The Application of Chemometrics to Volatile Compound Analysis for the Recognition of Specific Markers for Cultivar Differentiation of Greek Virgin Olive Oil Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Oil Samples
2.2. Determination of Free Acidity, Peroxide Value and Absorption Coefficients (K232, K270)
2.3. HS-SPME-GC/MS
2.4. Statistical Analysis
3. Results
3.1. Analysis of Conventional Quality Parameters
3.2. Analysis Volatile Compound Analysis
3.3. Multivariate Analysis of Variance
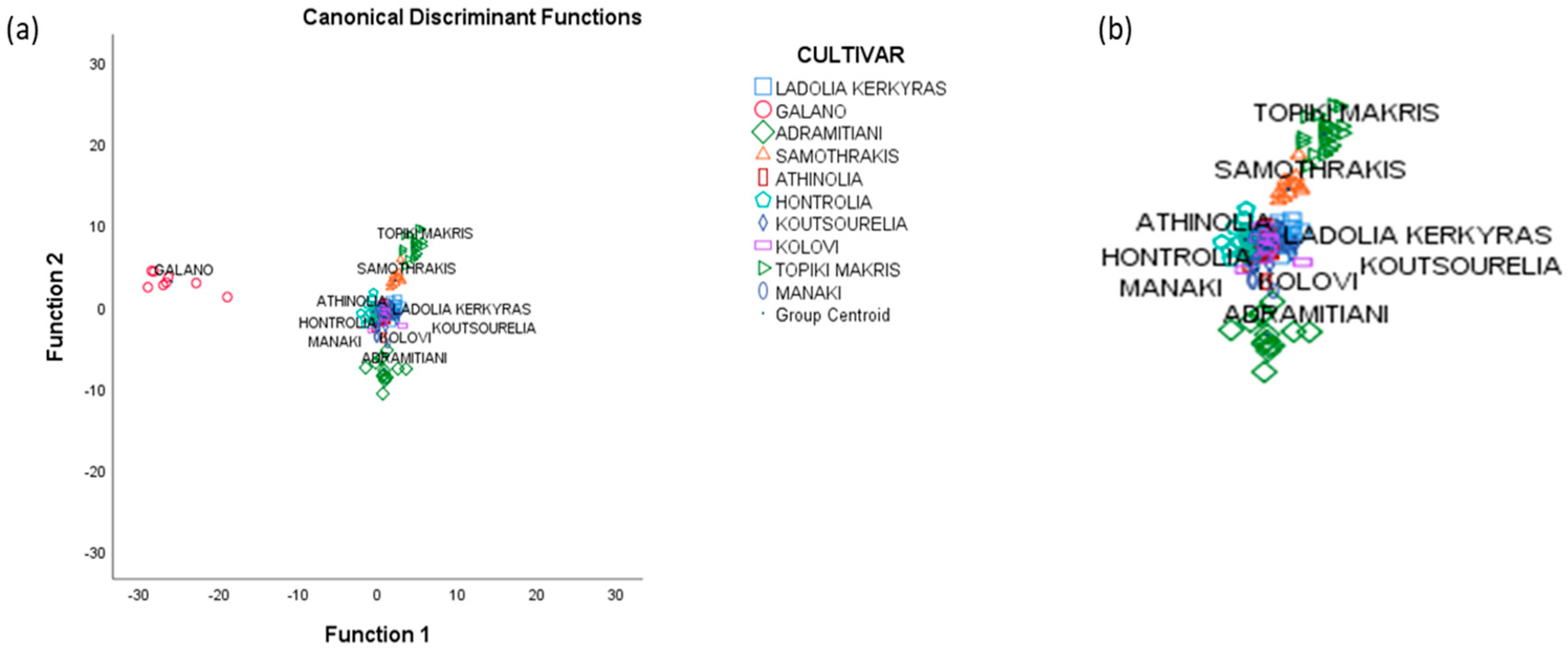
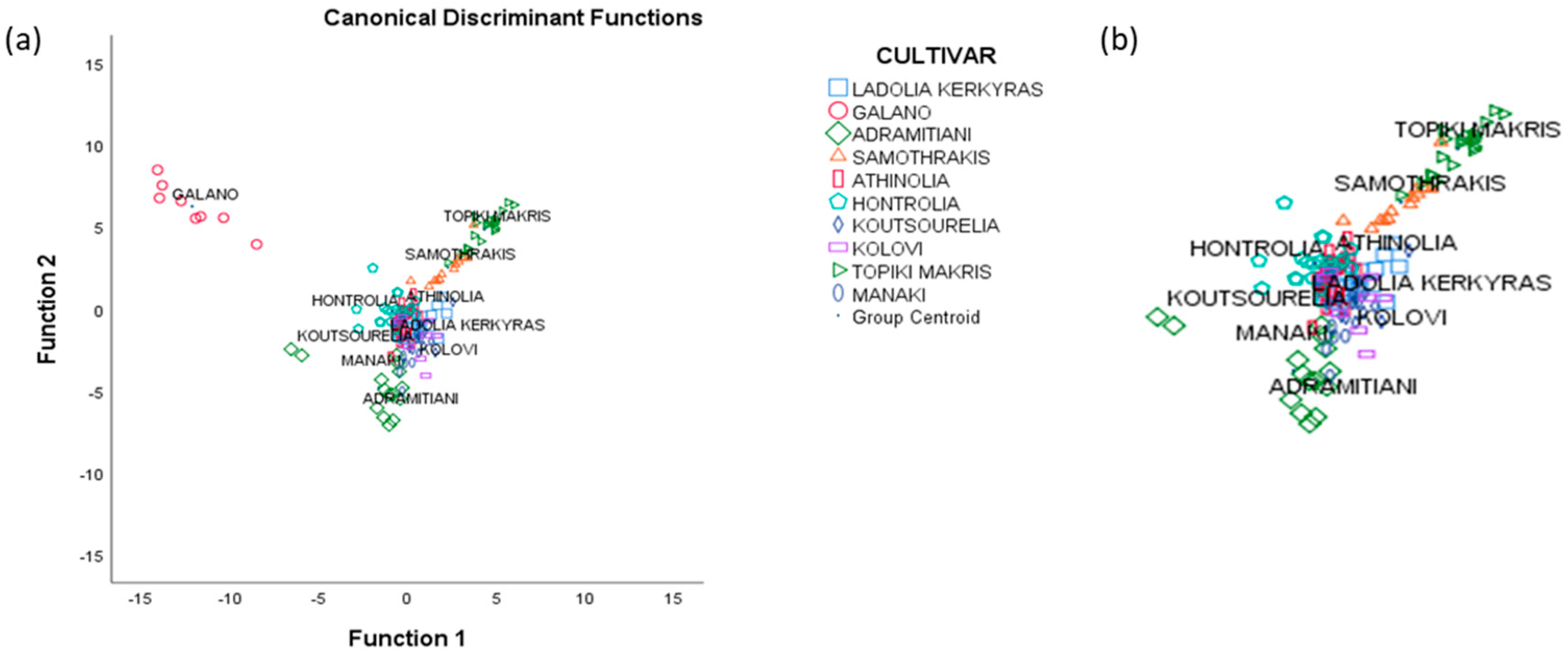
3.4. Linear Discriminant Analysis
3.5. Stepwise Linear Discriminant Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kosma, I.; Badeka, A.; Vatavali, K.; Kontakos, S.; Kontominas, M. Differentiation of Greek extra virgin olive oils according to cultivar based on volatile compound analysis and fatty acid composition. Eur. J. Lipid Sci. Technol. 2016, 118, 849–861. [Google Scholar] [CrossRef]
- Kosma, I.; Vavoura, M.; Kontakos, S.; Karabagias, I.; Kontominas, M.; Kiritsakis, A.; Badeka, A. Characterization and classification of extra virgin olive oil from five less well-known Greek olive cultivars. J. Am. Oil Chem. Soc. 2016, 93, 837–848. [Google Scholar] [CrossRef]
- International Food Authenticity Assurance Organization. Available online: https://uia.org/s/or/en/1122281809 (accessed on 30 October 2020).
- European Union No. 178/2002 of 28 January 2002, laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Union 2002, L31, 1–24.
- Ruiz-Samblás, C.; Tres, A.; Koot, A.; van Ruth, S.M.; González-Casado, A.; Luis Cuadros-Rodríguez, L. Proton transfer reaction-mass spectrometry volatile organic compound fingerprinting for monovarietal extra virgin olive oil identification. Food Chem. 2012, 134, 589–596. [Google Scholar] [CrossRef]
- Camin, F.; Larcher, R.; Perini, M.; Bontempo, L.; Bertoldi, D.; Gagliano, G.; Nicolini, G.; Versini, G. Characterisation of authentic Italian extra-virgin olive oils by stable isotope ratios of C, O and H and mineral composition. Food Chem. 2010, 118, 901–909. [Google Scholar] [CrossRef]
- Lukić, I.; Da Ros, A.; Guella, G.; Camin, F.; Masuero, D.; Mulinacci, N.; Vrhovsek, U.; Mattivi, F. Lipid Profiling and Stable Isotopic Data Analysis for Differentiation of Extra Virgin Olive Oils Based on Their Origin. Molecules 2019, 25, 4. [Google Scholar] [CrossRef] [Green Version]
- Lia, F.; Vella, B.; Zammit Mangion, M.; Farrugia, C. Application of 1H and 13C NMR fingerprinting as a tool for the authentication of Maltese extra virgin olive oil. Foods 2020, 9, 689. [Google Scholar] [CrossRef]
- Dais, P.; Hatzakis, E. Quality assessment and authentication of virgin olive oil by NMR spectroscopy: A critical review. Anal. Chim. Acta 2013, 765, 1–27. [Google Scholar] [CrossRef]
- Del Coco, L.; Schena, F.P.; Fanizzi, F.P. 1H Nuclear Magnetic Resonance Study of Olive Oils Commercially Available as Italian Products in the United States of America. Nutrients 2012, 4, 343–355. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.; Mafra, I.; Oliveira, M.B.P.P. Advances in vegetable oil authentication by DNA-based markers. Trends Food Sci. Technol. 2012, 26, 43–55. [Google Scholar] [CrossRef]
- Giménez, M.J.; Pistón, F.; Martín, A.; Atienza, S.G. Application of real-time PCR on the development of molecular markers and to evaluate critical aspects for olive oil authentication. Food Chem. 2010, 118, 482–487. [Google Scholar] [CrossRef]
- Raieta, K.; Muccillo, L.; Colantuoni, V. A novel reliable method of DNA extraction from olive oil suitable for molecular traceability. Food Chem. 2015, 172, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Stilo, F.; Cordero, C.; Sgorbini, B.; Bicchi, C.; Liberto, E.; Stilo, F.; Cordero, C.; Sgorbini, B.; Bicchi, C.; Liberto, E. Highly Informative Fingerprinting of Extra-Virgin Olive Oil Volatiles: The Role of High Concentration-Capacity Sampling in Combination with Comprehensive Two-Dimensional Gas Chromatography. Separations 2019, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Carranco, N.; Farrés-Cebrián, M.; Saurina, J.; Núñez, O. Authentication and quantitation of fraud in extra virgin olive oils based on HPLC-UV fingerprinting and multivariate calibration. Foods 2018, 7, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Villalba, R.; Carrasco-Pancorbo, A. Oliveras-Ferraros, C.; Vazquez-Martin, A.; Menendez, J.A.; Segura-Carretero, A.; Fernandez- Gutierrez, A. Characterization and quantification of phenolic compounds of extra-virgin olive oils with anticancer properties by a rapid and resolutive LC-ESI-TOF MS method. J. Pharmaceut. Biomed. Anal. 2010, 51, 416–429. [Google Scholar]
- Aceto, M.; Calà, E.; Musso, D.; Regalli, N.; Matteo Oddone, M. A preliminary study on the authentication and traceability of extra virgin olive oil made from Taggiasca olives by means of trace and ultra-trace elements distribution. Food Chem. 2019, 298, 125047. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochemistry 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- International Olive Oil Council. Organoleptic Assessment of Virgin Olive Oil; IOOC Standard Procedure: Madrid, Spain, 1996. [Google Scholar]
- European Union No. 2568/91 of 11 July 1992 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 1991, L248, 1–83.
- European Union No. 796/2002 of 6 May 2002 amending Regulation (EEC) No. 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis and additional notes in the Annex to Council regulation (EEC) No. 2658/91 on the tariff and statistical nomenclature and on the Common Customs Tariff. Off. J. Eur. Union 2002, L128, 23–28.
- Lawless, H.T. Descriptive analysis of complex odors: Reality, model or illusion? Food Qual. Pref. 1992, 10, 325–332. [Google Scholar] [CrossRef]
- Kosma, I.; Vatavali, K.; Kontakos, S.; Kontominas, M.; Kiritsakis, A.; Badeka, A. Geographical Differentiation of Greek Extra Virgin Olive Oil from Late-Harvested Koroneiki Cultivar Fruits. J. Am. Oil Chem. Soc. 2017, 94, 1373–1384. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Brahmi, F.; Dabbou, S.; Hassine, K.B.; Taamali, A.; Chehab, H.; Ellouz, M.; Zarrouk, M.; Hammami, M. Effect of the growing area conditions on differentiation between Chemlali and Chétoui olive oils. Food Chem. 2010, 119, 220–225. [Google Scholar] [CrossRef]
- Baccouri, B.; Temime, S.B.; Campeol, E.; Cioni, P.L.; Daoud, D.; Zarrouk, M. Application of solid-phase microextraction to the analysis of volatile compounds in virgin olive oils from five new cultivars. Food Chem. 2007, 102, 850–856. [Google Scholar] [CrossRef]
- Berlioz, B.; Cordella, C.; Cavalli, J.; Lizzani-Cuvelier, L.; Loiseau, A.; Fernandez, X. Comparison of the amounts of volatile compounds in French protected designation of origin virgin olive oils. J. Agric. Food. Chem. 2006, 54, 10092–100101. [Google Scholar] [CrossRef]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterization of 39 Varietal Virgin Olive Oils by their Volatile Compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Guerrini, L.; Masella, P.; Angeloni, G.; Zanoni, B.; Breschi, C.; Calamia, L.; Parrenti, A. The effect of an increase in paste temperature between malaxation and centrifugation on olive oil quality and yield: Preliminary results. Ital. J. Food Sci. 2019, 31, 451–458. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS; Sage Publications Ltd.: London, UK, 2009. [Google Scholar]
- Pizarro, C.; Rodríguez-Tecedor, S.; Pérez-del-Notario, N.; González-Sáiz, J.M. Recognition of volatile compounds as markers in geographical discrimination of Spanish extra virgin olive oils by chemometric analysis of non-specific chromatography volatile profiles. J. Chromatogr. A. 2011, 1218, 518–523. [Google Scholar] [CrossRef]
- Guo, J.; Yue, T.; Yuan, Y.; Sun, N.; Liu, P. Characterization of volatile and sensory profiles of apple juices to trace fruit origins and investigation of the relationship between the aroma properties and volatile constituents. LWT Food Sci. Technol. 2020, 124, 109203. [Google Scholar] [CrossRef]
- IBM Knowledge Center. Available online: https://www.ibm.com/support/knowledgecenter/en/SSLVMB_23.0.0/spss/tutorials/discrim_telco_stepwise.html (accessed on 19 February 2020).
- Pouliarekou, E.; Badeka, A.; Tasioula-Margari, M.; Kontakos, S.; Longobardi, F.; Kontominas, M.G. Characterization and classification of Western Greek olive oils according to cultivar and geographical origin based on volatile compounds. J. Chromatogr. 2011, 1218, 7534–7542. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.T.; Rios, J.J.; Aparicio, R. Changes in the volatile composition of virgin olive oil during oxidation: Flavors and off-flavors. J. Agric. Food. Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Vichi, S.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; Lopez-Tamames, E. Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: Characterization of virgin olive oils from two distinct geographical areas of northern Italy. J. Agric. Food. Chem. 2003, 51, 6572–6577. [Google Scholar] [CrossRef] [PubMed]
- Kiritsakis, A. Flavor components of olive oil. A review. J. Am. Oil Chem. Soc. 1998, 75, 673–681. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavor development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Tanouti, K.; Serghini-Caid, H.; Sindic, M.; Wathelet, J.P.; Bouseta, A.; Elamrani, A. Volatile compounds, profiles of virgin olive oils produced in the eastern Morocco: Oxidative stability and sensory defects. J. Food Res. 2012, 1, 194–206. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Kesen, S.; Kelebek, H.; Selli, S. Characterization of the volatile, phenolic and antioxidant properties of monovarietal olive oil obtained from cv. Halhali. J. Am. Oil Chem. Soc. 2013, 90, 1685–1696. [Google Scholar] [CrossRef]
- Morales, M.T.; Aparicio-Ruiz, R.; Aparicio, R. Chromatographic Methodologies: Compounds for Olive Oil Odor Issues. In Handbook of Olive Oil. Analysis and Properties, 2nd ed.; Aparicio, R., Harwood, J., Eds.; Springer: New York, NY, USA, 2013; pp. 261–310. [Google Scholar]
- Bubola, K.B.; Krapac, M.; Lukić, I.; Sladonja, B.; Autino, A.; Cantini, C.; Poljuha, D. Morphological and Molecular Characterization of Bova Olive Cultivar and Aroma Fingerprint of Its Oil. Food Technol. Biotech. 2014, 52, 342–350. [Google Scholar]
- Zunin, P.; Boggia, R.; Salvadeo, P.; Evangelisti, F. Geographical Traceability of West Liguria Extra Virgin Olive Oils by the Analysis of Volatile Terpenoid Hydrocarbons. J. Chromatogr. A. 2005, 1089, 243–249. [Google Scholar] [CrossRef]
| Cultivars (Origin) | Number of Samples |
|---|---|
| Topiki Makris (Evros) | 21 |
| Samothraki (Samothraki isle) | 13 |
| Galano (Metagitsi-Chalkidiki) | 8 |
| Hontrolia (Chalikidiki) | 21 |
| Adramitiani (Lesvos isle) | 15 |
| Kolovi (Lesvos isle) | 8 |
| Ladolia Kerkyras (Corfu isle) | 21 |
| Koutsourelia (Etoloakarnania) | 18 |
| Manaki (Korinthos) | 6 |
| Athinolia (Lakonia) | 36 |
| Cultivars | % Acidity | PV (meq O2/kg) | Κ232 | Κ270 |
|---|---|---|---|---|
| Topiki Makris | 0.6 ± 0.3 a | 12.3 ± 3.6 ab | 2.38 ± 0.38 bc | 0.15 ± 0.03 ab |
| Samothraki | 0.9 ± 0.6 ab | 8.7 ± 2.9 a | 1.89 ± 0.31 ab | 0.20 ± 0.03 b |
| Galano | 0.3 ± 0.2 a | 10.7 ± 2.6 a | 1.97 ± 0.21 ab | 0.14 ± 0.03 a |
| Hontrolia | 0.4 ± 0.2 a | 9.0 ± 3.2 a | 1.77 ± 0.27 a | 0.15 ± 0.03 a |
| Adramitiani | 1.8 ± 1.6 c | 11.2 ± 7.4 ab | 2.39 ± 0.86 bc | 0.17 ± 0.05 ab |
| Kolovi | 0.4 ± 0.2 a | 10.2 ± 5.0 a | 2.04 ± 0.45 ab | 0.16 ± 0.04 ab |
| Ladolia Kerkyras | 1.3 ± 1.5 bc | 15.6 ± 8.7 b | 2.76 ± 1.02 c | 0.20 ± 0.06 b |
| Koutsourelia | 0.5 ± 0.4 a | 10.0 ± 4.5 a | 2.04 ± 0.82 ab | 0.17 ± 0.04 ab |
| Manaki | 0.4 ± 0.2 a | 7.8 ± 1.2 a | 1.68 ± 0.22 a | 0.13 ± 0.03 a |
| Athinolia | 0.8 ± 0.6 ab | 10.8 ± 5.2 a | 1.93 ± 0.25 ab | 0.13 ± 0.02 a |
| Topiki Makris | Samothrakis | Galano | Hontrolia | Adramitiani | Kolovi | Ladolia Kerkyras | Koutsourelia | Manaki | Athinolia | RIlit1 | RIexp2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | ||||||||||||
| Ethanol | 22.8 ± 104.4 a | 83.9 ± 205.4 a | 238.9 ± 258.5 a | 153.8 ± 300.9 a | 1348.4 ± 1117.8 b | 2282.2 ± 2386.9 c | 104.5 ± 412.7 a | 172.9 ± 244.5 a | 1323.7 ± 682.0 b | 286.6 ± 453.3 a | <500 | -3 |
| 1-Penten-3-ol | 87.3 ± 46.2 b | 6.5 ± 23.5 a | 77.9 ± 51.6 b | 40.8 ± 65.8 ab | 64.2 ± 50.6 ab | 103.1 ± 72.6 b | 73.3 ± 77.9 b | 165.5 ± 125.4 c | 83.5 ± 44.7 b | 66.7 ± 65.3 b | 682 | 686 |
| 1-Pentanol | 3.3 ± 15.0 a | 31.5 ± 69.3 ab | 52.2 ± 62.4 b | 17.3 ± 59.6 ab | 62.4 ± 131.0 b | 16.5 ± 40.4 ab | 768 | 766 | ||||
| (Z)-2-Pentenol | 25.9 ± 48.0 a | 6.6 ± 30.4 a | 6.3 ± 16.8 a | 101.7 ± 86.5 b | 25.6 ± 50.2 a | 100.4 ± 116.9 b | 14.1 ± 36.4 a | 770 | 767 | |||
| (E)-2-Hexenol | 855.7 ± 584.6 ab | 1387.1 ± 844.2b | 519.6 ± 466.5 a | 1336.0 ± 1068.0 b | 505.6 ± 457.8 a | 419.2 ± 278.4 a | 829.5 ± 551.5 ab | 1397.7 ± 1385.0b | 632.0 ± 321.9 a | 327.3 ± 220.6 a | 867 | 862 |
| Hexanol | 655.0 ± 378.5 ab | 1222.4 ± 1064.1cd | 325.4 ± 339.3 a | 637.5 ± 684.1 ab | 603.0 ± 525.3 ab | 1704.6 ± 1079.5 cd | 460.8 ± 778.3 ab | 935.7 ± 918.4 bc | 1335.0 ± 658.3 d | 320.9 ± 230.3 a | 870 | 862 |
| Total Alcohols | 1646.7 | 2699.9 | 1161.9 | 2178.0 | 2600.6 | 4663.1 | 1511.1 | 2834.5 | 3390.6 | 1027.2 | ||
| Aldehydes | ||||||||||||
| Butanal, 3-methyl- | 1039.8 ± 4765.0 a | 6.9 ± 18.9 a | 3.4 ± 9.7 a | 15.3 ± 34.8 a | 39.4 ± 69.5 a | 7.7 ± 20.3 a | 656 | 660 | ||||
| Pentanal | 151.7 ± 171.8 b | 155.2 ± 89.4 b | 68.8 ± 121.9 ab | 9.2 ± 42.1 a | 141.7 ± 161.2 ab | 36.0 ± 70.1 ab | 122.0 ± 315. 7 ab | 71.7 ± 84.2 ab | 7.8 ± 19.2 a | 16.5 ± 43.9 a | 699 | 695 |
| (E)-2-Pentenal | 67.7 ± 55.5 b | 10.0 ± 9.0 a | 7.6 ± 34.7 a | 7.7 ± 21.8 a | 25.3 ± 50.5 a | 758 | 754 | |||||
| (Z)-3-Hexenal | 181.8 ± 226.3 c | 32.3 ± 78.9 a | 116.5 ± 115.7 abc | 117.7 ± 129.2 abc | 59.1 ± 167.2 ab | 89.6 ± 121.1 abc | 152.8 ± 198.8bc | 14.4 ± 64.3 a | 800 | 798 | ||
| Hexanal | 3189.8 ± 1057.7 cd | 2376.5 ± 913.3 abc | 1716.4 ± 1281.4 ab | 1523.6 ± 822.2 ab | 2451.1 ± 1281.2 bc | 1441.9 ± 1515.3 ab | 3774.7 ± 2983.8 d | 1457.7 ± 973.8 ab | 1101.3 ± 1008.3 a | 2539.4 ± 938.2 bc | 803 | 798 |
| (E)-2-Hexenal | 27,638.6 ± 4366.2 c | 14,650.8 ± 6449.5 b | 8189.5 ± 5980.5 a | 15,450.5 ± 11,180.0 b | 2905.4 ± 1912.1 a | 5506.3 ± 3055.5 a | 19,133.1 ± 11,778.4 b | 17,274.3 ± 9844.7 b | 4486.1 ± 2568.5 a | 7010.0 ± 5588.7 a | 858 | 852 |
| Heptanal | 64.5 ± 52.6 a | 45.2 ± 59.9 a | 43.0 ± 121.7 a | 10.6 ± 33.5 a | 57.8 ± 75.5 a | 20.2 ± 43.4 a | 270.2 ± 382.0 b | 60.2 ± 134.6 a | 140.1 ± 113.2 a | 904 | 899 | |
| (E,E)-2,4-Hexadienal | 323.0 ± 106.4 b | 76.7 ± 123.9 a | 102.6 ± 141.9 a | 85.4 ± 175.4 a | 8.5 ± 15.9 a | 92.0 ± 133.0 a | 272.7 ± 247.0b | 916 | 916 | |||
| (E)-2-Heptenal | 139.2 ± 111.2 b | 81.6 ± 78.3 ab | 27.3 ± 77.3 ab | 61.5 ± 108.6 ab | 58.5 ± 90.1 ab | 261.4 ± 241.0 c | 53.6 ± 78.4 ab | 93.0 ± 136.8 ab | 961 | 963 | ||
| Benzaldehyde | 24.7 ± 40.6 ab | 3.0 ± 11.7 a | 30.5 ± 77.4 ab | 62.5 ± 102.9 b | 4.7 ± 20.4 a | 974 | 970 | |||||
| (E,E)-2,4-Heptadienal | 364.8 ± 514.6 ab | 207.1 ± 292.3 ab | 377.5 ± 162.3 ab | 394.9 ± 183.9 ab | 91.1 ± 131.2 a | 289.0 ± 206.9 ab | 161.9 ± 113.3 ab | 457.4 ± 203.7 b | 177.0 ± 64.4 ab | 144.5 ± 137.4 a | 1002 | 1008 |
| Octanal | 20.7 ± 51.8 a | 144.2 ± 318.0 a | 75.5 ± 154.7 a | 83.3 ± 175.5 a | 100.5 ± 120.8 a | 1006 | 1004 | |||||
| Nonanal | 334.3 ± 120.1 a | 291.5 ± 168.2 a | 244.3 ± 277.6 a | 123.0 ± 138.8 a | 435.1 ± 438.2 a | 246.9 ± 338.2 a | 1203.8 ± 1528.3 a | 330.3 ± 469.5 a | 66.2 ± 74.9 a | 1028.2 ± 699.5 b | 1108 | 1099 |
| Total Aldehydes | 32552.7 | 17937.7 | 11030.2 | 19447.0 | 6293.1 | 7642.5 | 25439.9 | 20277.7 | 5838.4 | 11889. 9 | ||
| Ketones | ||||||||||||
| 2-Propanone | 1437.9 ± 1814.9 ab | 3406.4 ± 3664.4 b | 1781.0 ± 1682.8 ab | 2409.3 ± 720.9 ab | 3199.5 ± 1683.8 b | 342.3 ± 815.7 a | 356.2 ± 388.9 a | 2049.5 ± 2950.5 ab | <500 | - | ||
| 1-Penten-3-one | 284.3 ± 148.8 b | 9.1 ± 22.4 a | 232.1 ± 232.3b | 68.1 ± 126.0 a | 27.7 ± 63.4 a | 69.6 ± 118.6 a | 41.3 ± 57.6 a | 262.2 ± 252.4b | 26.4 ± 64.7 a | 75.0 ± 100.4 a | 685 | 678 |
| 2-Pentanone | 61.8 ± 222.8 a | 31.2 ± 60.0 a | 69.0 ± 130.0 a | 10.5 ± 33.3 a | 46.5 ± 109.2 a | 686 | 689 | |||||
| 3-Pentanone | 105.7 ± 76.8 abc | 69.9 ± 64.2 ab | 144.1 ± 111.0 abc | 117.5 ± 109.2 abc | 103.7 ± 62.7 abc | 410.4 ± 271.3d | 38.7 ± 77.5 a | 217.3 ± 190.2 c | 189.5 ± 65.9 bc | 60.2 ± 58.8 ab | 696 | 694 |
| 2-Heptanone | 10.6 ± 30.1 a | 264.9 ± 559.7 b | 14.3 ± 39.8 a | 1.6 ± 9.8 a | 891 | 889 | ||||||
| 6-Methyl-5-hepten-2-one | 130.0 ± 91.6 abc | 65.2 ± 84.6 a | 84.0 ± 107.1 a | 38.1 ± 66.9 a | 260.6 ± 240.3 c | 47.8 ± 57.1 a | 245.7 ± 350.8 bc | 112.7 ± 183.2 ab | 57.6 ± 86.2 a | 986 | 985 | |
| 2-Octanone | 275.9 ± 588.4 b | 20.3 ± 49.9 a | 12.4 ± 30.1 a | 992 | 989 | |||||||
| Total Ketones | 1957.8 | 206.0 | 470.8 | 3630.0 | 2745.2 | 3006.7 | 3570.2 | 934.4 | 572.2 | 2302.9 | ||
| Esters | ||||||||||||
| (Z)-3-Hexenyl acetate | 425.0 ± 333.9 a | 258.8 ± 261.4 a | 262.9 ± 298.5 a | 2698.5 ± 1110.6 c | 311.0 ± 431.4 a | 1986.2 ± 1224.8 b | 2021.0 ± 1291.3 b | 232.4 ± 390.8 a | 1004 | 1005 | ||
| Hexyl acetate | 93.9 ± 73.7 a | 93.7 ± 87.1 a | 76.5 ± 104.4 a | 608.6 ± 307.8 bc | 69.3 ± 142.1 a | 724.3 ± 393.5 bc | 849.7 ± 367.2 c | 61.1 ± 131.3 a | 1010 | 1011 | ||
| Total Esters | 518.9 | 352.4 | 339.4 | 3307.1 | 380.3 | 2710.4 | 2870.6 | 293.5 | ||||
| Terpenes | ||||||||||||
| α-Pinene | 57.7 ± 135.4 a | 29.1 ± 104.8 a | 31.2 ± 99.9 a | 3997.5 ± 5975.8 b | 154.6 ± 300.7 a | 654.6 ± 1452.6 a | 53.2 ± 195.0 a | 28.8 ± 44.9 a | 945 | 932 | ||
| δ-3-Carene | 6.8 ± 24.4 a | 130.6 ± 273.6 b | 133.0 ± 310.1 b | 1022 | 1010 | |||||||
| p-Cymene | 37.3 ± 66.6 a | 50.1 ± 64.9 a | 4.4 ± 20.1 a | 49.0 ± 88.8 a | 176.4 ± 407.6 b | 47.9 ± 63.1 a | 9.1 ± 28.1 a | 36.4 ± 59.0 a | 1035 | 1023 | ||
| dl-Limonene | 39.0 ± 55.2 a | 874.0 ± 3061.2 a | 27.1 ± 50.2 a | 63.6 ± 72.0 a | 420.0 ± 1095.6 a | 3515.7 ± 5393.3 b | 665.9 ± 1757.8 a | 76.3 ± 224.9 a | 87.6 ± 326.7 a | 1041 | 1035 | |
| (E)-β-Ocimene | 258.5 ± 77.9 a | 157.0 ± 201.4 a | 411.6 ± 299.2 a | 310.1 ± 286.0 a | 262.8 ± 136.4 a | 172.9 ± 148.7 a | 2009.3 ± 2244.1 b | 179.4 ± 315.4 a | 18.8 ± 29.4 a | 13.4 ± 33.5 a | 1049 | 1035 |
| γ-Terpinene | 0.9 ± 3.9 a | 16.9 ± 65.5 a | 306.6 ± 729.1 b | 44.2 ± 174.3 a | 2.0 ± 12.0 a | 1068 | 1048 | |||||
| allo-Ocimene | 7.5 ± 29.1 a | 47.5 ± 87.6 b | 1146 | 1129 | ||||||||
| α-Copaene | 182.7 ± 47.2 b | 101.9 ± 87.9 ab | 748.0 ± 309.4c | 197.4 ± 193.2 b | 31.1 ± 40.7 a | 16.6 ± 2.9 a | 4.4 ± 20.2 a | 3.8 ± 16.2 a | 37.7 ± 70.2 a | 1.1 ± 6.6 a | 1404 | 1392 |
| (E)-β-Farnesene | 31.4 ± 46.7 b | 1467 | 1471 | |||||||||
| (E,E)-α-Farnesene | 282.8 ± 221.8 bcd | 325.8 ± 278.9 cd | 142.8 ± 157.7 abc | 194.6 ± 257.2 abc | 518.4 ± 371.7 d | 62.5 ± 67.9 ab | 151.9 ± 135.6 abc | 18.5 ± 45.1 a | 1512 | 1509 | ||
| α-Muurolene | 6.7 ± 30.7 a | 72.9 ± 80.3 b | 20.2 ± 44.4 a | 11.2 ± 43.4 a | 22.6 ± 73.7 a | 7.3 ± 31.0 a | 1526 | 1530 | ||||
| Total Terpens | 865.5 | 1544.7 | 1402.3 | 821.5 | 5476.3 | 4405.3 | 3781.4 | 347.6 | 85.4 | 140.5 | ||
| Hydrocarbons | ||||||||||||
| 1,3-Butadiene, 2-methyl- | 35.2 ± 64.0b | 13.4 ± 43.1 a | <500 | - | ||||||||
| (Z)-1,3-Pentadiene | 26.4 ± 36.7 a | 5.6 ± 25.7 a | 17.2 ± 48.7 a | 56.3 ± 81.5b | <500 | - | ||||||
| Pentane, 2,2,4-trimethyl- | 1335.7 ± 3943.5 a | 4438.7 ± 5333.5 a | 1078.9 ± 1220.1 a | 14,039.8 ± 12,531.5 b | 22.9 ± 61.2 a | 10,369.6 ± 13,701.5 b | 639.9 ± 1204.6 a | 149.1 ± 73.4 a | 510.7 ± 955.7 a | 689 | 668 | |
| Heptane | 80.7 ± 121.1 a | 236.4 ± 595.5 a | 198.4 ± 246.2 a | 87.2 ± 161.4 a | 165.7 ± 327.8 a | 23.8 ± 56.3 a | 180.1 ± 289.8 a | 700 | 700 | |||
| Toluene | 69.9 ± 80.4 a | 15.6 ± 50.8 a | 10.3 ± 21.8 a | 106.8 ± 119.6 a | 256.8 ± 774.6 a | 212.3 ± 295.1 a | 12.6 ± 31.0 a | 10.3 ± 29.9 a | 773 | 771 | ||
| 1-Octene | 27.3 ± 34.2 a | 39.2 ± 69.1 a | 68.4 ± 143.3 a | 70.5 ± 110.4 a | 793 | 792 | ||||||
| 2-Octene | 17.9 ± 64.6 a | 7.0 ± 26.9 a | 150.2 ± 313.4 b | 22.0 ± 75.5 a | 818 | 815 | ||||||
| Xylene | 34.8 ± 47.4 a | 5.1 ± 16.0 a | 21.1 ± 38.4 a | 92.6 ± 111.1 a | 311.6 ± 1013.2 a | 259.9 ± 581.8 a | 9.5 ± 21.8 a | 879 | 870 | |||
| Cyclopentane, 2-propenyl- | 235.7 ± 91.2 c | 76.9 ± 53.5 ab | 108.7 ± 48.9 b | 49.2 ± 53.7 ab | 30.7 ± 42.6b | 76.5 ± 49.8 ab | 48.7 ± 58.9 ab | 111.6 ± 42.4 b | 21.6 ± 34.2 b | 17.4 ± 34.4 a | 898 | 850 |
| Styrene | 22.3 ± 43.8 a | 57.5 ± 158.2 ab | 165.5 ± 373.3 b | 39.5 ± 74.5 a | 901 | 895 | ||||||
| Nonane | 6.8 ± 24.6 a | 130.8 ± 211.1 b | 900 | 900 | ||||||||
| 3-Ethyl-1,5-octadiene | 858.9 ± 343.2 c | 430.9 ± 232.7 ab | 618.6 ± 160.0 bc | 379.2 ± 184.6 ab | 214.2 ± 175.7 a | 412.3 ± 200.2 ab | 265.4 ± 195.5 a | 595.4 ± 247.4 bc | 283.8 ± 84.7 a | 198.7 ± 148.4 a | 939 | 949 |
| Benzene, 1,3,5-trimethyl- | 27.9 ± 27.8 a | 53.9 ± 208.9 a | 978 | 976 | ||||||||
| 1,7-Nonadiene, 4,8-dimethyl | 569.4 ± 524.8 b | 330.7 ± 347.0 ab | 530.2 ± 121.4 b | 402.1 ± 170.3 ab | 139.4 ± 179.4 a | 345.5 ± 286.5 ab | 166.8 ± 221.3 a | 550.1 ± 273.4 b | 247.9 ± 78.7 ab | 154.3 ± 165.0 a | 998 | 1026 |
| Undecane | 186.5 ± 36.5 c | 118.3 ± 162.3 b | 1100 | 1100 | ||||||||
| (E)-4,8-Dimethyl-1,3,7-nonatriene | 546.1 ± 208.7 c | 404.9 ± 431.0 bc | 57.1 ± 84.4 a | 19.3 ± 43.5 a | 10.1 ± 27.4 a | 50.3 ± 56.7 a | 385.9 ± 336.9 bc | 203.2 ± 158.5 ab | 193.8 ± 120.2 ab | 1116 | - | |
| Dodecane | 120.1 ± 24.3 b | 12.5 ± 33.2 a | 1200 | 1200 | ||||||||
| Cyclodecane, methyl- | 276.2 ± 189.3 a | 229.0 ± 288.6 a | 1368.3 ± 896.3 c | 814.3 ± 471.2 b | 3451.8 ± 1527.7 d | 162.8 ± 182.4 a | 598.0 ± 439.9 ab | 274.7 ± 349.7 a | 881.4 ± 483.7 b | 73.7 ± 302.1 a | 1208 | 1202 |
| Total Hydrocarbons | 4069.1 | 6013.5 | 4068.4 | 15966.5 | 4464.2 | 1351.1 | 13006.4 | 2940.6 | 1596.5 | 1480.5 | ||
| Miscellaneous | ||||||||||||
| Ethyl ether | 87.4 ± 169.9 a | 11.1 ± 31.4 a | 89.9 ± 175.7 a | 43.6 ± 123.4 a | 76.7 ± 162.8 a | 39.2 ± 77.0 a | 27.7 ± 67.8 a | 97.6 ± 177.0 a | <500 | - | ||
| Hexane, 1-methoxy- | 1120.3 ± 600.6 c | 867.9 ± 432.2 b | 4.1 ± 15.9 a | 192.3 ± 206.0 a | 56.4 ± 138.1 a | 831 | 832 | |||||
| (Z)-3-Hexene, 1-methoxy- | 353.7 ± 342.8 b | 354.0 ± 283.0 b | 3.0 ± 11.6 a | 19.6 ± 55.5 a | 832 | 801 | ||||||
| Total Miscellaneous | 1561.4 | 1221.8 | 11.1 | 89.9 | 7.1 | 255.6 | 76.7 | 39.2 | 84.0 | 97.6 | ||
| Total Volatiles | 43,172.2 | 29,976.0 | 18,154.6 | 42,132.9 | 21,925.8 | 24,631.4 | 47,765.9 | 30,084.5 | 14,437.8 | 17,232.2 | ||
| Discriminant Function | Eigenvalue | Variance % | Cumulative % | Can. Correlation | Wilks’ Lambda | X2 | df | p < 0.05 |
|---|---|---|---|---|---|---|---|---|
| LDA | ||||||||
| 1 | 38.110 | 48.2 | 48.2 | 0.987 | 0.001 | 2146.769 | 495 | 0.000 |
| 2 | 15.276 | 19.3 | 67.6 | 0.969 | 0.001 | 1657.308 | 432 | 0.001 |
| 3 | 9.070 | 11.5 | 79.0 | 0.949 | 0.004 | 1284.882 | 371 | 0.001 |
| SLDA | ||||||||
| 1 | 10.900 | 33.8 | 33.8 | 0.957 | 0.001 | 1649.290 | 162 | 0.000 |
| 2 | 8.938 | 27.7 | 61.6 | 0.948 | 0.001 | 1272.862 | 136 | 0.000 |
| 3 | 4.778 | 14.8 | 76.4 | 0.909 | 0.002 | 923.820 | 112 | 0.000 |
| Step | Variables in the Analysis | F-Statistic | df1 | df2 | p |
|---|---|---|---|---|---|
| 1 | Dodecane | 98.135 | 9 | 157.000 | 0.001 |
| 2 | Hexane, 1-methoxy- | 69.985 | 18 | 312.000 | 0.000 |
| 3 | Cyclodecane, methyl- | 60.438 | 27 | 453.322 | 0.001 |
| 4 | Hexyl acetate | 55.047 | 36 | 578.847 | 0.000 |
| 5 | α-Copaene | 46.783 | 45 | 687.509 | 0.001 |
| 6 | Cyclopentane, 2-propenyl- | 40.908 | 54 | 779.645 | 0.001 |
| 7 | (E)-β-Ocimene | 36.183 | 63 | 856.548 | 0.001 |
| 8 | Nonanal | 33.016 | 72 | 919.994 | 0.000 |
| 9 | Ethanol | 30.783 | 81 | 971.886 | 0.001 |
| 10 | (E,Ε)-2,4-Heptadienal | 29.134 | 90 | 1014.043 | 0.000 |
| 11 | (E)-2-Hexenal | 25.685 | 108 | 1075.417 | 0.001 |
| 12 | (E)-2-Hexenol | 24.204 | 117 | 1097.196 | 0.001 |
| 13 | 3-Pentanone | 23.118 | 126 | 1114.392 | 0.000 |
| 14 | dl-Limonene | 22.118 | 135 | 1127.794 | 0.001 |
| 15 | 1-Penten-3-ol | 21.362 | 144 | 1138.046 | 0.000 |
| 16 | (E)-2-Pentenal | 20.647 | 153 | 1145.675 | 0.001 |
| 17 | 2-Propanone | 20.022 | 162 | 1151.106 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosma, I.S.; Kontominas, M.G.; Badeka, A.V. The Application of Chemometrics to Volatile Compound Analysis for the Recognition of Specific Markers for Cultivar Differentiation of Greek Virgin Olive Oil Samples. Foods 2020, 9, 1672. https://doi.org/10.3390/foods9111672
Kosma IS, Kontominas MG, Badeka AV. The Application of Chemometrics to Volatile Compound Analysis for the Recognition of Specific Markers for Cultivar Differentiation of Greek Virgin Olive Oil Samples. Foods. 2020; 9(11):1672. https://doi.org/10.3390/foods9111672
Chicago/Turabian StyleKosma, Ioanna S., Michael G. Kontominas, and Anastasia V. Badeka. 2020. "The Application of Chemometrics to Volatile Compound Analysis for the Recognition of Specific Markers for Cultivar Differentiation of Greek Virgin Olive Oil Samples" Foods 9, no. 11: 1672. https://doi.org/10.3390/foods9111672






