Co-Ingestion of Black Carrot and Strawberry. Effects on Anthocyanin Stability, Bioaccessibility and Uptake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Set-Up
2.3. Anthocyanin Chemical Extraction from Undigested Samples
2.4. Identification and Quantification of Anthocyanins
2.5. In Vitro Digestion Procedure
2.5.1. Gastric and Intestinal Stability Calculation
2.5.2. Bioaccessibility Calculation
2.6. Maintenance of Cell Culture
2.7. Toxicity Test
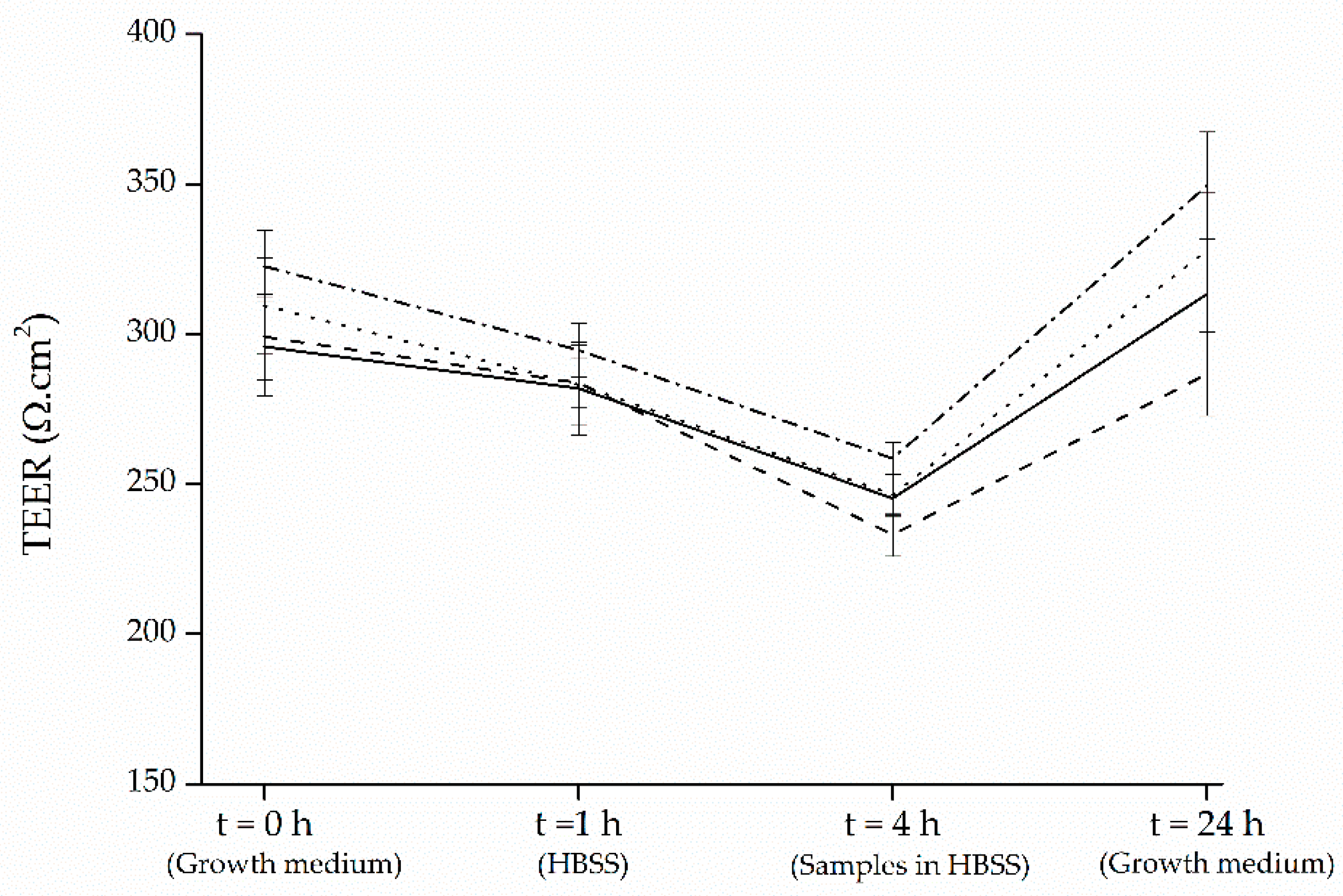
2.8. Transport Experiments
2.9. Statistical Analysis
3. Results and Discussion
3.1. Identification and Quantification of Black Carrot and Strawberry Anthocyanins
3.2. Stability of Black Carrot and Strawberry Anthocyanins, Effect of Co-Digestion
3.3. In Vitro Bioaccessibility of Black Carrot and Strawberry Anthocyanins and Effect of Co-Digestion
3.4. Transport of Black Carrot and Strawberry Anthocyanins through Intestinal Caco-2 Cells. Effect of Co-Administration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Jordheim, M. The anthocyanins. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 471–551. [Google Scholar]
- de Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The Colors of Health: Chemistry, 560 Bioactivity, and Market Demand for Colorful Foods and Natural Food Sources of Colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.; Riso, P. Factors influencing the bioavailability of antioxidants in foods: A critical appraisal. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 647–650. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef] [Green Version]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef] [Green Version]
- Zoubiri, L.; Bakir, S.; Barkat, M.; Carrillo, C.; Capanoglu, E. Changes in the phenolic profile, antioxidant capacity and in vitro bioaccessibility of two Algerian grape varieties, Cardinal and Dabouki (Sabel), during the production of traditional sun-dried raisins and homemade jam. J. Berry Res. 2019, 9, 563–574. [Google Scholar] [CrossRef]
- Kamiloglu, S. Effect of different freezing methods on the bioaccessibility of strawberry polyphenols. Int. J. Food Sci. Technol. 2019, 54, 2652–2660. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Ozkan, G.; Isik, H.; Horoz, O.; Van Camp, J.; Capanoglu, E. Black carrot pomace as a source of polyphenols for enhancing the nutritional value of cake: An in vitro digestion study with a standardized static model. LWT 2017, 77, 475–481. [Google Scholar] [CrossRef]
- Tomas, M.; Beekwilder, J.; Hall, R.D.; Sagdic, O.; Boyacioglu, D.; Capanoglu, E. Industrial processing versus home processing of tomato sauce: Effects on phenolics, flavonoids and in vitro bioaccessibility of antioxidants. Food Chem. 2017, 220, 51–58. [Google Scholar] [CrossRef]
- Carrillo, C.; Buvé, C.; Panozzo, A.; Grauwet, T.; Hendrickx, M. Role of structural barriers in the in vitro bioaccessibility of anthocyanins in comparison with carotenoids. Food Chem. 2017, 227, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Gui-Fang, D.; Lei, N.; Wen, A.; Yuan-Huan, W.; Rui-Fang, S.; Xiu-Juan, D.; Hua-Bin, L. Effects of simulated gastrointestinal digestion on antioxidant activities of individual and mixed fruits. N. Am. J. Med. Sci. 2019, 12, 14–20. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Bucknall, M.P.; Arcot, J. Co-ingestion of red cabbage with cherry tomato enhances digestive bioaccessibility of anthocyanins but decreases carotenoid bioaccessibility after simulated in vitro gastro-intestinal digestion. Food Chem. 2019, 298, 125040. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Frei, B.; Wrolstad, R.E. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J. Agric. Food Chem. 2002, 50, 6172–6181. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Pasli, A.; Ozcelik, B.; Van Camp, J.; Capanoglu, E. Colour retention, anthocyanin stability and antioxidant capacity in black carrot (Daucus carota) jams and marmalades: Effect of processing, storage conditions and in vitro gastrointestinal digestion. J. Funct. Foods 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Grootaert, C.; Capanoglu, E.; Ozkan, C.; Smagghe, G.; Raes, K.; Van Camp, J. Anti-inflammatory potential of black carrot (Daucus carota L.) polyphenols in a co-culture model of intestinal Caco-2 and endothelial EA.hy926 cells. Mol. Nutr. Food Res. 2017, 61, 1600455. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Buvé, C.; Kebede, B.T.; De Batselier, C.; Carrillo, C.; Pham, H.T.T.; Hendrickx, M.; Grauwet, T.; Van Loey, A. Kinetics of colour changes in pasteurised strawberry juice during storage. J. Food Eng. 2018, 216, 42–51. [Google Scholar] [CrossRef]
- Montilla, E.C.; Arzaba, M.R.; Hillebrand, S.; Winterhalter, P. Anthocyanin composition of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) cultivars Antonina, Beta Sweet, Deep Purple, and Purple Haze. J. Agric. Food Chem. 2011, 59, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Algarra, M.; Fernandes, A.; Mateus, N.; de Freitas, V.; Esteves da Silva, J.C.G.; Casado, J. Anthocyanin profile and antioxidant capacity of black carrots (Daucus carota L. ssp. sativus var. atrorubens Alef.) from Cuevas Bajas, Spain. J. Food Compos. Anal. 2014, 33, 71–76. [Google Scholar] [CrossRef]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef]
- Dzhanfezova, T.; Barba-Espín, G.; Müller, R.; Joernsgaard, B.; Hegelund, J.N.; Madsen, B.; Larsen, D.H.; Martínez Vega, M.; Toldam-Andersen, T.B. Anthocyanin profile, antioxidant activity and total phenolic content of a strawberry (Fragaria × ananassa Duch) genetic resource collection. Food Biosci. 2020, 36, 100620. [Google Scholar] [CrossRef]
- Marhuenda, J.; Alemán, M.D.; Gironés-Vilaplana, A.; Pérez, A.; Caravaca, G.; Figueroa, F.; Mulero, J.; Zafrilla, P. Phenolic composition, antioxidant activity, and in vitro availability of four different berries. J. Chem. 2016, 14, 5194901. [Google Scholar] [CrossRef] [Green Version]
- Kosińska-Cagnazzo, A.; Diering, S.; Prim, D.; Andlauer, W. Identification of bioaccessible and uptaken phenolic compounds from strawberry fruits in in vitro digestion/Caco-2 absorption model. Food Chem. 2015, 170, 288–294. [Google Scholar] [CrossRef]
- Josuttis, M.; Carlen, C.; Crespo, P.; Nestby, R.; Toldam-Andersen, T.B.; Dietrich, H.; Krüger, E. A comparison of bioactive compounds of strawberry fruit from Europe affected by genotype and latitude. J. Berry Res. 2012, 2, 73–95. [Google Scholar] [CrossRef] [Green Version]
- Stintzing, F.C.; Trichterborn, J.; Carle, R. Characterisation of anthocyanin–betalain mixtures for food colouring by chromatic and HPLC-DAD-MS analyses. Food Chem. 2006, 94, 296–309. [Google Scholar] [CrossRef]
- Kammerer, D.; Schillmöller, S.; Maier, O.; Schieber, A.; Carle, R. Colour stability of canned strawberries using black carrot and elderberry juice concentrates as natural colourants. Eur. Food Res. Technol. 2007, 224, 667–679. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine-their stability under simulated gastrointestinal digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef]
- Pérez-Vicente, A.; Gil-Izquierdo, A.; García-Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red cabbage-stability to simulated gastrointestinal digestion. Phytochemistry 2007, 68, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tang, C.; Zhang, J.; Zhou, Q.; Zhang, Z. Stability and antioxidant activity of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) subjected to simulated in vitro gastrointestinal digestion. Int. J. Food Sci. Technol. 2019, 54, 2604–2614. [Google Scholar] [CrossRef]
- Kammerer, D.; Carle, R.; Schieber, A. Quantification of anthocyanins in black carrot extracts (Daucus carota ssp. sativus var. atrorubens Alef.) and evaluation of their color properties. Eur. Food Res. Technol. 2004, 219, 479–486. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.-J.; Tomás-Barberán, F.-A.; García-Conesa, M.-T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of various dietary anthocyanins in vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef]
- Charron, C.S.; Kurilich, A.C.; Clevidence, B.A.; Simon, P.W.; Harrison, D.J.; Britz, S.J.; Baer, D.J.; Novotny, J.A. Bioavailability of anthocyanins from purple carrot juice: Effects of acylation and plant matrix. J. Agric. Food Chem. 2009, 57, 1226–1230. [Google Scholar] [CrossRef]
- Singh, A.; Kitts, D.D. In vitro Bioaccessibility of Tart Cherry Anthocyanins in a Health Supplement Mix Containing Mineral Clay. J. Food Sci. 2019, 84, 475–480. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Mikkelsen, D.; Gidley, M.J. Lack of release of bound anthocyanins and phenolic acids from carrot plant cell walls and model composites during simulated gastric and small intestinal digestion. Food Funct. 2013, 4, 906–916. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber—Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Bobrich, A.; Fanning, K.J.; Rychlik, M.; Russell, D.; Topp, B.; Netzel, M. Phytochemicals in Japanese plums: Impact of maturity and bioaccessibility. Food Res. Int. 2014, 65, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Podsȩdek, A.; Redzynia, M.; Klewicka, E.; Koziołkiewicz, M. Matrix effects on the stability and antioxidant activity of red cabbage anthocyanins under simulated gastrointestinal digestion. Biomed. Res. Int. 2014, 2014, 365738. [Google Scholar] [CrossRef] [PubMed]
- Toydemir, G.; Boyacioglu, D.; Capanoglu, E.; Van Der Meer, I.M.; Tomassen, M.M.M.; Hall, R.D.; Mes, J.J.; Beekwilder, J. Investigating the transport dynamics of anthocyanins from unprocessed fruit and processed fruit juice from sour cherry (Prunus cerasus L.) across intestinal epithelial cells. J. Agric. Food Chem. 2013, 61, 11434–11441. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Clevidence, B.A.; Britz, S.J.; Simon, P.W.; Novotny, J.A. Plasma and urine responses are lower for acylated vs nonacylated anthocyanins from raw and cooked purple carrots. J. Agric. Food Chem. 2005, 53, 6537–6542. [Google Scholar] [CrossRef]
- Dudonné, S.; Dal-Pan, A.; Dubé, P.; Varin, T.V.; Calon, F.; Desjardins, Y. Potentiation of the bioavailability of blueberry phenolic compounds by co-ingested grape phenolic compounds in mice, revealed by targeted metabolomic profiling in plasma and feces. Food Funct. 2016, 7, 3421–3430. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin absorption and metabolism by human intestinal Caco-2 cells-a review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzaid, F.; Cheung, H.-M.; Preedy, V.R.; Sharp, P.A. Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: Extensive presystemic metabolism reduces apparent bioavailability. J. Agric. Food Chem. 2014, 62, 3904–3911. [Google Scholar] [CrossRef]

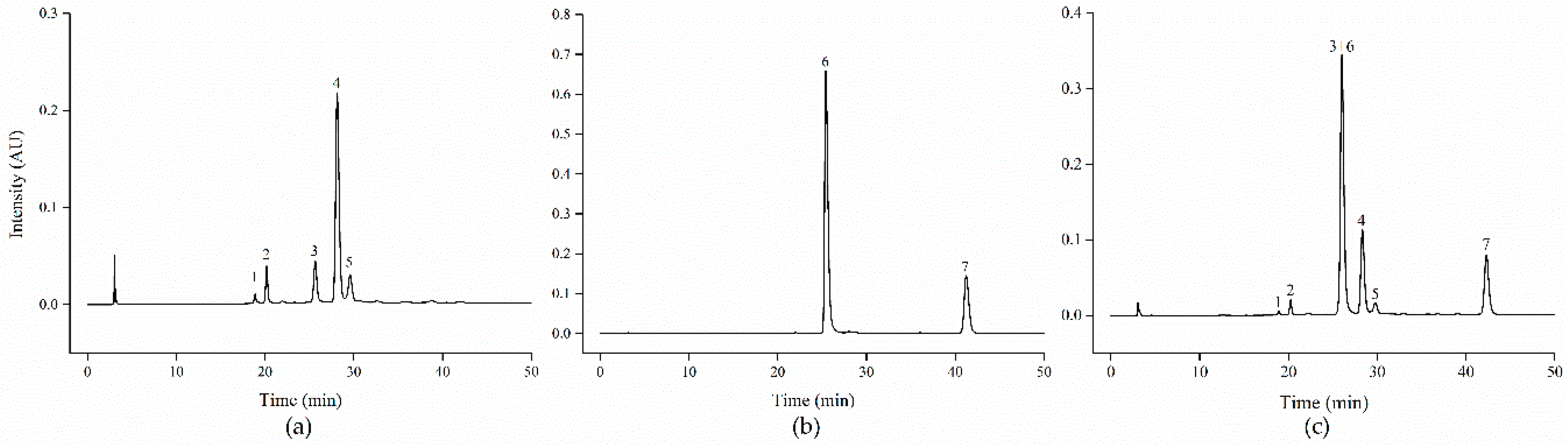

| Peak | Mass (m/z) | Compound Identity | Source | mg/g dw |
|---|---|---|---|---|
| 1 | 743 | Cyanidin-3-xylosyl-glucosyl-galactoside | Black carrot | 0.48 ± 0.05 |
| 2 | 581 | Cyanidin-3-xylosyl-galactoside | Black carrot | 1.35 ± 0.08 |
| 3 | 949 | Cyanidin-3-xylosyl-sinapoyl-glucosyl-galactoside | Black carrot | 2.16 ± 0.01 |
| 4 | 919 | Cyanidin-3-xylosyl-feruloyl-glucosyl-galactoside | Black carrot | 10.99 ± 0.02 |
| 5 | 889 | Cyanidin-3-xylosyl-coumaroyl-glucosyl-galactoside | Black carrot | 2.02 ± 0.01 |
| 6 | 433 | Pelargonidin-3-glucoside | Strawberry | 7.14 ± 0.01 |
| 7 | 519 | Pelargonidin-3-malonyl-glucoside | Strawberry | 2.24 ± 0.01 |
| Cyanidin-3-xylosyl-glucosyl-galactoside | Cyanidin-3-xylosyl-galactoside | Cyanidin-3-xylosyl-feruloyl-glucosyl-galactoside | Cyanidin-3-xylosyl-coumaroyl-glucosyl-galactoside | Pelargonidin-3-glucoside | Pelargonidin-3-malonyl-glucoside | |
|---|---|---|---|---|---|---|
| Undigested | ||||||
| 0.42 ± 0.04 | 1.18 ± 0.07 | 9.60 ± 0.02 | 1.76 ± 0.01 | 12.31 ± 0.01 | 3.87 ± 0.02 | |
| Post-Gastric | ||||||
| Single digestion | 0.25 ± 0.01 (59%) a | 0.95 ± 0.00 (81%) a | 8.23 ± 0.04 (86%) a | 1.14 ± 0.01 (65%) a | 11.13 ± 0.09 (90%) a | 3.37 ± 0.01 (87%) b |
| Co-digestion | 0.24 ± 0.02 (56%) a | 0.98 ± 0.02 (83%) a | 8.28 ± 0.03 (86%) a | 1.16 ± 0.02 (66%) a | 12.63 ± 0.46 (76%) b | 3.57 ± 0.07 (92%) a |
| Post-Intestinal | ||||||
| Single digestion | 0.36 ± 0.00 (86%) b | 0.94 ± 0.01 (79%) b | 5.54 ± 0.05 (58%) b | 0.85 ± 0.07 (48%) a | 6.01 ± 0.02 (49%) a | 1.77 ± 0.01 (46%) b |
| Co-digestion | 0.42 ± 0.00 (99%) a | 1.09 ± 0.01 (92%) a | 5.81 ± 0.03 (60%) a | 0.75 ± 0.04 (42%) a | 5.78 ± 0.13 (35%) b | 1.91 ± 0.02 (49%) a |
| Cyanidin-3-xylosyl-glucosyl-galactoside | Cyanidin-3-xylosyl-galactoside | Cyanidin-3-xylosyl-feruloyl-glucosyl-galactoside | Cyanidin-3-xylosyl-coumaroyl-glucosyl-galactoside | Pelargonidin-3-glucoside | Pelargonidin-3-malonyl-glucoside | |
|---|---|---|---|---|---|---|
| Undigested | ||||||
| 48.2 ± 5.0 | 135.3 ± 7.6 | 1099.5 ± 2.0 | 201.7 ± 0.9 | 714.0 ± 0.5 | 224.2 ± 1.0 | |
| Post-digestion | ||||||
| Single digestion | 29.7 ± 3.7 (62%) b | 44.1 ± 4.4 (33%) b | 378.4 ± 26.1 (34%) b | 55.8 ± 5.2 (28%) b | 256.6 ± 8.3 (36%) a | 76.2 ± 1.8 (34%) a |
| Co-digestion | 50.5 ± 9.2 (105%) a | 113.8 ± 0.2 (84%) a | 528.3 ± 15.0 (48%) a | 105.2 ± 0.9 (52%) a | 272.5 ± 19.7 (28%) b | 81.3 ± 6.6 (36%) a |
| Anthocyanin | Source | 0 h | 2 h | 4 h |
|---|---|---|---|---|
| Cyanidin-3-xylosyl-glucosyl-galactoside | BC | 3.1 ± 0.0 | nd | 0.28 ± 0.02 (9.0%) a |
| Mix BC-ST | 3.0 ± 0.0 | nd | 0.27 ± 0.01 (9.1%) a | |
| Cyanidin-3-xylosyl-galactoside | BC | 11.9 ± 0.1 | 0.28 ± 0.03 (2.3%) a | 0.52 ± 0.07 (4.4%) a |
| Mix BC-ST | 11.7 ± 0.0 | 0.29 ± 0.01 (2.5%) a | 0.48 ± 0.03 (4.1%) a | |
| Cyanidin-3-xylosyl-feruloyl-glucosyl-galactoside | BC | 91.6 ± 1.2 | 0.56 ± 0.12 (0.6%) a | 1.93 ± 0.44 (2.1%) a |
| Mix BC-ST | 88.9 ± 0.3 | 0.52 ± 0.05 (0.6%) a | 1.67 ± 0.18 (1.9%) a | |
| Cyanidin-3-xylosyl-coumaroyl-glucosyl-galactoside | BC | 13.5 ± 0.2 | 0.27 ± 0.02 (2.0%) a | 0.47 ± 0.07 (3.5%) a |
| Mix BC-ST | 13.1 ± 0.0 | 0.28 ± 0.02 (2.2%) a | 0.43 ± 0.04 (3.3%) a | |
| Pelargonidin-3-glucoside | ST | 81.0 ± 1.0 | 0.69 ± 0.17 (0.9%) a | 1.94 ± 0.53 (2.4%) a |
| Mix BC-ST | 138.1 ± 2.1 | 0.53 ± 0.12 (0.4%) a | 2.56 ± 0.31 (1.9%) a | |
| Pelargonidin-3-malonyl-glucoside | ST | 21.8 ± 0.1 | nd | 0.60 ± 0.07 (2.7%) a |
| Mix BC-ST | 24.2 ± 0.1 | nd | 0.46 ± 0.07 (1.9%) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo, C.; Kamiloglu, S.; Grootaert, C.; Van Camp, J.; Hendrickx, M. Co-Ingestion of Black Carrot and Strawberry. Effects on Anthocyanin Stability, Bioaccessibility and Uptake. Foods 2020, 9, 1595. https://doi.org/10.3390/foods9111595
Carrillo C, Kamiloglu S, Grootaert C, Van Camp J, Hendrickx M. Co-Ingestion of Black Carrot and Strawberry. Effects on Anthocyanin Stability, Bioaccessibility and Uptake. Foods. 2020; 9(11):1595. https://doi.org/10.3390/foods9111595
Chicago/Turabian StyleCarrillo, Celia, Senem Kamiloglu, Charlotte Grootaert, John Van Camp, and Marc Hendrickx. 2020. "Co-Ingestion of Black Carrot and Strawberry. Effects on Anthocyanin Stability, Bioaccessibility and Uptake" Foods 9, no. 11: 1595. https://doi.org/10.3390/foods9111595
APA StyleCarrillo, C., Kamiloglu, S., Grootaert, C., Van Camp, J., & Hendrickx, M. (2020). Co-Ingestion of Black Carrot and Strawberry. Effects on Anthocyanin Stability, Bioaccessibility and Uptake. Foods, 9(11), 1595. https://doi.org/10.3390/foods9111595





