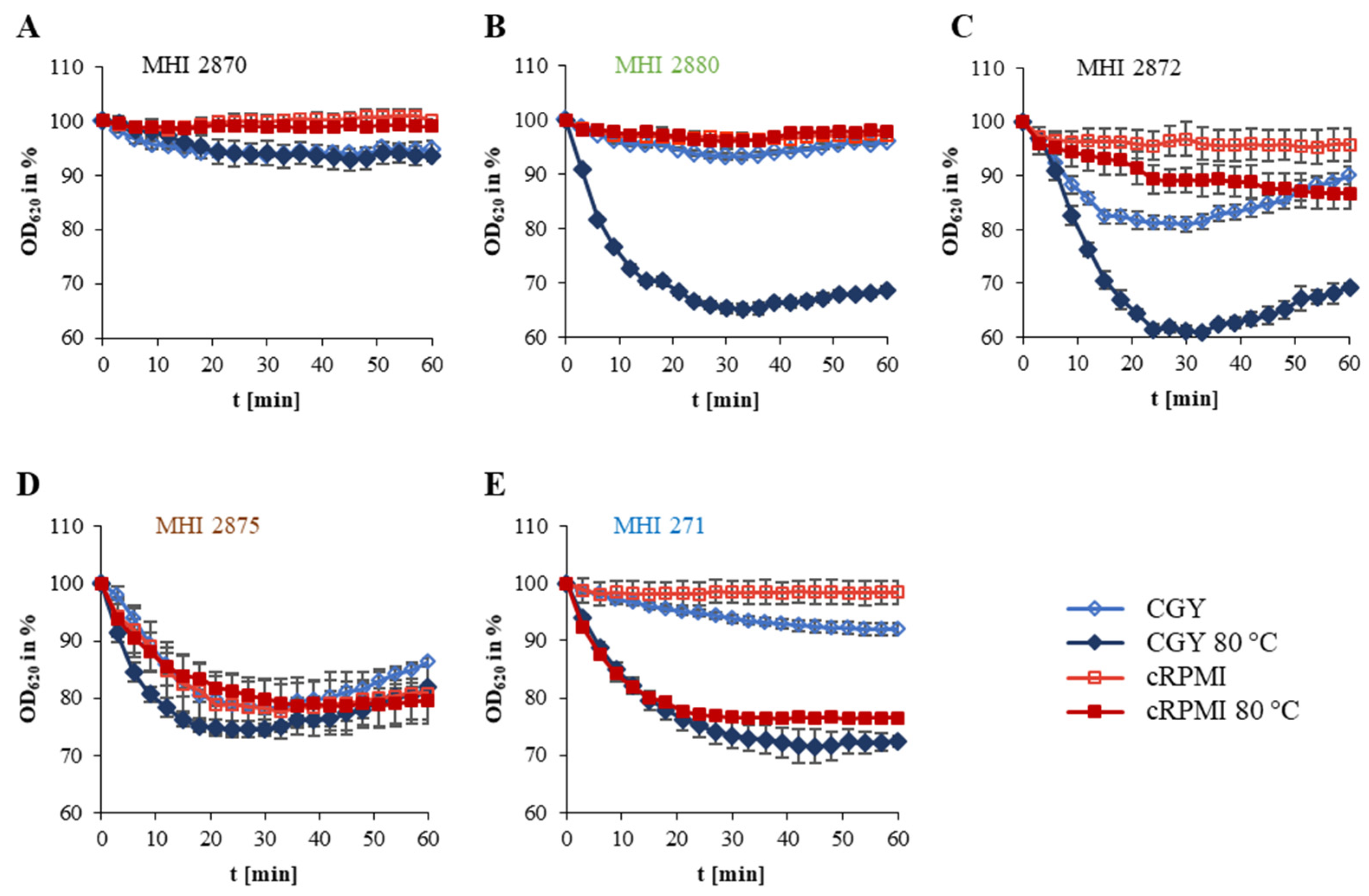
3.3. Growth under Simulated Intestinal Conditions and Production of Enterotoxins
3.4. Exhibition of Cytotoxic and Haemolytic Activity
3.5. Growth Inhibition by Essential Oils (EOs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chattopadhyay, P.; Banerjee, G. Recent advancement on chemical arsenal of Bt toxin and its application in pest management system in agricultural field. 3 Biotech 2018, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711. [Google Scholar] [CrossRef]
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Wang, B.C.; Yu, Z.; Sun, M. Structural insights into Bacillus thuringiensis Cry, Cyt and parasporin toxins. Toxins 2014, 6, 2732–2770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, W.P.; Engleman, J.T.; Donovan, J.C.; Baum, J.A.; Bunkers, G.J.; Chi, D.J.; Clinton, W.P.; English, L.; Heck, G.R.; Ilagan, O.M.; et al. Discovery and characterization of Sip1A: A novel secreted protein from Bacillus thuringiensis with activity against coleopteran larvae. Appl. Microbiol. Biotechnol. 2006, 72, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, C.S.; Boets, A.; Van Rie, J.; Ferré, J. Screening and identification of vip genes in Bacillus thuringiensis strains. J. Appl. Microbiol. 2009, 107, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Höfte, H.; Whiteley, H.R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 1989, 53, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Ellar, D.J.; Bishop, A.; Johnson, C.; Lin, S.; Hart, E.R. Characterization of a Bacillus thuringiensis delta-endotoxin which is toxic to insects in three orders. J. Invertebr. Pathol. 2000, 76, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Guinebretière, M.H.; Auger, S.; Galleron, N.; Contzen, M.; De Sarrau, B.; De Buyser, M.L.; Lamberet, G.; Fagerlund, A.; Granum, P.E.; Lereclus, D.; et al. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus group occasionally associated with food poisoning. Int. J. Syst. Evol. Microbiol. 2013, 63, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.; Urdiain, M.; Cifuentes, A.; López-López, A.; Blanch, A.R.; Tamames, J.; Kämpfer, P.; Kolstø, A.B.; Ramón, D.; Martínez, J.F.; et al. Description of Bacillus toyonensis sp. nov., a novel species of the Bacillus cereus group, and pairwise genome comparisons of the species of the group by means of ANI calculations. Syst. Appl. Microbiol. 2013, 36, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lai, Q.; Göker, M.; Meier-Kolthoff, J.P.; Wang, M.; Sun, Y.; Wang, L.; Shao, Z. Genomic insights into the taxonomic status of the Bacillus cereus group. Sci. Rep. 2015, 5, 14082. [Google Scholar] [CrossRef] [Green Version]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [Green Version]
- Beecher, D.J.; Schoeni, J.L.; Wong, A.C. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 1995, 63, 4423–4428. [Google Scholar] [CrossRef] [Green Version]
- Jessberger, N.; Kranzler, M.; da Riol, C.; Schwenk, V.; Buchacher, T.; Dietrich, R.; Ehling-Schulz, M.; Märtlbauer, E. Assessing the toxic potential of enteropathogenic Bacillus cereus. Food Microbiol. 2019, 84, 103276. [Google Scholar] [CrossRef]
- Jessberger, N.; Rademacher, C.; Krey, V.M.; Dietrich, R.; Mohr, A.K.; Böhm, M.E.; Scherer, S.; Ehling-Schulz, M.; Märtlbauer, E. Simulating intestinal growth conditions enhances toxin production of enteropathogenic Bacillus cereus. Front. Microbiol. 2017, 8, 627. [Google Scholar] [CrossRef] [Green Version]
- Lund, T.; De Buyser, M.L.; Granum, P.E. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 2000, 38, 254–261. [Google Scholar] [CrossRef]
- Lund, T.; Granum, P.E. Characterisation of a non-haemolytic enterotoxin complex from Bacillus cereus isolated after a foodborne outbreak. FEMS Microbiol. Lett. 1996, 141, 151–156. [Google Scholar] [CrossRef]
- Cho, S.H.; Kang, S.H.; Lee, Y.E.; Kim, S.J.; Yoo, Y.B.; Bak, Y.S.; Kim, J.B. Distribution of toxin genes and enterotoxins in Bacillus thuringiensis isolated from microbial insecticide products. J. Microbiol. Biotechnol. 2015, 25, 2043–2048. [Google Scholar] [CrossRef]
- Kim, M.J.; Han, J.K.; Park, J.S.; Lee, J.S.; Lee, S.H.; Cho, J.I.; Kim, K.S. Various enterotoxin and other virulence factor genes widespread among Bacillus cereus and Bacillus thuringiensis strains. J. Microbiol. Biotechnol. 2015, 25, 872–879. [Google Scholar] [CrossRef]
- Swiecicka, I.; Van der Auwera, G.A.; Mahillon, J. Hemolytic and nonhemolytic enterotoxin genes are broadly distributed among Bacillus thuringiensis isolated from wild mammals. Microb. Ecol. 2006, 52, 544–551. [Google Scholar] [CrossRef]
- McIntyre, L.; Bernard, K.; Beniac, D.; Isaac-Renton, J.L.; Naseby, D.C. Identification of Bacillus cereus group species associated with food poisoning outbreaks in British Columbia, Canada. Appl. Environ. Microbiol. 2008, 74, 7451–7453. [Google Scholar] [CrossRef] [Green Version]
- EFSA, European Foods Safety Authority, Panel on Biological Hazards. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, 4524. [Google Scholar] [CrossRef]
- Johler, S.; Kalbhenn, E.M.; Heini, N.; Brodmann, P.; Gautsch, S.; Bagcioglu, M.; Contzen, M.; Stephan, R.; Ehling-Schulz, M. Enterotoxin production of Bacillus thuringiensis isolates from biopesticides, foods, and outbreaks. Front. Microbiol. 2018, 9, 1915. [Google Scholar] [CrossRef] [Green Version]
- Ben-Dov, E.; Zaritsky, A.; Dahan, E.; Barak, Z.; Sinai, R.; Manasherob, R.; Khamraev, A.; Troitskaya, E.; Dubitsky, A.; Berezina, N.; et al. Extended screening by PCR for seven cry-group genes from field-collected strains of Bacillus thuringiensis. Appl. Environ. Microbiol. 1997, 63, 4883–4890. [Google Scholar] [CrossRef] [Green Version]
- Wehrle, E.; Moravek, M.; Dietrich, R.; Bürk, C.; Didier, A.; Märtlbauer, E. Comparison of multiplex PCR, enzyme immunoassay and cell culture methods for the detection of enterotoxinogenic Bacillus cereus. J. Microbiol. Methods 2009, 78, 265–270. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Guinebretiére, M.H.; Monthan, A.; Berge, O.; Fricker, M.; Svensson, B. Toxin gene profiling of enterotoxic and emetic Bacillus cereus. FEMS Microbiol. Lett. 2006, 260, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Guinebretiére, M.H.; Thompson, F.L.; Sorokin, A.; Normand, P.; Dawyndt, P.; Ehling-Schulz, M.; Svensson, B.; Sanchis, V.; Nguyen-The, C.; Heyndrickx, M.; et al. Ecological diversification in the Bacillus cereus Group. Environ. Microbiol. 2008, 10, 851–865. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef]
- Miller, R.A.; Jian, J.; Beno, S.M.; Wiedmann, M.; Kovac, J. Intraclade variability in toxin production and cytotoxicity of Bacillus cereus group type strains and dairy-associated isolates. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Fricker, M.; Agren, J.; Segerman, B.; Knutsson, R.; Ehling-Schulz, M. Evaluation of Bacillus strains as model systems for the work on Bacillus anthracis spores. Int. J. Food Microbiol. 2011, 145, 129–136. [Google Scholar] [CrossRef] [PubMed]
- da Riol, C.D.; Dietrich, R.; Märtlbauer, E.; Jessberger, N. Consumed foodstuffs have a crucial impact on the toxic activity of enteropathogenic Bacillus cereus. Front. Microbiol. 2018, 9, 1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, R.; Fella, C.; Strich, S.; Märtlbauer, E. Production and characterization of monoclonal antibodies against the hemolysin BL enterotoxin complex produced by Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4470–4474. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, R.; Moravek, M.; Bürk, C.; Granum, P.E.; Märtlbauer, E. Production and characterization of antibodies against each of the three subunits of the Bacillus cereus nonhemolytic enterotoxin complex. Appl. Environ. Microbiol. 2005, 71, 8214–8220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tausch, F.; Dietrich, R.; Schauer, K.; Janowski, R.; Niessing, D.; Märtlbauer, E.; Jessberger, N. Evidence for complex formation of the Bacillus cereus haemolysin BL components in solution. Toxins 2017, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Dussault, D.; Vu, K.D.; Lacroix, M. In vitro evaluation of antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Lereclus, D.; Arantes, O.; Chaufaux, J.; Lecadet, M. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol. Lett. 1989, 51, 211–217. [Google Scholar] [CrossRef]
- Sheppard, A.E.; Poehlein, A.; Rosenstiel, P.; Liesegang, H.; Schulenburg, H. Complete Genome Sequence of Bacillus thuringiensis Strain 407 Cry. Genome Announc. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Jessberger, N.; Krey, V.M.; Rademacher, C.; Böhm, M.E.; Mohr, A.K.; Ehling-Schulz, M.; Scherer, S.; Märtlbauer, E. From genome to toxicity: A combinatory approach highlights the complexity of enterotoxin production in Bacillus cereus. Front. Microbiol. 2015, 6, 560. [Google Scholar] [CrossRef] [Green Version]
- Wijnands, L.M.; Dufrenne, J.B.; Zwietering, M.H.; van Leusden, F.M. Spores from mesophilic Bacillus cereus strains germinate better and grow faster in simulated gastro-intestinal conditions than spores from psychrotrophic strains. Int. J. Food Microbiol. 2006, 112, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Wijnands, L.M.; Dufrenne, J.B.; van Leusden, F.M.; Abee, T. Germination of Bacillus cereus spores is induced by germinants from differentiated Caco-2 Cells, a human cell line mimicking the epithelial cells of the small intestine. Appl. Environ. Microbiol. 2007, 73, 5052–5054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornstra, L.M.; van der Voort, M.; Wijnands, L.M.; Roubos-van den Hil, P.J.; Abee, T. Role of germinant receptors in Caco-2 cell-initiated germination of Bacillus cereus ATCC 14579 endospores. Appl. Environ. Microbiol. 2009, 75, 1201–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessberger, N.; Dietrich, R.; Mohr, A.K.; da Riol, C.; Märtlbauer, E. Porcine gastric mucin triggers toxin production of enteropathogenic Bacillus cereus. Infect. Immun. 2019, 87, e00765-18. [Google Scholar] [CrossRef] [Green Version]
- Ghelardi, E.; Celandroni, F.; Salvetti, S.; Ceragioli, M.; Beecher, D.J.; Senesi, S.; Wong, A.C. Swarming behavior of and hemolysin BL secretion by Bacillus cereus. Appl. Environ. Microbiol. 2007, 73, 4089–4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzantini, D.; Celandroni, F.; Salvetti, S.; Gueye, S.A.; Lupetti, A.; Senesi, S.; Ghelardi, E. FlhF is required for swarming motility and full pathogenicity of Bacillus cereus. Front. Microbiol. 2016, 7, 1644. [Google Scholar] [CrossRef]
- Salvetti, S.; Ghelardi, E.; Celandroni, F.; Ceragioli, M.; Giannessi, F.; Senesi, S. FlhF, a signal recognition particle-like GTPase, is involved in the regulation of flagellar arrangement, motility behaviour and protein secretion in Bacillus cereus. Microbiology 2007, 153, 2541–2552. [Google Scholar] [CrossRef] [Green Version]
- Beecher, D.J.; Macmillan, J.D. Characterization of the components of hemolysin BL from Bacillus cereus. Infect. Immun. 1991, 59, 1778–1784. [Google Scholar] [CrossRef] [Green Version]
- Beecher, D.J.; Wong, A.C. Tripartite hemolysin BL from Bacillus cereus. Hemolytic analysis of component interactions and a model for its characteristic paradoxical zone phenomenon. J. Biol. Chem. 1997, 272, 233–239. [Google Scholar] [CrossRef] [Green Version]
- BVL (Federal Office of Consumer Protection and Food Safety). List of Authorised Plant Protection Products in Germany with Information on Terminated Authorisations. Available online: www.bvl.bund.de (accessed on 12 October 2020).
- Ankolekar, C.; Rahmati, T.; Labbe, R.G. Detection of toxigenic Bacillus cereus and Bacillus thuringiensis spores in U.S. rice. Int. J. Food Microbiol. 2009, 128, 460–466. [Google Scholar] [CrossRef]
- Böhm, M.E.; Huptas, C.; Krey, V.M.; Scherer, S. Massive horizontal gene transfer, strictly vertical inheritance and ancient duplications differentially shape the evolution of Bacillus cereus enterotoxin operons hbl, cytK and nhe. BMC Evol. Biol. 2015, 15, 246. [Google Scholar] [CrossRef] [Green Version]
- Ngamwongsatit, P.; Buasri, W.; Pianariyanon, P.; Pulsrikarn, C.; Ohba, M.; Assavanig, A.; Panbangred, W. Broad distribution of enterotoxin genes (hblCDA, nheABC, cytK, and entFM) among Bacillus thuringiensis and Bacillus cereus as shown by novel primers. Int. J. Food Microbiol. 2008, 121, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, K.; Rosenquist, H.; Jorgensen, K.; Wilcks, A. Occurrence of natural Bacillus thuringiensis contaminants and residues of Bacillus thuringiensis-based insecticides on fresh fruits and vegetables. Appl. Environ. Microbiol. 2006, 72, 3435–3440. [Google Scholar] [CrossRef] [Green Version]
- Frentzel, H.; Juraschek, K.; Pauly, N.; Kelner-Burgos, Y.; Wichmann-Schauer, H. Indications of biopesticidal Bacillus thuringiensis strains in bell pepper and tomato. Int. J. Food Microbiol. 2020, 321, 108542. [Google Scholar] [CrossRef]
- Zhou, G.; Yan, J.; Dasheng, Z.; Zhou, X.; Yuan, Z. The residual occurrences of Bacillus thuringiensis biopesticides in food and beverages. Int. J. Food Microbiol. 2008, 127, 68–72. [Google Scholar] [CrossRef]
- Hendriksen, N.B.; Hansen, B.M. Detection of Bacillus thuringiensis kurstaki HD1 on cabbage for human consumption. FEMS Microbiol. Lett. 2006, 257, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.B.; Larsen, P.; Jacobsen, B.L.; Madsen, B.; Wilcks, A.; Smidt, L.; Andrup, L. Isolation and characterization of Bacillus cereus-like bacteria from faecal samples from greenhouse workers who are using Bacillus thuringiensis-based insecticides. Int. Arch. Occup. Environ. Health 2002, 75, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, E.; Celandroni, F.; Salvetti, S.; Beecher, D.J.; Gominet, M.; Lereclus, D.; Wong, A.C.; Senesi, S. Requirement of flhA for swarming differentiation, flagellin export, and secretion of virulence-associated proteins in Bacillus thuringiensis. J. Bacteriol. 2002, 184, 6424–6433. [Google Scholar] [CrossRef] [Green Version]
- Bouillaut, L.; Ramarao, N.; Buisson, C.; Gilois, N.; Gohar, M.; Lereclus, D.; Nielsen-Leroux, C. FlhA influences Bacillus thuringiensis PlcR-regulated gene transcription, protein production, and virulence. Appl. Environ. Microbiol. 2005, 71, 8903–8910. [Google Scholar] [CrossRef] [Green Version]
- Gaviria Rivera, A.M.; Granum, P.E.; Priest, F.G. Common occurrence of enterotoxin genes and enterotoxicity in Bacillus thuringiensis. FEMS Microbiol. Lett. 2000, 190, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Pang, J.C.; Kao, S.S.; Tsen, H.Y. Enterotoxigenicity and cytotoxicity of Bacillus thuringiensis strains and development of a process for Cry1Ac production. J. Agric. Food Chem. 2003, 51, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, P.H. Diarrhoeal enterotoxin production by strains of Bacillus thuringiensis isolated from commercial Bacillus thuringiensis-based insecticides. FEMS Immunol. Med. Microbiol. 1995, 12, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, P.H.; Larsen, H.D.; Hansen, B.M.; Bresciani, J.; Jørgensen, K. Enterotoxin-producing strains of Bacillus thuringiensis isolated from food. Lett. Appl. Microbiol. 1996, 23, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.E.; Krey, V.M.; Jessberger, N.; Frenzel, E.; Scherer, S. Comparative bioinformatics and experimental analysis of the intergenic regulatory regions of Bacillus cereus hbl and nhe enterotoxin operons and the impact of CodY on virulence heterogeneity. Front. Microbiol. 2016, 7, 768. [Google Scholar] [CrossRef]
- Bravo, A.; Gomez, I.; Porta, H.; Garcia-Gomez, B.I.; Rodriguez-Almazan, C.; Pardo, L.; Soberon, M. Evolution of Bacillus thuringiensis Cry toxins insecticidal activity. Microb. Biotechnol. 2013, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.; Federici, B.A. In defense of Bacillus thuringiensis, the safest and most successful microbial insecticide available to humanity—A response to EFSA. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unlu, M.; Ergene, E.; Unlu, G.V.; Zeytinoglu, H.S.; Vural, N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem. Toxicol. 2010, 48, 3274–3280. [Google Scholar] [CrossRef]
- Valero, M.; Giner, M.J. Effects of antimicrobial components of essential oils on growth of Bacillus cereus INRA L2104 in and the sensory qualities of carrot broth. Int. J. Food Microbiol. 2006, 106, 90–94. [Google Scholar] [CrossRef]
- Valero, M.; Salmeron, M.C. Antibacterial activity of 11 essential oils against Bacillus cereus in tyndallized carrot broth. Int. J. Food Microbiol. 2003, 85, 73–81. [Google Scholar] [CrossRef]
- Ghosh, I.N.; Patil, S.D.; Sharma, T.K.; Srivastava, S.K.; Pathania, R.; Navani, N.K. Synergistic action of cinnamaldehyde with silver nanoparticles against spore-forming bacteria: A case for judicious use of silver nanoparticles for antibacterial applications. Int. J. Nanomedicine 2013, 8, 4721–4731. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.A.; Yu, C.B.; Park, H.D. Bacteriocidal effects and inhibition of cell separation of cinnamic aldehyde on Bacillus cereus. Lett. Appl. Microbiol. 2003, 37, 61–65. [Google Scholar] [CrossRef]
- Friedman, M.; Buick, R.; Elliott, C.T. Antibacterial activities of naturally occurring compounds against antibiotic-resistant Bacillus cereus vegetative cells and spores, Escherichia coli, and Staphylococcus aureus. J. Food Prot. 2004, 67, 1774–1778. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.J.; Haroutounian, S.A. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef]
- Oke, F.; Aslim, B.; Ozturk, S.; Altundag, S. Essential oil composition, antimicrobial and antioxidant anctivities of Satureja cuneifolia Ten. Food Chem. 2009, 112, 874–879. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef]
- Singh, G.; Marimuthu, P.; Murali, H.S.; Bawa, A.S. Antioxidative and antibacterial potentials of essential oils and extracts isolated from various spice materials. J. Food Saf. 2005, 25, 130–145. [Google Scholar] [CrossRef]
- Singh, S.; Das, S.S.; Singh, G.; Schuff, C.; de Lampasona, M.P.; Catalan, C.A. Composition, in vitro antioxidant and antimicrobial activities of essential oil and oleoresins obtained from black cumin seeds (Nigella sativa L.). Biomed. Res. Int. 2014, 2014, 918209. [Google Scholar] [CrossRef] [Green Version]
- El Kolli, M.; Laouer, H.; El Kolli, H.; Akkal, S.; Sahli, F. Chemical analysis, antimicrobial and anti-oxidative properties of Daucus gracilis essential oil and its mechanism of action. Asian Pac. J. Trop. Biomed. 2016, 6, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Ultee, A.; Bennik, M.H.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Ultee, A.; Gorris, L.G.; Smid, E.J. Bactericidal activity of carvacrol towards the food-borne pathogen Bacillus cereus. J. Appl. Microbiol. 1998, 85, 211–218. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.; Alberda, M.; Hoekstra, F.A.; Smid, E.J. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 2000, 174, 233–238. [Google Scholar] [CrossRef]
- Ultee, A.; Smid, E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Antimicrobial activity of the essential oils of Thymus vulgaris L. measured using a bioimpedometric method. J. Food Prot. 1999, 62, 1017–1023. [Google Scholar] [CrossRef]
- Moghaddam, M.; Mehdizadeh, L.; Mirzaei Najafgholi, H.; Ghasemi Pirbalouti, A. Chemical composition, antibacterial and antifungal activities of seed essential oil of Ferulago angulata. Int. J. Food Prop. 2018, 21, 158–170. [Google Scholar] [CrossRef] [Green Version]



| Internal Number (MHI) | Other Designation | Source | Origin | panC Type | cry | nhe | hbl | ces | cytK1 | cytK2 | NheB * | Toxicity ** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 271 | B. thuringiensis Berliner 1915, DSM 6029 | Animal tissue | A | IV | 1 | + | + | − | − | + | High | High |
| 2873 | B. thuringiensis Berliner 1915, ATCC® 10792™, DSM 2046 | Mediterranean flour moth | A | IV | 1 | + | + | − | − | + | High | High |
| 2882 | B. thuringiensis ssp. israelensis, BGSC 4Q2 (HD500) | Culicidae larva | A | IV | 4 | + | + | − | − | + | High | Medium |
| 3370 | B. thuringiensis Bt 407 | Ephestia kuehniella larva; Cry- | A | IV | − | + | + | − | − | + | High | High |
| 2880 | B. thuringiensis WSBC 28025 | Vegetables for infant food | F | IV | 1, 2 | + | + | − | − | + | Medium | High |
| 3163 | B. thuringiensis | Ready-to-eat salad 1 | F | IV | 1, 2 | + | + | − | − | + | High | High |
| 3164 | B. thuringiensis | Ready-to-eat salad 2 | F | IV | 1, 2 | + | + | − | − | + | Medium | High |
| 3189 | B. thuringiensis | Ready-to-eat salad 3 | F | IV | 1, 2 | + | + | − | − | + | Low | Medium |
| 3190 | B. thuringensis | Ready-to-eat salad 4 | F | IV | 1, 2 | + | + | − | − | + | Low | Medium |
| 3191 | B. thuringiensis | Ready-to-eat salad 5 | F | IV | 1 | + | + | − | − | + | Low | High |
| 2878 | B. thuringiensis ssp. tenebrionis | Undefined biopesticide | P | IV | 3 | + | + | − | − | − | Low | Medium |
| 3186 | B. thuringiensis ssp. aizawai | Biopesticide granulate 1 | P | IV | 1, 2 | + | + | − | − | + | Low | High |
| 3187 | B. thuringiensis ssp. aizawai | Biopesticide granulate 1 | P | IV | 1, 2 | + | + | − | − | + | Low | Medium |
| 3188 | B. thuringensis | Isolate from Biopesticide 1 | P | IV | 1, 2 | + | + | − | − | + | Low | High |
| 3240 | B. thuringensis ssp. kurstaki | Biopesticide 2 | P | IV | 1, 2 | + | + | − | − | + | Medium | High |
| 3241 | B. thuringiensis ssp. tenebrionis | Biopesticide 3, solids | P | IV | 3 | + | + | − | − | − | Medium | Low |
| 3369 | B. thuringiensis ssp. aizawai | Biopesticide 4 | P | IV | 1, 2 | + | + | − | − | + | Low | High |
| 2874 | B. thuringiensis ssp. kurstaki, PO1 | Soil | S | IV | 1 | + | + | − | − | + | High | Medium |
| 2875 | B. thuringiensis ssp. kurstaki, PO6 | Soil | S | IV | 1 | + | + | − | − | + | High | High |
| 2876 | B. thuringiensis ssp. kurstaki, PO10 | Soil | S | IV | 1 | + | + | − | − | + | Medium | High |
| 2877 | B. thuringiensis ssp. kurstaki, PO14 | Soil | S | IV | 1 | + | + | − | − | + | High | High |
| 2870 | B. thuringiensis ssp. entomocidus bv. subtoxicus, IEBC-T06A001 | Canada | U | IV | 1, 2 | + | + | − | − | + | Medium | Low |
| 2871 | B. thuringiensis | Undefined | U | IV | 1, 2 | + | + | − | − | + | Medium | Medium |
| 2872 | B. thuringiensis HER 1404 | Undefined | U | IV | 1, 2 | + | + | − | − | + | Medium | Medium |
| Internal Number (MHI) | Origin | Germination (OD620 in %) after 15 min | Germination Pattern | |||
|---|---|---|---|---|---|---|
| CGY | CGY 80 °C | cRPMI | cRPMI 80 °C | |||
| 2870 | U | 94.2 ± 0.6 | 96.4 ± 0.5 | 97.8 ± 1.3 | 98.1 ± 0.6 | A |
| 2873 | A | 96.6 ± 0.8 | 96.8 ± 0.1 | 98.5 ± 1.3 | 94.2 ± 1.9 | A |
| 2880 | F | 93.5 ± 2.8 | 71.4 ± 1.4 | 98.1 ± 1.5 | 98.9 ± 1.4 | B |
| 2882 | A | 85.2 ± 4.1 | 78.0 ± 1.0 | 97.2 ± 1.6 | 96.4 ± 2.8 | B |
| 3163 | F | 94.8 ± 0.6 | 86.5 ± 1.2 | 98.8 ± 0.7 | 97.6 ± 1.3 | B |
| 3164 | F | 94.1 ± 0.3 | 86.9 ± 2.5 | 98.6 ± 1.7 | 96.6 ± 1.1 | B |
| 3186 | P | 98.5 ± 0.3 | 88.5 ± 2.5 | 99.8 ± 0.3 | 100.1 ± 0.6 | B |
| 3187 | P | 98.9 ± 0.9 | 90.4 ± 2.7 | 99.4 ± 0.7 | 99.7 ± 0.5 | B |
| 3188 | P | 96.0 ± 0.4 | 78.8 ± 0.3 | 98.4 ± 0.5 | 100.5 ± 1.1 | B |
| 3189 | F | 96.2 1± 1.6 | 88.2 ± 2.3 | 98.6 ± 0.4 | 100.6 ± 0.8 | B |
| 3190 | F | 98.4 ± 0.6 | 94.8 ± 2.0 | 99.1 ±0.9 | 99.6 ± 0.2 | B |
| 3191 | F | 93.1 ± 0.4 | 77.2 ± 0.7 | 99.3 ± 0.8 | 99.2 ± 0.1 | B |
| 2871 | U | 90.6 ± 3.2 | 82.4 ± 2.1 | 89.7 ± 4.4 | 91.3 ± 3.0 | C |
| 2872 | U | 81.5 ± 1.2 | 72.6 ± 2.9 | 96.5 ± 0.2 | 93.4 ± 0.4 | C |
| 3240 | P | 98.1 ± 1.9 | 79.2 ± 1.3 | 90.3 ± 5.8 | 93.0 ± 1.9 | C |
| 3241 | P | 86.5 ± 7.1 | 75.6 ± 10.9 | 119.1 ± 24.7 | 85.2 ± 11.1 | C |
| 3369 | P | 87.3 ± 1.6 | 75.2 ± 2.7 | 96.9 ± 1.9 | 95.0 ± 1.6 | C |
| 2874 | S | 82.9 ± 31.5 | 62.0 ± 4.1 | 74.4 ± 1.5 | 81.7 ± 1.5 | D |
| 2875 | S | 78.8 ± 6.2 | 74.8 ± 1.9 | 83.9 ± 2.0 | 82.7 ± 1.8 | D |
| 2876 | S | 87.1 ± 1.9 | 89.9 ± 1.0 | 90.1 ± 4.9 | 89.1 ± 0.4 | D |
| 2877 | S | 81.3 ± 7.8 | 75.6 ± 9.6 | 91.3 ± 2.2 | 93.5 ± 1.4 | D |
| 3370 | A | 71.9 ± 6.8 | 64.5 ± 8.2 | 78.6 ± 9.1 | 78.2 ± 9.5 | D |
| 271 | A | 95.2 ± 1.3 | 81.9 ± 3.3 | 97.7 ± 0.6 | 80.2 ± 0.1 | E |
| 2878 | P | 96.1 ± 1.8 | 88.8 ± 2.2 | 96.4 ± 2.0 | 92.3 ± 2.1 | E |
| A | WST-1 CGY | NheB CGY | |
| NheB CGY Hbl L2 CGY | R = 0.37 (ns) R = 0.237 (ns) | NheB cRPMI Hbl L2 CGY | R = 0.35 (ns) R = 0.297 (ns) |
| WST-1 cRPMI | NheB cRPMI | ||
| NheB cRPMI | R = 0.381 (ns) | Hbl L2 cRPMI | R = 0.283 (ns) |
| Hbl L2 cRPMI | R = 0.375 (ns) | ||
| WST-1 CGY | Hbl L2 CGY | ||
| WST-1 cRPMI | R = 0.237 (ns) | Hbl L2 cRPMI | R = 0.13 (ns) |
| B | MIC Citral | MIC Oregano | |
| MTC Citral | R = 0.35 (ns) | MTC Oregano | R = 0.65 (*) |
| MIC Cassia | MIC Winter s. | ||
| MIC Citral | R = 0.126 (ns) | MIC Citral | R = 0.322 (ns) |
| MIC Oregano | R = 0.446 (*) | ||
| MTC Cassia | MTC Winter s. | ||
| MTC Oregano | R = 0.285 (ns) | MTC Oregano | R = 0.16 (ns) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwenk, V.; Riegg, J.; Lacroix, M.; Märtlbauer, E.; Jessberger, N. Enteropathogenic Potential of Bacillus thuringiensis Isolates from Soil, Animals, Food and Biopesticides. Foods 2020, 9, 1484. https://doi.org/10.3390/foods9101484
Schwenk V, Riegg J, Lacroix M, Märtlbauer E, Jessberger N. Enteropathogenic Potential of Bacillus thuringiensis Isolates from Soil, Animals, Food and Biopesticides. Foods. 2020; 9(10):1484. https://doi.org/10.3390/foods9101484
Chicago/Turabian StyleSchwenk, Valerie, Janina Riegg, Monique Lacroix, Erwin Märtlbauer, and Nadja Jessberger. 2020. "Enteropathogenic Potential of Bacillus thuringiensis Isolates from Soil, Animals, Food and Biopesticides" Foods 9, no. 10: 1484. https://doi.org/10.3390/foods9101484
APA StyleSchwenk, V., Riegg, J., Lacroix, M., Märtlbauer, E., & Jessberger, N. (2020). Enteropathogenic Potential of Bacillus thuringiensis Isolates from Soil, Animals, Food and Biopesticides. Foods, 9(10), 1484. https://doi.org/10.3390/foods9101484





