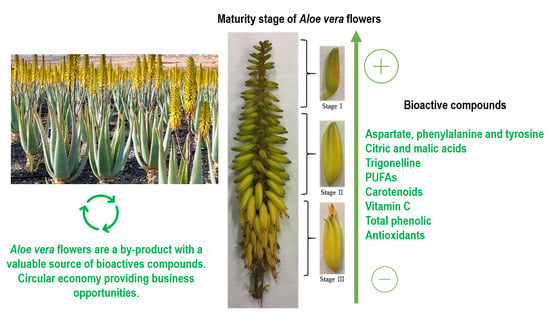
Aloe vera Flowers, a Byproduct with Great Potential and Wide Application, Depending on Maturity Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Primary Metabolites Analyses
2.3. Volatile Profile
2.4. Fatty Acid Profile
2.5. Carotenoids and RAE
2.6. Vitamin C
2.7. Total Phenolic Compounds
2.8. Total Antioxidant Capacity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Primary Metabolites
3.1.1. Amino Acids
3.1.2. Free Sugar Composition
3.1.3. Organic Acids
3.2. Volatile Compounds
3.3. Fatty Acid Profile
3.4. Carotenoids and RAE
3.5. Total Vitamin C
3.6. Total Phenolic Content
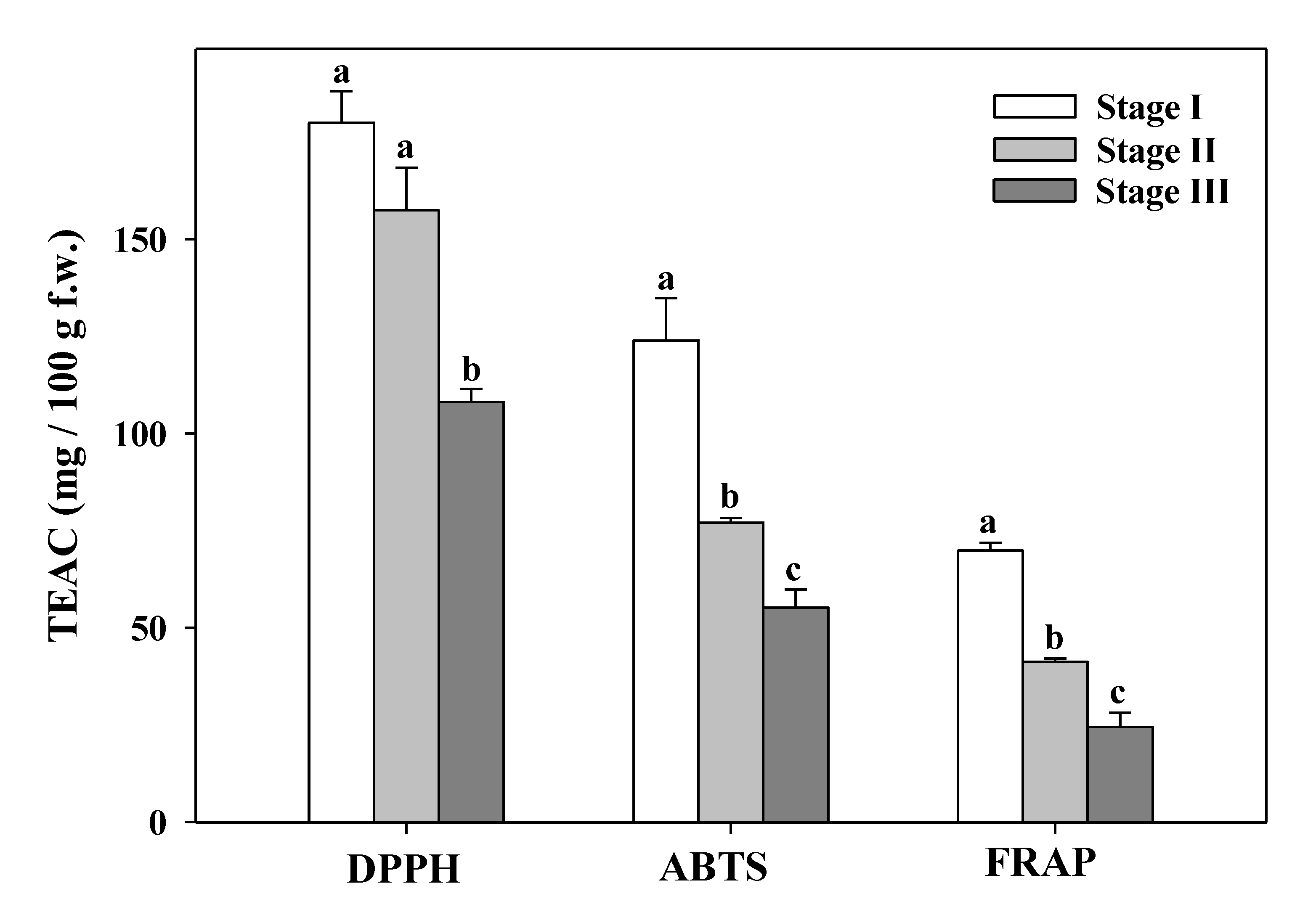
3.7. Total Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Therapeutic potential of Aloe vera—A miracle gift of nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Suga, T. Biologically active constituents of leaves and roots of Aloe arborescens var. natalensis. Z. Nat. C Biosci. 1977, 32, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Quispe, C.; Villalobos, M.; Bórquez, J.; Simirgiotis, M. Chemical Composition and Antioxidant Activity of Aloe vera from the Pica Oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J. Chem. 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bozzi, A.; Perrin, C.; Austin, S.; Arce Vera, F. Quality and authenticity of commercial Aloe vera gel powders. Food Chem. 2007, 103, 22–30. [Google Scholar] [CrossRef]
- Khajeeyana, R.; Salehia, A.; Movahhedi Dehnavia, M.; Farajee, H.; Amin Kohanmoob, M. Physiological and yield responses of Aloe vera plant to biofertilizers under different irrigation regimes. Agric. Water Manag. 2019, 225, 105768. [Google Scholar] [CrossRef]
- Sahu, P.; Giri, D.; Singh, R.; Pandey, P.; Gupta, S.; Shrivastava, A.; Kumar, A.; Pandey, K. Therapeutic and Medicinal Uses of Aloe vera: A Review. Pharmacol. Pharm. 2013, 4, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Chacón, O.; Forno, N.; Lapierre, L.; Muñoz, R.; Fresno, M.; San Martín, B. Effect of Aloe barbadensis Miller (Aloe vera) associated with beta-lactam antibiotics on the occurrence of resistance in strains of Staphylococcus aureus and Streptococcus uberis. Eur. J. Integr. Med. 2019, 32, 100996. [Google Scholar]
- Minjares-fuentes, R.; Femenia, A.; Vera, A.; Barbadensis, A. Aloe vera. Nonvitamin and Nonmineral Nutritional Supplements; Elsevier Inc.: Philadelphia, PA, USA, 2019. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.; López-Cervantes, J.; Sendón, R.; Sánchez-Silva, A. Aloe vera: Ancient knowledge with new frontiers. Trends Food Sci. Tech. 2017, 61, 94–102. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Safety of hydroxyanthracene derivatives for use in food. EFSA J. 2018, 16, 05090. [Google Scholar] [CrossRef] [Green Version]
- Debnath, T.; Ghosh, M.; Lee, Y.M.; Nath, N.C.; Lee, K.G.; Lim, B.O. Identification of phenolic constituents and antioxidant activity of Aloe barbadensis flower extracts. Food Agric. Immunol. 2017, 29, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants—A Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ćirić, A.; Silva, S.P.; Coelho, E.; Mocan, A.; Calhelha, R.C.; Sokovic, M.; Coimbra, M.A.; et al. Compositional Features and Bioactive Properties of Aloe vera Leaf (Fillet, Mucilage, and Rind) and Flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- López-Cervantes, J.; Sánchez-Machado, D.I.; Cruz-Flores, P.; Mariscal-Domínguez, M.F.; Servín de la Mora-López, G.; Campas-Baypoli, O.N. Antioxidant capacity, proximate composition, and lipid constituents of Aloe vera flowers. J. Appl. Res. Med. Aroma 2018, 10, 93–98. [Google Scholar] [CrossRef]
- Sotelo, A.; López-García, S.; Basurto-Peña, F. Content of Nutrient and Antinutrient in Edible Flowers of Wild Plants in Mexico. Plant Foods Hum. Nutr. 2007, 62, 133–138. [Google Scholar] [CrossRef] [PubMed]
- López, A.; de Tangil, M.; Vega-Orellana, O.; Ramírez, A.; Rico, M. Phenolic constituents, antioxidant and preliminary antimycoplasmic activities of leaf skin and flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules 2013, 18, 4942–4954. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, C.; Menichini, F.; Xiao, J.; Tundis, R. Edible Flowers: A Rich Source of Phytochemicals with Antioxidant and Hypoglycaemic properties. J. Agric. Food Chem. 2016, 64, 2467–2474. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Moreno, A.; López, M.Y.; Jiménez, L. Aloe vera (Sábila): Cultivo y Utilización; MundiPrensa, Ed.; Ediciones Paraninfo: Madrid, Spain, 2016; p. 127. [Google Scholar]
- Biais, B.; Allwood, J.W.; Deborde, C.; Xu, Y.; Maucourt, M.; Beauvoit, B.; Dunn, W.B.; Jacob, D.; Goodacre, R.; Rolin, D.; et al. 1H NMR, GC-EI-TOFMS, and Data Set Correlation for Fruit Metabolomics: Application to Spatial Metabolite Analysis in Melon. Anal. Chem. 2009, 81, 2884–2894. [Google Scholar] [CrossRef]
- Chun, M.H.; Kim, E.K.; Lee, K.R.; Jung, J.H.; Hong, J. Quality control of Schizonepeta tenuifolia Briq by solid phase microextraction gas chromatography/mass spectrometry and principal component analysis. Microchem. J. 2010, 95, 25–31. [Google Scholar] [CrossRef]
- Smooker, A.M.; Wells, R.; Morgan, C.; Beaudoin, F.; Cho, K.; Fraser, F.; Bancroft, I. The identification and mapping of candidate genes and QTL involved in the fatty acid desaturation pathway in Brassica napus. Theor. Appl. Genet. 2011, 122, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Santos, L.E.; Martínez-Girón, J.; Arias-Jaramillo, M.E. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017, 233, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.J.; Scott, K.J. Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK. Food Chem. 1995, 54, 101–111. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Aguayo, E. Effect of irrigation with ozonated water on the quality of capsicum seedlings grown in the nursery. Agric. Water Manag. 2019, 221, 547–555. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.; Ferreres, F. A Comparative Study of Flavonoid Compounds, Vitamin C, and Antioxidant Properties of Baby Leaf Brassicaceae Species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagments. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New Insights into the Shikimate and Aromatic Amino Acids Biosynthesis Pathways in Plants. Mol. Plant 2020, 3, 956–972. [Google Scholar] [CrossRef]
- Joshi, R.; Poonam; Gulati, A. Biochemical attributes of tea flowers (Camellia sinensis) at different developmental stages in the Kangra region of India. Sci. Hortic. 2011, 130, 266–274. [Google Scholar] [CrossRef]
- Mulu, T.; Teshale, F.; Gemeda, S.; Sahu, O. Medicated Evaluation of Aloe vera: Overview on Characteristics and Application. J. Nutr. Health 2015, 3, 1–7. [Google Scholar]
- Wang, L.; Xu, R.J.; Hu, B.; Li, W.; Sun, Y.; Tu, Y.Y.; Zeng, X.X. Analysis of free amino acids in Chinese teas and flower of tea plant by high performance liquid chromatography combined with solid-phase extraction. Food Chem. 2010, 123, 1259–1266. [Google Scholar] [CrossRef]
- Christiaens, A.; De Keyser, E.; Pauwels, E.; De Riek, J.; Gobin, B.; Van Labeke, M.-C. Suboptimal Light Conditions Influence Source-Sink Metabolism during Flowering. Front. Plant Sci. 2016, 7, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.L.; Chen, B.Y.; Feng, Y.M. Water-soluble polysaccharides isolated from skin juice, gel juice and flower of Aloe vera Miller. J. Taiwan Inst. Chem. Eng. 2011, 42, 197–203. [Google Scholar] [CrossRef]
- Javed, S. Aloe vera gel in food, health products, and cosmetics industry. In Studies in Natural Products Chemistry; Atta-Ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 261–285. [Google Scholar]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. Borage, camellia, centaurea and pansies: Nutritional, fatty acids, free sugars, vitamin E, carotenoids and organic acids characterization. Food Res. Int. 2020, 132, 109070. [Google Scholar] [CrossRef] [PubMed]
- Flores-López, M.L.; Romaní, A.; Cerqueira, M.A.; Rodríguez-García, R.; de Rodríguez, D.J.; Vicente, A.A. Compositional features and bioactive properties of whole fraction from Aloe vera processing. Ind. Crops Prod. 2016, 91, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Jiao, P.; Jia, Q.; Randel, G.; Diehl, B.; Weaver, S.; Milligan, G. Quantitative1H-NMR spectrometry method for quality control of Aloe vera products. J. AOAC Int. 2010, 93, 842–848. [Google Scholar] [PubMed]
- Hu, Y.Y.; Rawal, A.; Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22425–22429. [Google Scholar] [CrossRef] [Green Version]
- Eswaranandam, S.; Hettiarachchy, N.S.; Johnson, M.G. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella gaminara. J. Food Sci. 2004, 69, 79–84. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Lin, C.; Li, L.; Lin, C.; Zheng, Y.; Hou, J.; Li, D. The cardioprotective effects of citric acid and l-malic acid on myocardial ischemia/reperfusion injury. Evid.-Based Complementary Altern. Med. 2013, 820695. [Google Scholar] [CrossRef] [Green Version]
- Salzano, F.A.; Marino, L.; Salzano, G.; Botta, R.M.; Cascone, G.; D’Agostino Fiorenza, U.; Casolaro, V. Microbiota Composition and the Integration of Exogenous and Endogenous Signals in Reactive Nasal Inflammation. J. Immunol. Res. 2018, 2724951. [Google Scholar] [CrossRef]
- Harel-Beja, R.; Tzuri, G.; Portnoy, V.; Lotan-Pompan, M.; Lev, S.; Cohen, S.; Katzir, N. A genetic map of melon highly enriched with fruit quality QTLs and EST markers, including sugar and carotenoid metabolism genes. Theor. Appl. Genet. 2010, 121, 511–533. [Google Scholar] [CrossRef]
- Yang, C.; Li, Z.; Shi, Z.; He, K.; Tian, A.; Wu, J.; Li, Z. Regulation of cell survival by the HIP-55 signaling network. Mol. Biosyst. 2014, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Alavi, M.; Esmaeil-Jamaatc, E.; Baluchnejadmojarad, T.; Roghania, M. Trigonelline mitigates lipopolysaccharide-induced learning and memory impairment in the rat due to its anti-oxidative and anti-inflammatory effect. Int. Immunopharmacol. 2018, 61, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Nazir, L.A.; Tanveer, M.A.; Shahid, N.H.; Sharma, R.R.; Tasduq, A.S. Trigonelline, a naturally occurring alkaloidal agent protects ultraviolet-B (UV-B)-irradiation-induced apoptotic cell death in human skin fibroblasts via attenuation of oxidative stress, restoration of cellular calcium homeostasis and prevention of endoplasmic reticulum (ER) stress. J. Photochem. Photobiol. B Biol. 2020, 202, 111720. [Google Scholar] [CrossRef]
- Mathur, M.; Kamal, R. Studies on trigonelline from Moringa oleifera and its in vitro regulation by feeding precursor in cell cultures. Rev. Bras. Farmacogn. 2012, 222, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- del Campo, G.; Berregi, I.; Caracena, R.; Zuriarrain, J. Quantitative determination of caffeine, formic acid, trigonelline and 5-(hydroxymethyl) furfural in soluble coffees by 1H NMR spectrometry. Talanta 2010, 81, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.A.B.M.; Boyce, A.N.; Osman, N. Postharvest quality, vase life and photosynthetic yield (chlorophyll fluorescence) of bougainvillea flower by applying ethanol. Aust. J. Basic Appl. Sci. 2007, 1, 733–740. [Google Scholar]
- Jiménez-Aguilar, D.M.; Grusak, M.A. Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of Amaranthus leafy vegetables. J. Food Compos. Anal. 2017, 58, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Ho, C.; Schwab, W.; Song, C.; Wan, X. Aroma compositions of large-leaf yellow tea and potential effect of theanine on volatile formation in tea. Food Chem. 2019, 280, 73–82. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Park, J.G.; Lee, J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Mekni, M.; Flamini, G.; Garrab, M.; Hmida, R.B.; Cheraief, I.; Mastouri, M.; Hammami, M. Aroma volatile components, fatty acids and antibacterial activity of four Tunisian Punica granatum L. flower cultivars. Ind. Crop. Prod. 2013, 48, 111–117. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Oliveira Rodrigues, L.L.; De Oliveira, A.C.L.; Tabrez, S.; Shakil, S.; Khan, M.I.; Asghar, M.N.; Matias, B.D.; Alves da Silva Batista, J.M.; Modesto Rosal, M.; Fulgencio de Lima, M.M.D.; et al. Mutagenic, antioxidant and wound healing properties of Aloe vera. J. Ethnopharmacol. 2018, 227, 191–197. [Google Scholar] [CrossRef]
- Egert, S.; Baxheinrich, A.; Lee-Barkey, Y.H.; Tschoepe, D.; Stehle, P.; Stratmann, B.; Wahrburg, U. Effects of a hypoenergetic diet rich in α-linolenic acid on fatty acid composition of serum phospholipids in overweight and obese patients with metabolic syndrome. Nutrition 2018, 49, 74–80. [Google Scholar] [CrossRef]
- Prasanna, G.; Saraswathi, N.T. Linolenic acid prevents early and advanced glycation end-products (AGEs) modification of albumin. Int. J. Biol. Macromol. 2017, 95, 121–125. [Google Scholar] [CrossRef]
- Jana, T.; Zuzana, H.; Anna, S.; Barbora, K.; Irina, G.; Ivetac, W.; Katarína, S.; Iveta, G.; Jána, S.; Zdeňka, D. Omega-3 fatty-acids modulate symptoms of depressive disorder, serum levels of omega-3 fatty acids and omega-6/omega-3 ratio in children. A randomized, double-blind and controlled trial. Psychiatry Res. 2020, 287, 112911. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Jang, H.; Park, K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 765–773. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; Núñez-Gastélum, J.A.; Reyes-Moreno, C.; Ramírez-Wong, B.; López-Cervantes, J. Nutritional Quality of Edible Parts of Moringa oleifera. Food Anal. Methods 2010, 3, 175–180. [Google Scholar] [CrossRef]
- Lee, H.H.; Ahn, J.H.; Kwon, A.R.; Lee, E.S.; Kwak, J.H.; Min, Y.H. Chemical Composition and Antimicrobial Activity of the Essential Oil of Apricot Seed. Phytother. Res. 2014, 28, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Lorenzo, J.M.; Afolayan, A.J.; Muchenje, V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products. Food Res. Int. 2018, 106, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; He, Z.; Zhao, Y.; Yang, J.; Mao, Y. Antioxidant properties and involved compounds of daylily flowers in relation to maturity. Food Chem. 2009, 114, 1192–1197. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Review: Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef] [Green Version]
- Walid, R.; Ha, M.; Haci, E.; Abdelhamid, I.; Reda, B.; Rachid, A. Beneficial effects of Aloe vera gel on lipid profile, lipase activities and oxidant/antioxidant status in obese rats. J. Funct. Foods 2018, 48, 525–532. [Google Scholar] [CrossRef]
- Kumar, S.R.; Vallikannan, B. Carotenoid composition and retinol equivalent in plants of nutritional and medicinal importance: Efficacy of β-carotene from Chenopodium album in retinol-deficient rats. Food Chem. 2010, 119, 1584–1590. [Google Scholar] [CrossRef]
- Wertz, K.; Hunziker, P.B.; Seifert, N.; Riss, G.; Neeb, M.; Steiner, G.; Hunziker, W.; Goralczyk, R. Beta-Carotene interferes with ultraviolet light A-induced gene expression by multiple pathways. J. Investig. Dermatol. 2005, 124, 428–434. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. In WHO Global Database on Vitamin A Deficiency; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Bartoli, C.G.; Simontacchi, M.; Montaldi, E.R.; Puntarulo, S. Oxidants and antioxidants during aging of chrysanthemum petals. Plant Sci. 1997, 129, 157–165. [Google Scholar] [CrossRef]
- De Ancos, B.; Rodrigo, M.J.; Sánchez-Moreno, C.; Cano, M.P.; Zacarías, L. Effect of high-pressure processing applied as pretreatment on carotenoids, flavonoids and vitamin C in juice of the sweet oranges ‘Navel’ and the red-fleshed ‘Cara Cara’. Food Res. Int. 2020, 132, 109105. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [Green Version]
- Cardarelli, M.; Rouphael, Y.; Pellizzoni, M.; Colla, G.; Lucini, L. Profile of bioactive secondary metabolites and antioxidant capacity of leaf exudates from eighteen Aloe species. Ind. Crops Prod. 2017, 108, 44–51. [Google Scholar] [CrossRef]
- Lucini, L.; Pellizzoni, M.; Pellegrino, R.; Molinari, G.P.; Colla, G. Phytochemical constituents and in vitro radical scavenging activity of different Aloe species. Food Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef]
- Fan, J.J.; Li, C.H.; Hu, Y.J.; Chen, H.; Yang, F.Q. Comparative assessment of in vitro thrombolytic and fibrinolysis activity of four aloe species and analysis of their phenolic compounds by LC–MS. S. Afr. J. Bot. 2018, 119, 325–334. [Google Scholar] [CrossRef]
- Corpus-González, V.; Pérez-Reyes, M.A.; Sáenz-Esqueda, M.; Candelas-Codillo, M.G.; Martínez-García, J. Capacidad antioxidante, rendimiento y adsorción de grasa en el gel de Aloe vera (Aloe ferox) secado por aspersión a diferentes temperaturas. Rev. Mex. Ing. Química 2016, 1, 795–800. Available online: http://www.fcb.uanl.mx/IDCyTA/files/volume1/2/9/138.pdf (accessed on 15 January 2020).
- Vega-Gálvez, A.; Miranda, M.; Aranda, M.; Henriquez, K.; Vergara, J.; Tabilo-Munizaga, G.; Pérez-Won, M. Effect of high hydrostatic pressure on functional properties and quality characteristics of Aloe vera gel (Aloe barbadensis Miller). Food Chem. 2011, 129, 1060–1065. [Google Scholar] [CrossRef]
- Jin, L.; Li, X.; Tian, D.; Fang, X.; Yu, Y.; Zhu, H. Antioxidant properties and color parameters of herbal teas in China. Ind. Crops Prod. 2016, 87, 198–209. [Google Scholar] [CrossRef]
- Baruah, A.; Bordoloi, M.; Deka Baruah, H.P. Aloe vera: A multipurpose industrial crop. Ind. Crops Prod. 2016, 94, 951–963. [Google Scholar] [CrossRef]
- Grajzer, M.; Prescha, A.; Korzonek, K.; Wojakowsk, A.; Dziadas, M.; Kulma, A.; Grajeta, H. Characteristics of rose hip (Rosa canina L.) cold-pressed oil and its oxidative stability studied by the differential scanning calorimetry method. Food Chem. 2015, 188, 459–466. [Google Scholar] [CrossRef]



 DHAA- dehydroascorbic acid and
DHAA- dehydroascorbic acid and  AA-ascorbic acid) at different maturity stages of Aloe vera flowers. Stage I: immature; stage II: mature; stage III: mature, with flowers buds opened (mean ± SD, n = 3). a-b Different letters show significant differences in ascorbic acid (p < 0.05) among the maturity stages.
AA-ascorbic acid) at different maturity stages of Aloe vera flowers. Stage I: immature; stage II: mature; stage III: mature, with flowers buds opened (mean ± SD, n = 3). a-b Different letters show significant differences in ascorbic acid (p < 0.05) among the maturity stages.
 DHAA- dehydroascorbic acid and
DHAA- dehydroascorbic acid and  AA-ascorbic acid) at different maturity stages of Aloe vera flowers. Stage I: immature; stage II: mature; stage III: mature, with flowers buds opened (mean ± SD, n = 3). a-b Different letters show significant differences in ascorbic acid (p < 0.05) among the maturity stages.
AA-ascorbic acid) at different maturity stages of Aloe vera flowers. Stage I: immature; stage II: mature; stage III: mature, with flowers buds opened (mean ± SD, n = 3). a-b Different letters show significant differences in ascorbic acid (p < 0.05) among the maturity stages.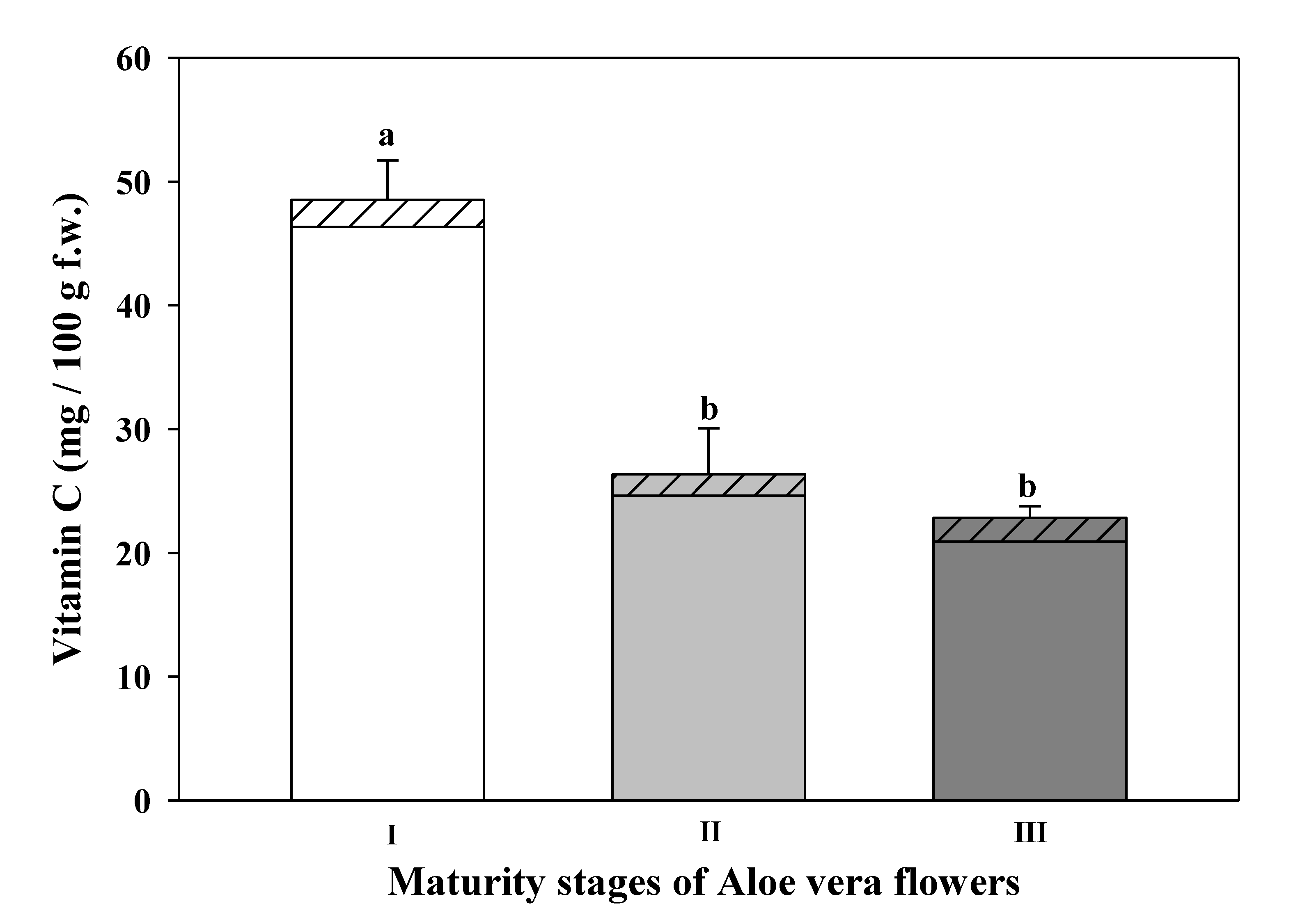

| Stage I | Stage II | Stage III | |
|---|---|---|---|
| Amino acids | |||
| Glutamine | 0.613 ± 0.027 a | 0.748 ± 0.076 a | 0.726 ± 0.186 a |
| Aspartate | 0.273 ± 0.029 a | 0.193 ± 0.027 b | 0.252 ± 0.042 ab |
| Alanine | 0.169 ± 0.027 a | 0.206 ± 0.030 a | 0.201 ± 0.013 a |
| Phenylalanine | 0.263 ± 0.020 a | 0.135 ± 0.029 b | 0.111 ± 0.011 b |
| GABA | 0.168 ± 0.032 a | 0.119 ± 0.018 a | 0.122 ± 0.022 a |
| Threonine | 0.119 ± 0.023 a | 0.091 ± 0.017 a | 0.109 ± 0.036 a |
| Tyrosine | 0.185 ± 0.013 a | 0.151 ± 0.005 b | 0.138 ± 0.025 b |
| Valine | 0.096 ± 0.021 a | 0.074 ± 0.018 a | 0.087 ± 0.007 a |
| Isoleucine | 0.064 ± 0.022 a | 0.047 ± 0.014 a | 0.064 ± 0.003 a |
| Free sugars Composition | |||
| Fructose | 8.14 ± 0.25 c | 11.81 ± 0.16 b | 28.57 ± 0.88 a |
| Glucose | 7.57 ± 0.53 c | 17.34 ± 0.74 b | 42.78 ± 7.24 a |
| Sucrose | 11.07 ± 0.92 a | 9.78 ± 0.19 a | 3.12 ± 0.83 b |
| Trehalose | 0.10 ± 0.00 c | 0.14 ± 0.03 b | 0.34 ± 0.02 a |
| Organic acids | |||
| Citric Acid | 4.315 ± 0.216 a | 2.919 ± 0.223 b | 1.552 ± 0.097 c |
| Malic acid | 2.134 ± 0.163 ab | 2.404 ± 0.183 a | 2.084 ± 0.087 b |
| Acetic acid | 0.023 ± 0.002 b | 0.026 ± 0.001 b | 0.044 ± 0.013 a |
| Formic acid | 0.009 ± 0.000 c | 0.010 ± 0.000 b | 0.015 ± 0.001 a |
| Fumaric acid | 0.014 ± 0.001 a | 0.013 ± 0.001 a | 0.012 ± 0.001 a |
| Nucleoside derivatives | |||
| AMP | 0.140 ± 0.016 a | 0.131 ± 0.003 ab | 0.116 ± 0.009 b |
| Adenosine | 0.105 ± 0.014 a | 0.096 ± 0.005 a | 0.087 ± 0.005 a |
| Other metabolites | |||
| Choline | 0.492 ± 0.024 a | 0.458 ± 0.020 ab | 0.426 ± 0.028 b |
| Trigonelline | 0.519 ± 0.014 a | 0.328 ± 0.004 b | 0.180 ± 0.014 c |
| Ethanol | 0.025 ± 0.004 a | 0.019 ± 0.003 b | 0.013 ± 0.001 c |
| Compound | CAS Number | RT (min) | Stage I (% Relative) | Stage II (% Relative) | Stage III (% Relative) |
|---|---|---|---|---|---|
| VOCs | |||||
| 1-Pentanal | 110-62-3 | 1.650 | 0.66 | 0.55 | 0.66 |
| Butanoic acid | 107-92-6 | 2.251 | 1.67 | 1.34 | 1.85 |
| 1-Hexanal | 66-25-1 | 2.488 | 1.82 | 1.75 | 2.08 |
| 2-Hexenaldehyde | 6728-26-3 | 3.185 | 6.79 | 4.64 | 5.37 |
| Benzene ethenyl | 629-20-9 | 3.786 | 2.61 | 1.89 | 2.02 |
| 1-heptanal | 111-71-7 | 3.980 | 1.35 | 1.28 | - |
| Benzaldehyde | 100-52-7 | 5.198 | 4.94 | 9.27 | 5.00 |
| Hexanoic acid ethyl ester | 123-66-0 | 6.090 | 0.75 | - | - |
| Benzyl alcohol | 100-51-6 | 6.860 | 23.88 | 16.99 | 27.52 |
| Benzeneacetaldehyde | 122-78-1 | 7.109 | 1.81 | 1.48 | 1.67 * |
| Acetophenone | 98-86-2 | 7.621 | 1.35 | 0.62 * | - |
| Phenol, 3-methyl | 108-39-4 | 7.807 | 0.71 | 0.69 | 0.65 |
| Formic acid phenylmethyl ester | 104-57-4 | 7.854 | 0.93 | 0.77 * | 1.21 |
| 1-Nonanal | 124-19-6 | 8.370 | 5.96 | 5.66 | 5.98 |
| Acetic acid benzyl ester | 140-11-4 | 9.731 | 0.75 * | - | 0.75 |
| Dodecane | 112-40-3 | 10.458 | 0.75 * | 0.57 | 0.53 * |
| Tetradecane | 629-59-4 | 15.237 | 1.67 | 2.93 | 1.60 |
| Fatty acids and fatty acid esters | |||||
| Caproic acid | 142-62-1 | 5.676 | 8.12 | 6.35 | 7.32 |
| Octanoic acid methyl ester | 111-11-5 | 8.831 | 0.83 | - | 0.55 |
| Caprylic acid | 124-07-2 | 9.900 | 4.39 | 3.04 | 4.40 |
| Decanoic acid methyl ester | 110-42-9 | 13.44 | 0.64 * | 0.90 | 0.72 * |
| Capric acid | 334-48-5 | 14.442 | 1.34 | 1.00 | 1.23 |
| Dodecanoic acid methyl ester | 111-82-0 | 18.269 | 3.75 | 4.95 | 3.63 |
| Lauric acid | 143-07-7 | 19.186 | 5.98 | 8.06 | 7.71 |
| Tetradecanoic acid methyl ester | 124-10-7 | 22.899 | - | 0.55 | - |
| Myristic acid | 544-63-8 | 23.652 | - | 0.70 | - |
| Maturity Stage | Caprylic acid (C8:0) | Capric acid (C10:0) | Lauric acid (C12:0) | Myristic acid (C14:0) | Palmitic acid (C16:0) | Stearic acid (C18:0) | Arachidic acid (C20:0) | Lignoceric acid (C24:0) |
|---|---|---|---|---|---|---|---|---|
| I | 59.5 ± 9.5 a | 51.9 ± 4.1 a | 26.0 ± 7.0 a | 1.5 ± 0.3 ab | 436.2 ± 7.3 a | 105.2 ± 5.4 b | 27.2 ± 7.1 a | 11.9 ± 1.4 b |
| II | 62.9 ± 12.3 a | 60.9 ± 3.9 a | 28.4 ± 5.5 a | 2.1 ± 0.2 a | 414.9 ± 98.1 a | 167.8 ± 22.9 a | 44.4 ± 5.4 a | 27.4 ± 8.9 a |
| III | 53.8 ± 4.7 a | 51.4 ± 8.4 a | 16.6 ± 3.8 a | 0.7 ± 0.2 b | 189.0 ± 30.6 b | 110.0 ± 5.7 b | 38.8 ± 5.7 a | - |
| Maturity Stage | Elaidic acid (C18:1n9) | Oleic acid (C18:1n9) | Linoleic acid (C18:2n6) | α-Linolenic acid (C18:3n3) | 11-Eicosenoic acid (C20:1n9) |
|---|---|---|---|---|---|
| I | 176.4 ± 35.4 a | 85.1 ± 5.4 a | 826.4 ± 22.8 a | 609.3 ± 8.8 a | 16.4 ± 3.2 b |
| II | 165.1 ± 26.6 a | 94.7 ± 12.0 a | 802.5 ± 108.3 a | 785.8 ± 139.0 a | 62.0 ± 3.0 a |
| III | 73.4 ± 20.9 b | 6.5 ± 5.0 b | 325.1 ± 46.5 b | 270.6 ± 55.6 b | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sánchez, A.; López-Cañavate, M.E.; Guirao-Martínez, J.; Roca, M.J.; Aguayo, E. Aloe vera Flowers, a Byproduct with Great Potential and Wide Application, Depending on Maturity Stage. Foods 2020, 9, 1542. https://doi.org/10.3390/foods9111542
Martínez-Sánchez A, López-Cañavate ME, Guirao-Martínez J, Roca MJ, Aguayo E. Aloe vera Flowers, a Byproduct with Great Potential and Wide Application, Depending on Maturity Stage. Foods. 2020; 9(11):1542. https://doi.org/10.3390/foods9111542
Chicago/Turabian StyleMartínez-Sánchez, Ascensión, María Elena López-Cañavate, Josefa Guirao-Martínez, María José Roca, and Encarna Aguayo. 2020. "Aloe vera Flowers, a Byproduct with Great Potential and Wide Application, Depending on Maturity Stage" Foods 9, no. 11: 1542. https://doi.org/10.3390/foods9111542