Pomegranate (Punica granatum L.) Extract and Its Anthocyanin and Copigment Fractions—Free Radical Scavenging Activity and Influence on Cellular Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Pomegranate (Punica granatum L.) Sample
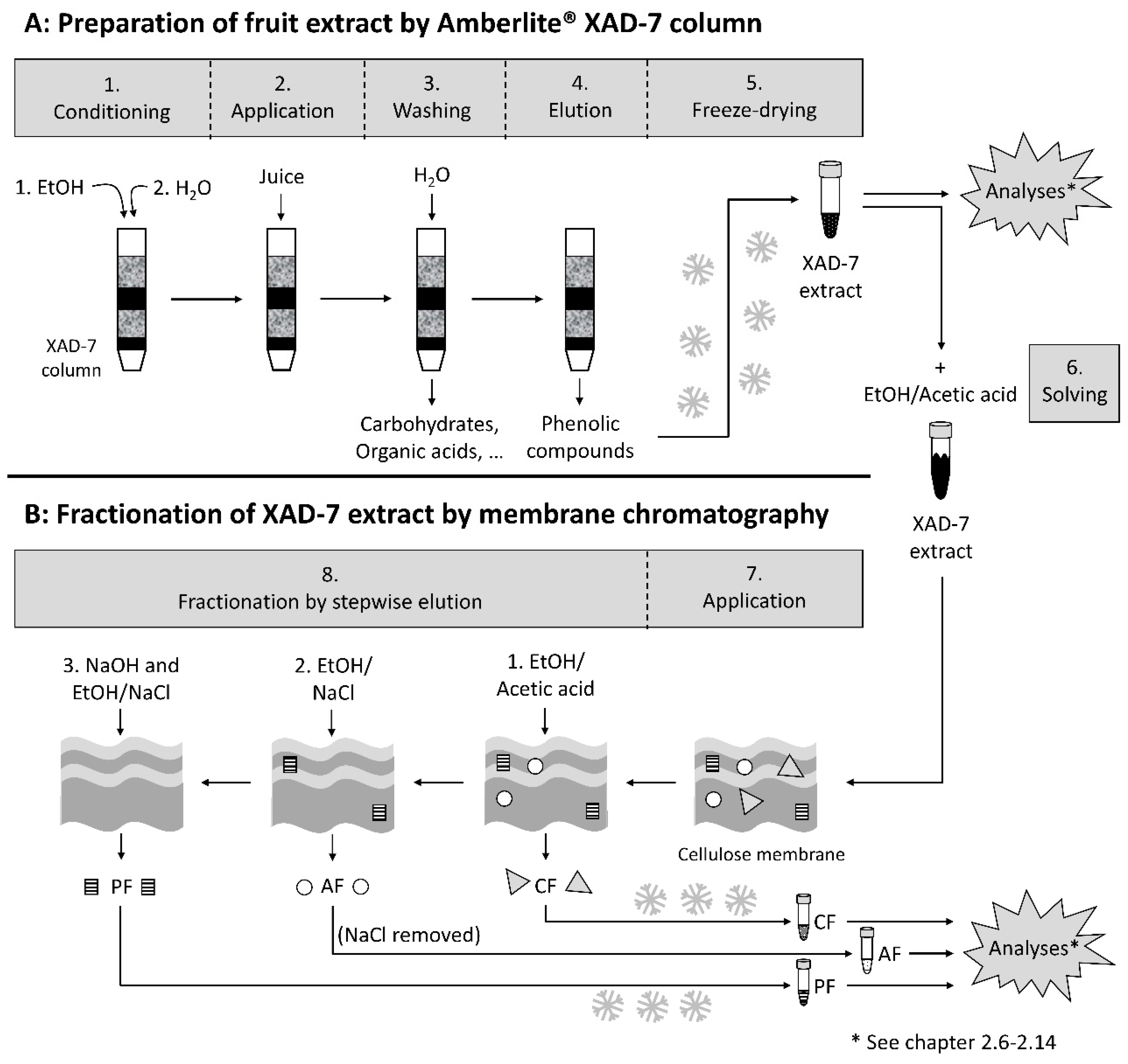
2.4. Preparation of a Phenolic Fruit Extract from Pomegranate Juice by Adsorption on an Amberlite XAD-7 Resin
2.5. Fractionation by Adsorptive Membrane Chromatography
2.6. Quantification of the Total Phenolic Content (TPC) Using the Folin–Ciocalteu Assay
2.7. Quantification of Polyphenols by UHPLC–PDA
2.8. Quantification of Polymeric Compounds by HPLC–PDA
2.9. Polyphenol Identification by HPLC Photodiode Array Electrospray Ionization Tandem Mass Spectrometry (HPLC–PDA–ESI–MS/MS)
2.10. Determination of the Radical Scavenging Capacity by the Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.11. Determination of the Free Radical Scavenging Activities by ESR Spectroscopy
2.12. Preparation of XAD-7 Extract and Its Separated Fractions for Cell Culture Experiments
2.13. Determination of the Cytotoxic Potential on HepG2 Cells in the MTT Viability Assay
2.14. Quantitative Determination of ROS Reducing Activity
2.15. Statistical Analysis
3. Results
3.1. Chemical Characterization of PE and Its Fractions Obtained by Membrane Chromatography
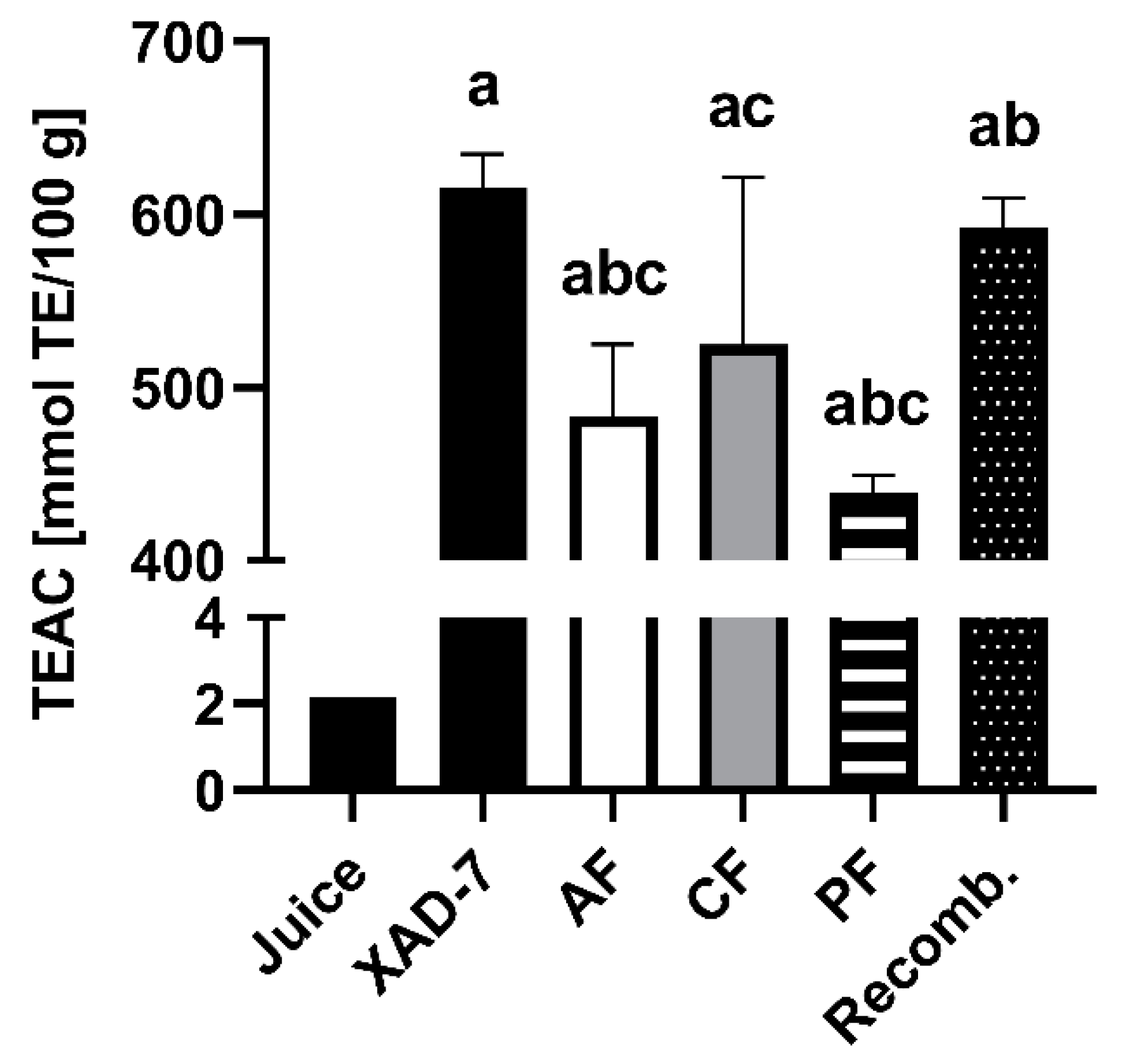
3.2. Detection of Free Radical Scavenging Activity
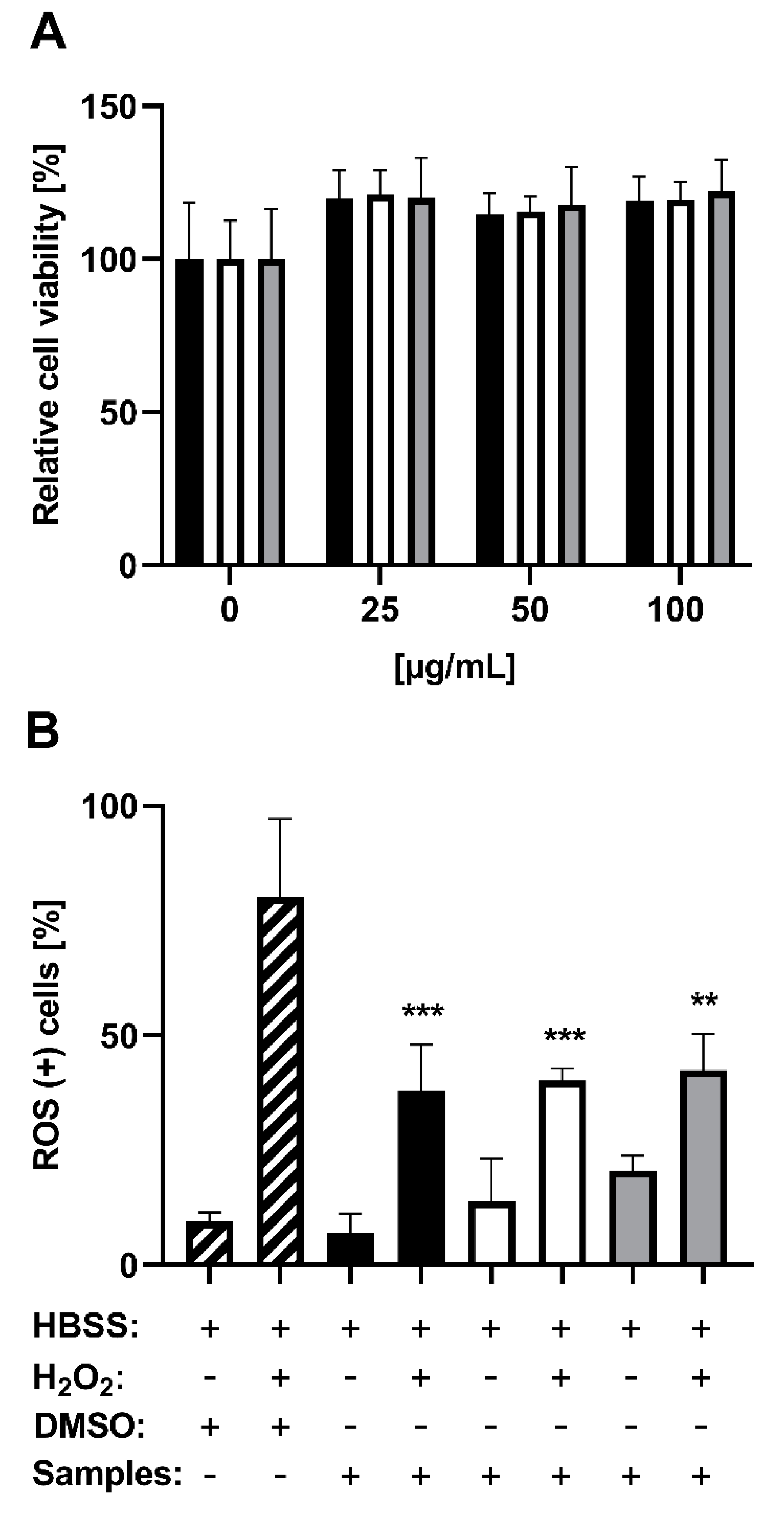
3.3. Analyzing the Protection Potential against ROS Induced by H2O2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van den Driessche, J.J.; Plat, J.; Mensink, R.P. Effects of Superfoods on Risk Factors of Metabolic Syndrome: A Systematic Review of Human Intervention Trials. Food Funct. 2018, 9, 1944–1966. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of Antioxidant Potency of Commonly Consumed Polyphenol-rich Beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Duman, A.D.; Ozgen, M.; Dayisoylu, K.S.; Erbil, N.; Durgac, C. Antimicrobial Activity of Six Pomegranate (Punica granatum L.) Varieties and their Relation to Some of their Pomological and Phytonutrient Characteristics. Molecules 2009, 14, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; McClees, S.F.; Afaq, F. Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef] [PubMed]
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Integrated Processing Technologies for Food and Agricultural By-Products, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–216. ISBN 9780128141380. [Google Scholar]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Wang, S.Y.; Feng, R.; Bowman, L.; Penhallegon, R.; Ding, M.; Lu, Y. Antioxidant Activity in Lingonberries (Vaccinium vitis-idaea L.) and its Inhibitory Effect on Activator Protein-1, Nuclear Factor-kappaB, and Mitogen-activated Protein Kinases Activation. J. Agric. Food Chem. 2005, 53, 3156–3166. [Google Scholar] [CrossRef]
- Dróżdż, P.; Šėžienė, V.; Pyrzynska, K. Phytochemical Properties and Antioxidant Activities of Extracts from Wild Blueberries and Lingonberries. Plant Foods Hum. Nutr. 2017, 72, 360–364. [Google Scholar] [CrossRef]
- Caruso, A.; Barbarossa, A.; Tassone, A.; Ceramella, J.; Carocci, A.; Catalano, A.; Basile, G.; Fazio, A.; Iacopetta, D.; Franchini, C.; et al. Pomegranate: Nutraceutical with Promising Benefits on Human Health. Appl. Sci. 2020, 10, 6915. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Zhang, Z.; Ravid, N.; Wetzstein, M.E. Characterization of Attributes Related to Fruit Size in Pomegranate. J. Am. Soc. Hortic. Sci. 2011, 46, 908–912. [Google Scholar] [CrossRef]
- Pool-Zobel, B.L.; Bub, A.; Schröder, N.; Rechkemmer, G. Anthocyanins are Potent Antioxidants in Model Systems but Do Not Reduce Endogenous Oxidative DNA Damage in Human Colon Cells. Eur. J. Nutr. 1999, 38, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Weisel, T.; Baum, M.; Eisenbrand, G.; Dietrich, H.; Will, F.; Stockis, J.-P.; Kulling, S.; Rüfer, C.; Johannes, C.; Janzowski, C. An Anthocyanin/Polyphenolic-rich Fruit Juice Reduces Oxidative DNA Damage and Increases Glutathione Level in Healthy Probands. Biotechnol. J. 2006, 1, 388–397. [Google Scholar] [CrossRef]
- Shirode, A.B.; Kovvuru, P.; Chittur, S.V.; Henning, S.M.; Heber, D.; Reliene, R. Antiproliferative Effects of Pomegranate Extract in MCF-7 Breast Cancer Cells are Associated with Reduced DNA Repair Gene Expression and Induction of Double Strand Breaks. Mol. Carcinog. 2014, 53, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Prieto, J.M.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Bioactive Properties of Commercialised Pomegranate (Punica granatum) Juice: Antioxidant, Antiproliferative and Enzyme Inhibiting Activities. Food Funct. 2015, 6, 2049–2057. [Google Scholar] [CrossRef]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human Intervention Study to Investigate the Intestinal Accessibility and Bioavailability of Anthocyanins from Bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef]
- González-Barrio, R.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of Anthocyanins and Ellagitannins Following Consumption of Raspberries by Healthy Humans and Subjects with an Ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.; Iacopetta, D.; La Torre, C.; Ceramella, J.; Muià, N.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Finding Solutions for Agricultural Wastes: Antioxidant and Antitumor Properties of Pomegranate Akko Peel Extracts and β-Glucan Recovery. Food Funct. 2018, 9, 6618–6631. [Google Scholar] [CrossRef]
- Dassprakash, M.V.; Arun, R.; Abraham, S.K.; Premkumar, K. In Vitro and In Vivo Evaluation of Antioxidant and Antigenotoxic Potential of Punica Granatum Leaf Extract. Pharm. Biol. 2012, 50, 1523–1530. [Google Scholar] [CrossRef]
- Navarro, M.; Amigo-Benavent, M.; Mesias, M.; Baeza, G.; Gökmen, V.; Bravo, L.; Morales, F.J. An Aqueous Pomegranate Seed Extract Ameliorates Oxidative Stress of Human Hepatoma HepG2 Cells. J. Sci. Food Agric. 2014, 94, 1622–1627. [Google Scholar] [CrossRef]
- Aly, F.A.E.; Salman, A.S.; Hassan, Z.M. Evaluation of the Antimutagenic Effect of Pomegranate Seed Oil against Genotoxicity Induced by Nitrobenzene in Mice. Glob. J. Pharmacol. 2014, 8, 189–195. [Google Scholar]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total Cranberry Extract versus its Phytochemical Constituents: Antiproliferative and Synergistic Effects against Human Tumor Cell Lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxid. Med. Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef]
- Juadjur, A.; Winterhalter, P. Development of a Novel Adsorptive Membrane Chromatographic Method for the Fractionation of Polyphenols from Bilberry. J. Agric. Food Chem. 2012, 60, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Ostberg-Potthoff, J.J.; Berger, K.; Richling, E.; Winterhalter, P. Activity-Guided Fractionation of Red Fruit Extracts for the Identification of Compounds Influencing Glucose Metabolism. Nutrients 2019, 11, 1166. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free Radical Scavenging and Antioxidant Activity of Betanin: Electron Spin Resonance Spectroscopy Studies and Studies in Cultured Cells. Food Chem. Toxicol. 2014, 73, 119–126. [Google Scholar] [CrossRef]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant Activities of Pomegranate Fruit Extract and Its Anthocyanidins: Delphinidin, Cyanidin, and Pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef]
- Ostberg-Potthoff, J.J. Aktivitätsgeleitete Fraktionierung und Charakterisierung von Phenolischen Verbindungen aus Fruchtsaftextrakten, 1. Auflage; Verlag Dr. Hut: München, Germany, 2020; ISBN 3843943192. [Google Scholar]
- Kozik, V.; Jarzembek, K.; Jędrzejowska, A.; Bąk, A.; Polak, J.; Bartoszek, M.; Pytlakowska, K. Investigation of Antioxidant Activity of Pomegranate Juices by Means of Electron Paramagnetic Resonance and UV-Vis Spectroscopy. J. AOAC Int. 2015, 98, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and Quantification of Phenolic Compounds from Pomegranate (Punica granatum L.) Peel, Mesocarp, Aril and Differently Produced Juices by HPLC-DAD-ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Pitonzo, R.; Novara, M.E.; Bongiorno, D.; Indelicato, S.; Gentile, C.; Avellone, G.; Bognanni, R.; Scandurra, S.; Melilli, M.G. Antioxidant Activity and Phenolic Composition in Pomegranate (Punica granatum L.) Genotypes from South Italy by UHPLC-Orbitrap-MS Approach. J. Sci. Food Agric. 2019, 99, 1038–1045. [Google Scholar] [CrossRef]
- Akhavan, H.; Barzegar, M.; Weidlich, H.; Zimmermann, B.F. Phenolic Compounds and Antioxidant Activity of Juices from Ten Iranian Pomegranate Cultivars Depend on Extraction. J. Chem. 2015, 2015, 907101. [Google Scholar] [CrossRef]
- Kerimi, A.; Nyambe-Silavwe, H.; Gauer, J.S.; Tomás-Barberán, F.A.; Williamson, G. Pomegranate Juice, but not an Extract, Confers a Lower Glycemic response on a High-Glycemic Index Food: Randomized, Crossover, Controlled Trials in Healthy Subjects. Am. J. Clin. Nutr. 2017, 106, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Liang, H.; Yuan, Q. Purification, Antioxidant Activity and Protein-Precipitating Capacity of Punicalin from Pomegranate Husk. Food Chem. 2013, 138, 437–443. [Google Scholar] [CrossRef]
- Lima, M.D.S.; Silani, I.d.S.V.; Toaldo, I.M.; Corrêa, L.C.; Biasoto, A.C.T.; Pereira, G.E.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic Compounds, Organic Acids and Antioxidant Activity of Grape Juices Produced from New Brazilian Varieties Planted in the Northeast Region of Brazil. Food Chem. 2014, 161, 94–103. [Google Scholar] [CrossRef]
- Aloqbi, A.; Omar, U.; Yousr, M.; Grace, M.; Lila, M.A.; Howell, N. Antioxidant Activity of Pomegranate Juice and Punicalagin. Nat. Sci. 2016, 8, 235–246. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Anthocyanin Profile, Antioxidant Activity and Enzyme Inhibiting Properties of Blueberry and Cranberry Juices: A Comparative Study. Food Funct. 2017, 8, 4187–4193. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-Based Assays for Measurement of Antiproliferative Activity of Green Tea Polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X.-H.; Chen, H.-D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) Phenolics Ameliorate Hydrogen Peroxide-Induced Oxidative Stress and Cytotoxicity in Human Keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef]
- Pontonio, E.; Montemurro, M.; Pinto, D.; Marzani, B.; Trani, A.; Ferrara, G.; Mazzeo, A.; Gobbetti, M.; Rizzello, C.G. Lactic Acid Fermentation of Pomegranate Juice as a Tool to Improve Antioxidant Activity. Front. Microbiol. 2019, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Hong, S.H.; Shin, S.S.; Lee, D.-S.; Han, M.H.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; Park, E.K.; et al. Activation of the Nrf2/HO-1 Signaling Pathway Contributes to the Protective Effects of Sargassum Serratifolium Extract against Oxidative Stress-Induced DNA Damage and Apoptosis in SW1353 Human Chondrocytes. Int. J. Environ. Res. Public Health 2018, 15, 1173. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Afkhami Goli, A.; Asadpour, E.; Ghorbani, A.; Sadeghnia, H.R. Protective Effect of Punica granatum L. against Serum/Glucose Deprivation-Induced PC12 Cells Injury. Evid. Based Complement. Alternat. Med. 2013, 2013, 716730. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, X.; He, G.; Gao, X.; Li, M.; Wu, J.; Li, Z.; Wu, J.; Wang, J.; Luo, C. Cytosolic Protection against Ultraviolet Induced DNA Damage by Blueberry Anthocyanins and Anthocyanidins in Hepatocarcinoma HepG2 Cells. Biotechnol. Lett. 2013, 35, 491–498. [Google Scholar] [CrossRef]
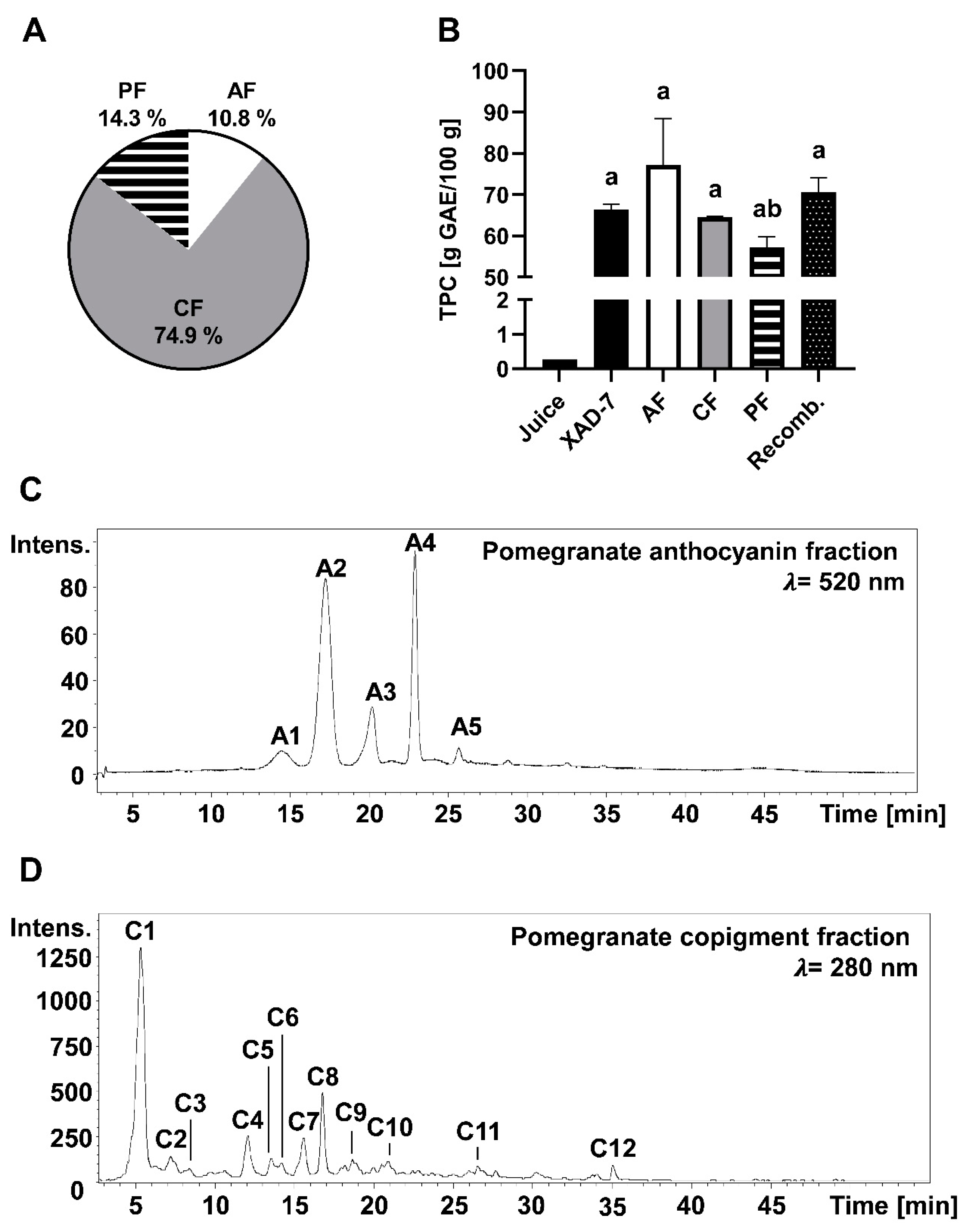

| Peak | [M + H]± | Fragments (m/z) | Compound |
|---|---|---|---|
| A1 | 627 | 465, 303 | delphinidin-3,5-diglucoside |
| A2 | 611 | 449, 287 | cyanidin-3,5-diglucoside |
| A3 | 465 | 303 | delphidin-3-glucoside |
| A4 | 449 | 287 | cyanidin-3-glucoside |
| A5 | 433 | 271 | pelargonidin-3-glucoside |
| C1 | 781 | 601, 271 | punicalin |
| C2 | 1083 | 601, 299, 271 | punicalagin I |
| C3 | 783 | 601, 301 | pedunculagin I |
| C4 | 1083 | 601, 299, 271 | punicalagin II |
| C5 | 783 | 601, 301 | pedunculagin II |
| C6 | 951 | 907, 301 | granatin B |
| C7 | 783 | 601, 301 | pedunculagin III |
| C8 | 1083 | 601, 301 | pedunculagin III |
| C9 | 799 | 479, 301 | ellagic acid derivative |
| C10 | 801 | 347, 301, 195 | punicluconin |
| C11 | 463 | 301, 257 | ellagic acid hexoside |
| C12 | 301 | 258 | ellagic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostka, T.; Ostberg-Potthoff, J.J.; Briviba, K.; Matsugo, S.; Winterhalter, P.; Esatbeyoglu, T. Pomegranate (Punica granatum L.) Extract and Its Anthocyanin and Copigment Fractions—Free Radical Scavenging Activity and Influence on Cellular Oxidative Stress. Foods 2020, 9, 1617. https://doi.org/10.3390/foods9111617
Kostka T, Ostberg-Potthoff JJ, Briviba K, Matsugo S, Winterhalter P, Esatbeyoglu T. Pomegranate (Punica granatum L.) Extract and Its Anthocyanin and Copigment Fractions—Free Radical Scavenging Activity and Influence on Cellular Oxidative Stress. Foods. 2020; 9(11):1617. https://doi.org/10.3390/foods9111617
Chicago/Turabian StyleKostka, Tina, Johanna Josefine Ostberg-Potthoff, Karlis Briviba, Seiichi Matsugo, Peter Winterhalter, and Tuba Esatbeyoglu. 2020. "Pomegranate (Punica granatum L.) Extract and Its Anthocyanin and Copigment Fractions—Free Radical Scavenging Activity and Influence on Cellular Oxidative Stress" Foods 9, no. 11: 1617. https://doi.org/10.3390/foods9111617
APA StyleKostka, T., Ostberg-Potthoff, J. J., Briviba, K., Matsugo, S., Winterhalter, P., & Esatbeyoglu, T. (2020). Pomegranate (Punica granatum L.) Extract and Its Anthocyanin and Copigment Fractions—Free Radical Scavenging Activity and Influence on Cellular Oxidative Stress. Foods, 9(11), 1617. https://doi.org/10.3390/foods9111617






