Antifungal Activity of Aromatic Plants of the Lamiaceae Family in Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. Isolation and Identification of Pencillum
2.3. Preparation of Essential Oils
2.4. Gas Chromatography/Mass Spectrometry
2.5. HPLC Analysis
2.6. Bread Preparation
2.7. Preparation of Media and Fungal Spore Suspension
2.8. Inoculation and Incubation of PDA Petri Dishes
2.9. Inoculation and Incubation of Bread Samples
2.10. Statistical Analysis
3. Results and Discussion
3.1. Composition of Essential Oil
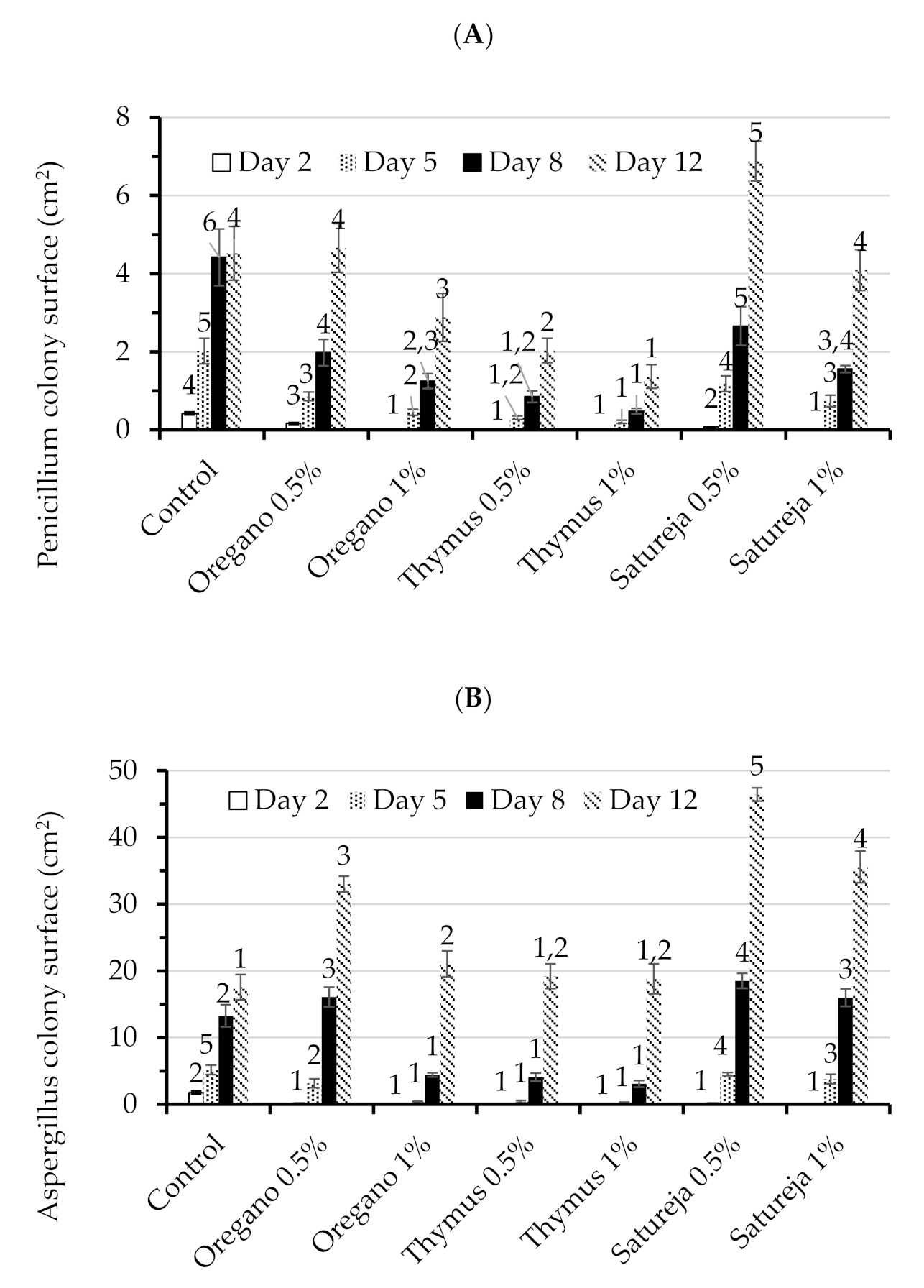
3.2. Antifungal Activity of Aromatic Plants in Petri Dishes
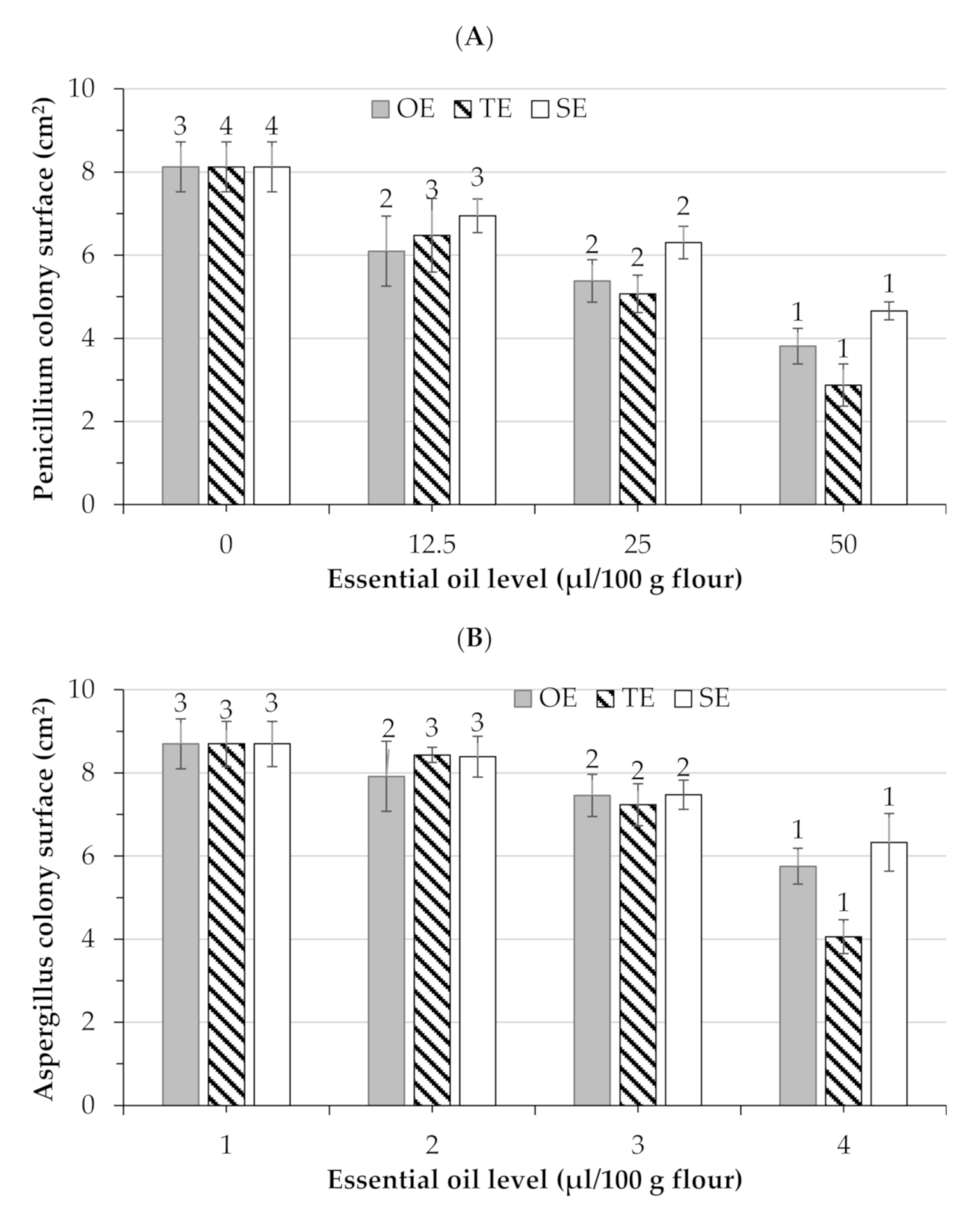
3.3. Antifungal Activity in Bread
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nieto, G. Biological Activities of Three Essential Oils of the Lamiaceae Family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J. Agric. Food Chem. 2005, 53, 9452–9458. [Google Scholar] [CrossRef] [PubMed]
- Murbach Teles Andrade, B.F.; Nunes Barbosa, L.; da Silva Probst, I.; Fernandes Júnior, A. Antimicrobial activity of essential oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Bounar, R.; Krimat, S.; Boureghda, H.; Dob, T. Chemical analyses, antioxidant and antifungal effects of oregano and thyme essential oils alone or in combination against selected Fusarium species. Int. Food Res. J. 2020, 27, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Jurado, F.; Cervantes-Rincón, T.; Bach, H.; López-Malo, A.; Palou, E. Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crop. Prod. 2019, 131, 90–95. [Google Scholar] [CrossRef]
- Císarová, M.; Hleba, L.; Tančinová, D.; Florková, M.; Foltinová, D.; Charousová, I.; Vrbová, K.; Božik, M.; Klouček, P. Inhibitory effect of essential oils from some Lamiaceae species on growth of Eurotium Spp. Isolated From Bread. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 857–862. [Google Scholar] [CrossRef]
- Debonne, E.; Van Bockstaele, F.; Samapundo, S.; Eeckhout, M.; Devlieghere, F. The use of essential oils as natural antifungal preservatives in bread products. J. Essent. Oil Res. 2018, 30, 309–318. [Google Scholar] [CrossRef]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Ristic, M.D.; Duletic-Lausevic, S.; Knezevic-Vukcevic, J.; Marin, P.D.; Simic, D.; Vukojevic, J.; Janackovic, P.; Vajs, V. Antimicrobial activity of essential oils and ethanol extract of Phlomis fruticosa L. (Lamiaceae). Phytother. Res. 2000, 14, 267–271. [Google Scholar] [CrossRef]
- Rota, C.; Carraminana, J.J.; Burillo, J.; Herrera, A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Debonne, E.; Van Bockstaele, F.; De Leyn, I.; Devlieghere, F.; Eeckhout, M. Validation of in-vitro antifungal activity of thyme essential oil on Aspergillus niger and Penicillium paneum through application in par-baked wheat and sourdough bread. LWT 2018, 87, 368–378. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Muslim, S.N.; Hussin, Z.S. Chemical compounds and synergistic antifungal properties of Thymus kotschanus essential oil plus ketoconazole against Candida spp. Gene Rep. 2020, 21, 100916. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Marinelli, L.; Di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Omidbeygi, M.; Barzegar, M.; Hamidi, Z.; Naghdibadi, H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control 2007, 18, 1518–1523. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P.; Papageorgiou, M. Aromatic plants of Lamiaceae family in a traditional bread recipe: Effects on quality and phytochemical content. J. Food Biochem. 2019, 43, e13020. [Google Scholar] [CrossRef]
- Tamil Selvi, A.; Joseph, G.S.; Jayaprakasha, G.K. Inhibition of growth and aflatoxin production in Aspergillus flavus by Garcinia indica extract and its antioxidant activity. Food Microbiol. 2003, 20, 455–460. [Google Scholar] [CrossRef]
- Vico, I.; Duduk, N.; Vasić, M.; Nikolić, M. Identification of Penicillium expansum causing postharvest blue mold decay of apple fruit. Pestic. Fitomed. 2014, 29, 257–266. [Google Scholar] [CrossRef]
- Bampidis, V.A.; Christodoulou, V.; Florou-Paneri, P.; Christaki, E.; Chatzopoulou, P.S.; Tsiligianni, T.; Spais, A.B. Effect of dietary dried oregano leaves on growth performance, carcase characteristics and serum cholesterol of female early maturing turkeys. Br. Poult. Sci. 2005, 46, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing: Carol Stream, IL, USA, 1995. [Google Scholar]
- Moosavi-Nasab, M.; Jamalian, J.; Heshmati, H.; Haghighi-Manesh, S. The inhibitory potential of Zataria multiflora and Syzygium aromaticum essential oil on growth and aflatoxin production by Aspergillus flavus in culture media and Iranian white cheese. Food Sci. Nutr. 2017, 6, 318–324. [Google Scholar] [CrossRef]
- Lagouri, V.; Blekas, G.; Tsimidou, M.; Kokkini, S.; Boskou, D. Composition and antioxidant activity of essential oils from Oregano plants grown wild in Greece. Z. Lebensm. Unters. Forsch. 1993, 197, 20–23. [Google Scholar] [CrossRef]
- Kokkini, S.; Vokou, D. Carvacrol-rich plants in Greece. Flavour Fragr. J. 1989, 4, 1–7. [Google Scholar] [CrossRef]
- Vokou, D.; Kokkini, S.; Bessiere, J.-M. Geographic variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Hanlidou, E. Herbs of the Labiatae. In Encyclopedia of Food Science and Nutrition; Academic Press: Amsterdam, The Nederlands, 2003; Volume 5, pp. 3082–3090. [Google Scholar]
- Vernet, P.; Gouyon, R.H.; Valdeyron, G. Genetic control of the oil content in Thymus vulgaris L: A case of polymorphism in a biosynthetic chain. Genetica 1986, 69, 227–231. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M. Characterization of several aromatic plants grown in northern Italy. Flavour Fragr. J. 1993, 8, 115–122. [Google Scholar] [CrossRef]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Cheng, Y.; Qian, H.; Yao, W. Simple microencapsulation of plant essential oil in porous starch granules: Adsorption kinetics and antibacterial activity evaluation. J. Food Process. Preserv. 2019, 43, e14156. [Google Scholar] [CrossRef]
- Gonçalves da Rosa, C.; Zapelini de Melo, A.P.; Sganzerla, W.G.; Machado, M.H.; Nunes, M.R.; de Oliveira Brisola Maciel, M.V.; Bertoldi, F.C.; Manique Barreto, P.L. Application in situ of zein nanocapsules loaded with Origanum vulgare Linneus and Thymus vulgaris as a preservative in bread. Food Hydrocoll. 2020, 99, 105339. [Google Scholar] [CrossRef]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of lavender (Lavandula angustifolia) and melissa (Melissa Officinalis) waste on quality and shelf life of bread. Food Chem. 2018, 253, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.F.; Tan, R.X.; Yang, L.; Liu, Z.L. Two Flavones from Artemisia giraldii and their Antimicrobial Activity. Planta Med. 1996, 62, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Cafarchia, C.; De Laurentis, N.; Milillo, M.A.; Losacco, V.; Puccini, V. Antifungal activity of Apulia region propolis. Parassitologia 1999, 41, 587–590. [Google Scholar] [PubMed]
- Afolayan, A.J.; Meyer, J.J.M. The antimicrobial activity of 3,5,7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. J. Ethnopharmacol. 1997, 57, 177–181. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Berkada, B. Preliminary report on warfarin for the treatment of herpes 210 simplex. J. Ir. Coll. Physicians Surg. 1978, 22, 56. [Google Scholar]

| Compound | Elution Time (min) | Origanum vulgare ssp. hirtum | Thymus capitatus | Satureja thymbra |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Monoterpene hydrocarbons | 12.62 | 19.35 | 44.99 | |
| α-thujene | 7.8 | 0.64 ± 0.03 a | 0.69 ± 0.01 a | 1.45 ± 0.01 b |
| α-pinene | 8.1 | 0.78 ± 0.03 a | 0.84 ± 0.01 b | 1.17 ± 0.02 c |
| camphene | 8.8 | 0.15 ± 0.00 a | 0.16 ± 0.00 b | 0.31 ± 0.01 c |
| β-pinene | 10.1 | 0.14 ± 0.00 a | 0.14 ± 0.01 a | 0.40 ± 0.01 b |
| cis-sabinene hydrate | 16.0 | - ± - a | - ± - a | 0.09 ± 0.01 b |
| trans-sabinene hydrate | 18.1 | - ± - a | 0.71 ± 0.04 b | 1.01 ± 0.05 c |
| β-myrcene | 10.9 | 1.61 ± 0.06 b | 1.97 ± 0.02 c | 0.33 ± 0.01 a |
| α-phellandrene | 11.8 | 0.19 ± 0.01 b | 0.29 ± 0.01 c | 0.09 ± 0.00 a |
| β-phellandrene | 13.3 | 0.38 ± 0.01 a | 0.60 ± 0.01 b | 0.67 ± 0.01 b |
| α-terpinene | 12.5 | 0.97 ± 0.04 a | 1.66 ± 0.04 b | 3.40 ± 0.01 c |
| p-cymene | 13.0 | 5.00 ± 0.07 a | 7.18 ± 0.16 c | 6.67 ± 0.01 b |
| γ-terpinene | 15.3 | 2.62 ± 0.05 a | 4.90 ± 0.11 b | 29.28 ± 0.19 c |
| α-terpinolene | 17.3 | 0.14 ± 0.01 b | 0.21 ± 0.00 c | 0.12 ± 0.00 a |
| Oxygenated monoterpenes | 1.12 | 1.17 | 0.98 | |
| borneol | 23.6 | 0.25 ± 0.03 a | 0.28 ± 0.03 a | 0.38 ± 0.02 a |
| terpinen-4-ol | 24.6 | 0.87 ± 0.04 b | 0.89 ± 0.05 b | 0.60 ± 0.02 a |
| Sesquiterpenes | 2.14 | 1.94 | 4.92 | |
| trans-caryophyllene | 44.4 | 1.23 ± 0.00 a | 1.80 ± 0.02 b | 4.52 ± 0.06 c |
| α-humulene | 46.4 | - ± - a | - ± - a | 0.19 ± 0.00 b |
| β-bisabolene | 49.8 | 0.91 ± 0.00 c | 0.14 ± 0.00 a | 0.21 ± 0.01 b |
| Oxygenated sesquiterpenes | ||||
| caryophyllene oxide | - ± - a | 0.16 ± 0.01 b | 0.21 ± 0.01 c | |
| Monoterpene phenols | 82.64 | 76.37 | 46.34 | |
| carvacrol | 36 | 82.48 ± 0.62 c | 76.19 ± 0.18 b | 44.93 ± 0.35 a |
| thymol | 35.1 | 0.16 ± 0.03 a | 0.18 ± 0.01 a | 1.41 ± 0.02 b |
| Alcohols | ||||
| 1-octen-3-ol | 10.3 | 0.45 ± 0.06 b | 0.42 ± 0.04 b | 0.21 ± 0.03 a |
| Ketones | ||||
| 3-octanone | 10.8 | 0.15 ± 0.01 b | - ± - a | 2.02 ± 0.04 c |
| Total | 99.10 | 99.37 | 97.18 | |
| Phytochemicals | Origanum vulgare ssp. hirtum | Thymus capitatus | Saturejathymbra |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Phenolic acids and their derivatives | 90.53 | 22.66 | 55.44 |
| 4-hydroxybenzoic acid | 0.23 ± 0.02 a | 0.32 ± 0.04 b | 1.02 ± 0.04 c |
| gallic acid | 0.14 ± 0.02 b | 0.36 ± 0.03 c | 0.08 ± 0.01 a |
| protocatechuic acid | 0.93 ± 0.06 b | 0.68 ± 0.04 a | 0.73 ± 0.05 a |
| syringic acid | 0.22 ± 0.02 a | 0.64 ± 0.02 c | 0.35 ± 0.04 b |
| vanillic acid | 0.75 ± 0.01 a | 1.05 ± 0.07 b | 0.75 ± 0.05 a |
| trans-cinnamic acid | ND | ND | 0.04 ± 0.01 |
| caffeic acid | 5.66 ± 0.09 b | 2.79 ± 0.06 a | 6.85 ± 0.03 c |
| ferulic acid | ND | ND | ND |
| sinapic acid | ND | ND | ND |
| p-coumaric acid | ND | ND | ND |
| chlorogenic acid | 0.27 ± 0.01 a | 0.34 ± 0.01 b | ND |
| rosmarinic acid | 82.34 ± 0.13 c | 16.48 ± 0.09 a | 45.61 ± 0.16 b |
| Flavonoids | 9.11 | 17.15 | 10.01 |
| luteolin | 0.41 ± 0.03 | LLQ | LLQ |
| apigenin | ND | ND | ND |
| quercetin | ND | 1.18 ± 0.07 b | 0.78 ± 0.04 a |
| kaempferol | 0.67 ± 0.02 a | 0.70 ± 0.04 a | 0.78 ± 0.03 b |
| myricetin | ND | 5.62 ± 0.09 b | 0.28 ± 0.05 a |
| rutin | 1.62 ± 0.01 | ND | ND |
| (±)-naringenin | ND | ND | 1.17 ± 0.03 |
| (+)-catechin | ND | 0.71 ± 0.03 a | 1.93 ± 0.12 b |
| (−)-epicatechin | 5.96 ± 0.08 b | 8.78 ± 0.18 c | 5.07 ± 0.14 a |
| (−)-epigallocatechine | 0.46 ± 0.05 b | 0.16 ± 0.03 a | ND |
| Phenolic monoterpenes | 105.19 | 82.17 | 91.37 |
| carvacrol | 105.19 ± 0.15 c | 82.17 ± 0.22 a | 91.37 ± 0.18 b |
| thymol | ND | ND | ND |
| Total phenolics | 204.83 | 121.99 | 156.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skendi, A.; Katsantonis, D.Ν.; Chatzopoulou, P.; Irakli, M.; Papageorgiou, M. Antifungal Activity of Aromatic Plants of the Lamiaceae Family in Bread. Foods 2020, 9, 1642. https://doi.org/10.3390/foods9111642
Skendi A, Katsantonis DΝ, Chatzopoulou P, Irakli M, Papageorgiou M. Antifungal Activity of Aromatic Plants of the Lamiaceae Family in Bread. Foods. 2020; 9(11):1642. https://doi.org/10.3390/foods9111642
Chicago/Turabian StyleSkendi, Adriana, Dimitrios Ν. Katsantonis, Paschalina Chatzopoulou, Maria Irakli, and Maria Papageorgiou. 2020. "Antifungal Activity of Aromatic Plants of the Lamiaceae Family in Bread" Foods 9, no. 11: 1642. https://doi.org/10.3390/foods9111642
APA StyleSkendi, A., Katsantonis, D. Ν., Chatzopoulou, P., Irakli, M., & Papageorgiou, M. (2020). Antifungal Activity of Aromatic Plants of the Lamiaceae Family in Bread. Foods, 9(11), 1642. https://doi.org/10.3390/foods9111642