The Influence of Two-Component Mixtures from Spanish Origanum Oil with Spanish Marjoram Oil or Coriander Oil on Antilisterial Activity and Sensory Quality of a Fresh Cut Vegetable Mixture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GC-FID Analysis
2.3. Determination of the Minimal Inhibitory Concentration (MIC)
2.4. Microbiological Analyses
2.4.1. In Vegetable Filtrate
2.4.2. In a Mixture of Fresh Cut Vegetables
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of Essential Oils
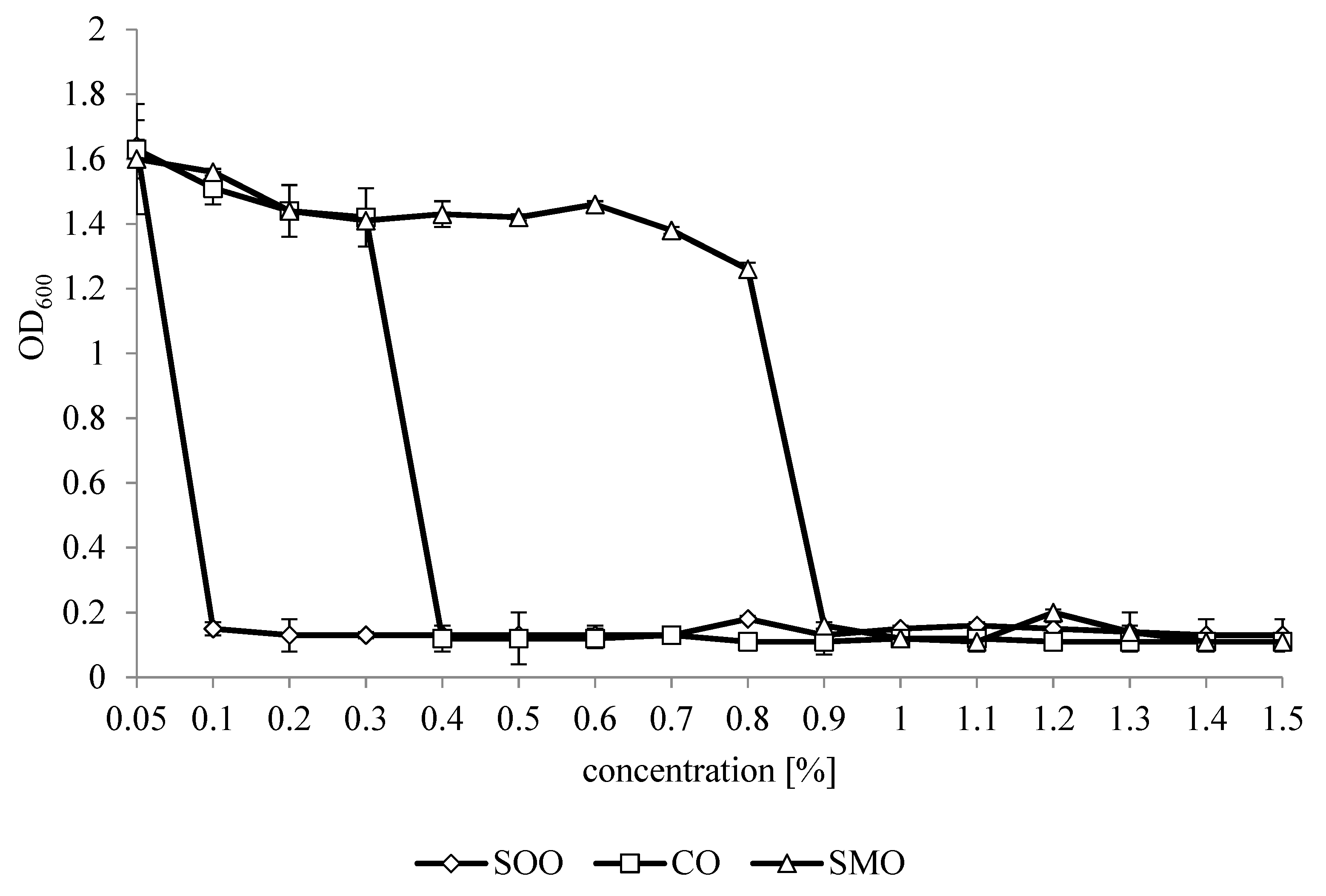
3.2. Inhibition of L. monocytogenes Growth by Essential Oils
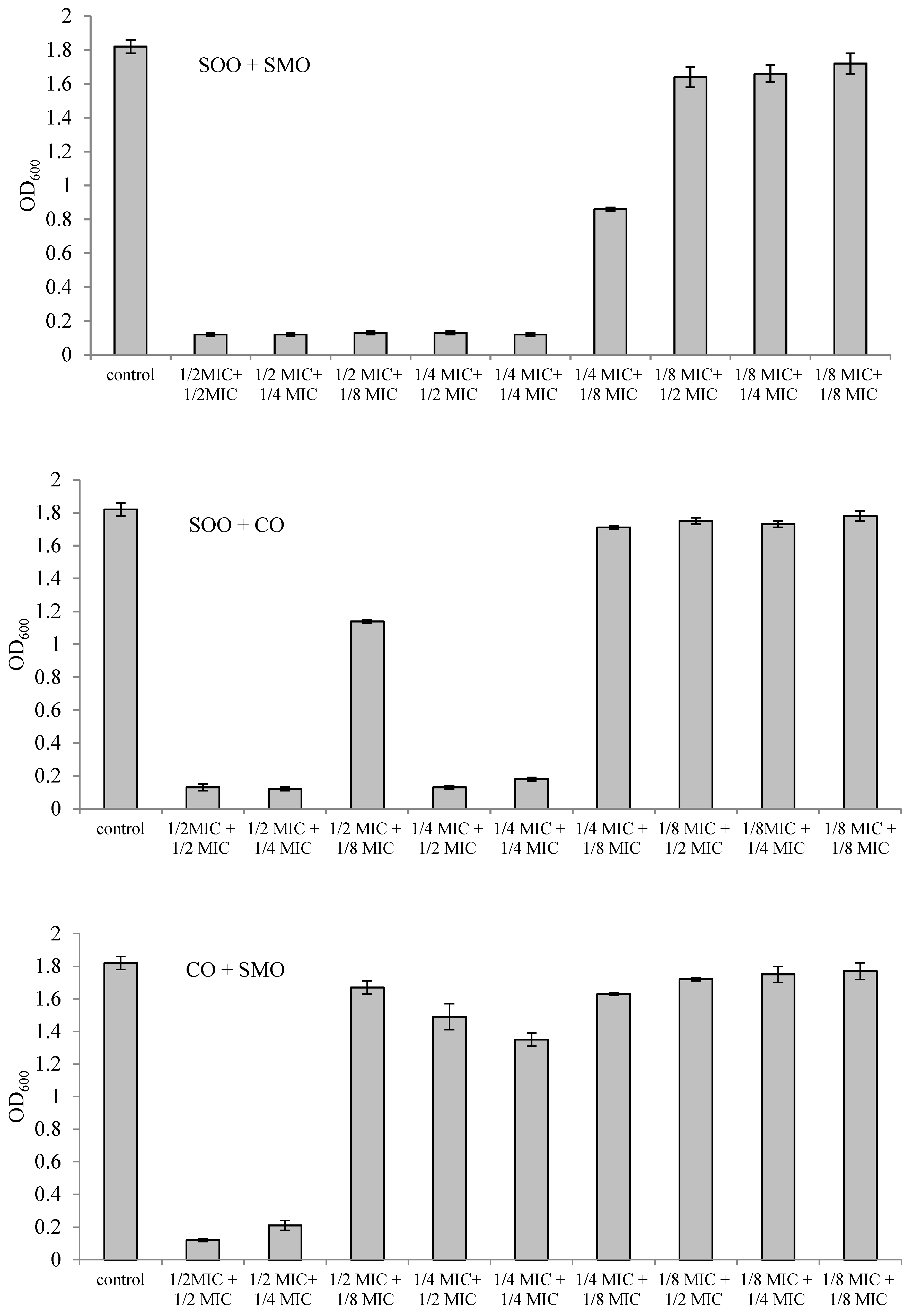
3.3. Inhibition of L. monocytogenes Growth by Mixtures of Essential Oils
3.4. Inactivation of L. monocytogenes in Vegetable Filtrate and Mixture of Fresh Cut Vegetables with EOs
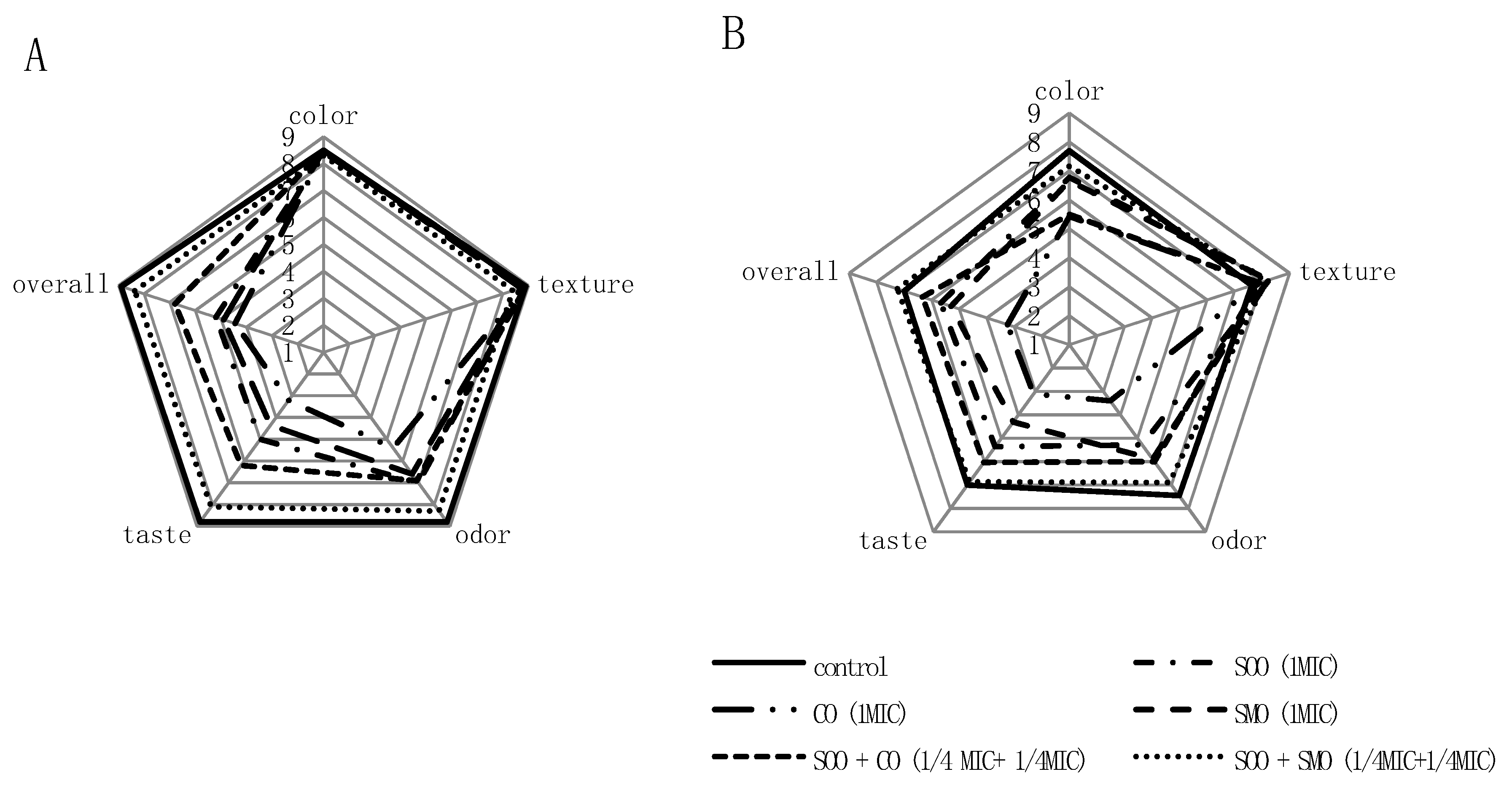
3.5. Sensory Evaluation of a Mixture of Fresh Cut Vegetables Treated with EOs or EO Mixtures
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zoellner, C.; Ceres, K.; Ghezzi-Kopel, K.; Wiedmann, M.; Ivanek, R. Design elements of Listeria environmental monitoring programs in food processing facilities: A scoping review of research and guidance materials. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1156–1171. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union one health 2018 zoonoses report. EFSA J. 2019, 17, 5926. [Google Scholar] [CrossRef]
- Oliveira, M.; Rodrigues, C.M.; Teixeira, P. Microbiological quality of raw berries and their products: A focus on foodborne pathogens. Heliyon 2019, 5, e02992. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, P.; Rodríguez-Herrera, J.J.; Vázquez-Sánchez, D.; López Cabo, M. Current knowledge on Listeria monocytogenes biofilms in food-related environments: Incidence, resistance to biocides, ecology and biocontrol. Foods 2018, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose–response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Balali, G.I.; Yar, D.D.; Dela, V.G.A.; Adjei-Kusi, P. Microbial contamination, an increasing treat to the consumption of fresh fruits and vegetables in today’s world. Int. J. Microbiol. 2020, 2020, 3029295. [Google Scholar] [CrossRef]
- Tucci Tucci, P.; Centorotola, G.; Salini, R.; Iannetti, L.; Sperandii, F.; D’Alterio, N.; Migliorati, G.; Pomilio, F. Challenge test studies on Listeria monocytogenes in ready-to-eat iceberg lettuce. Food Sci. Nutr. 2019, 7, 3845–3852. [Google Scholar] [CrossRef]
- Pignata, G.; Ertani, A.; Casale, M.; Piano, S.; Nicola, S. Mixing fresh-cut baby green and red leaf lettuce from soilless cultivation preserves phytochemical content and safety. Agric. Food Sci. 2020, 29, 55–65. [Google Scholar] [CrossRef]
- Nousiainen, L.L.; Joutsen, S.; Lunden, J.; Hänninen, M.-L.; Fredriksson-Ahomaa, M. Bacterial quality and safety of packaged fresh leafy vegetables at the retail level in Finland. Int. J. Food Microbiol. 2016, 232, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. 2015. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.F.; de Souza Santos, P.; Schmidt, C.A.; de Bittencourt, T.C.C.; Guimarães, A.G. Antimicrobial activity of marjoram (Origanum majorana) essential oil against the multidrug-resistant Salmonella enterica serovar Schwarzengrund inoculated in vegetables from organic farming. J. Food Saf. 2016, 36, 489–496. [Google Scholar] [CrossRef]
- Todd, J.; Friedman, M.; Patel, J.; Jaroni, D.; Ravishankar, S. The antimicrobial effects of cinnamon leaf oil against multi-drug resistant Salmonella Newport on organic leafy greens. Int. J. Food Microbiol. 2013, 166, 193–199. [Google Scholar] [CrossRef]
- Hyun, J.-E.; Bae, J.-M.; Song, H.; Yoon, J.-H.; Lee, S.-Y. Antibacterial effect of various essential oils against pathogens and spoilage microorganisms in fresh produce. J. Food Saf. 2015, 35, 206–219. [Google Scholar] [CrossRef]
- Bouayad Alam, S.; Dib, M.E.A.; Djabou, N.; Tabti, B.; Gaouar Benyelles, N.; Costa, J.; Muselli, A. Essential oils as biocides for the control of fungal infections and devastating pest (Tuta absoluta) of tomato (Lycopersicon esculentum Mill.). Chem. Biodivers 2017, 14, e1700065. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Gonzáles-Aguilar, G.A.; del-Toro-Sánchez, L. Enhancing safety and aroma appealing of fresh-cut fruits and vegetables using the antimicrobial and aromatic power of essential oils. J. Food Sci. 2009, 74, R84–R91. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Doern, C.D. When Does 2 Plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.P.P.; Campelo, T.A.; Frota, C.C. Evaluation of the antimicrobial activity Cinnamomum zeylanicum essential oil and trans-cinnamaldehyde against resistant Mycobacterium tuberculosis. Biosci. J. 2019, 35, 296–306. [Google Scholar] [CrossRef]
- Baryłko-Pikielna, N.; Matuszewska, I. Sensoryczne Badania Żywności. Podstawy. Metody. Zastosowania, 2nd ed.; Wyd. Naukowe PTTŻ: Cracow, Poland, 2014. [Google Scholar]
- Ibáñez, M.D.; Blázquez, M.A. Herbicidal value of essential oils from oregano-like flavour species. Food Agric. Immunol. 2017, 28, 1168–1180. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruíz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Chemical composition of the essential oils obtained from some spices widely used in Mediterranean region. Acta Chim. Slov. 2007, 54, 921–926. [Google Scholar]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2019, 60, 3042–3053. [Google Scholar] [CrossRef]
- Tserennadmid, R.; Takó, M.; Galgóczy, L.; Papp, T.; Pesti, M.; Vágvölgyi, C.; Almássy, K.; Krisch, J. Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int. J. Food Microbiol. 2011, 144, 480–486. [Google Scholar] [CrossRef]
- van Vuuren, S.F.; Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Fragr. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Yamazaki, K.; Yamamoto, T.; Kawai, Y.; Inoue, N. Enhancement of antilisterial activity of essential oil constituents by nisin and diglycerol fatty acid ester. Food Microbiol. 2004, 21, 283–289. [Google Scholar] [CrossRef]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. radiata. Food Control 2008, 19, 346–352. [Google Scholar] [CrossRef]
- Son, H.J.; Kang, J.H.; Song, K.B. Antimicrobial activity of safflower seed meal extract and its application as an antimicrobial agent for the inactivation of Listeria monocytogenes inoculated on fresh lettuce. LWT 2017, 85, 52–57. [Google Scholar] [CrossRef]
- de Azeredo, G.A.; Stamford, T.L.M.; Nunes, P.C.; Neto, N.J.G.; de Oliveira, M.E.G.; de Souza, E.L. Combined application of essential oils from Origanum vulgare L. and Rosmarinus officinalis L. to inhibit bacteria and autochthonous microflora associated with minimally processed vegetables. Food Res. Int. 2011, 44, 1541–1548. [Google Scholar] [CrossRef]
- Gutierrez, J.; Bourke, P.; Lonchamp, J.; Barry-Ryan, C. Impact of plant essential oils on microbiological, organoleptic and quality parameters of minimally processed vegetables. Innov. Food Sci. Emerg. Technol. 2009, 10, 195–202. [Google Scholar] [CrossRef]
| Sub-Inhibited Concentration | Mixture of EOs | ||
|---|---|---|---|
| SOO + SMO | SOO + CO | CO + SMO | |
| (%) | |||
| ½ MIC + ½ MIC | 0.05 + 0.45 | 0.05 + 0.2 | 0.2 + 0.45 |
| ½ MIC + ¼ MIC | 0.05 + 0.225 | 0.05 + 0.1 | 0.2 + 0.225 |
| ½ MIC + ⅛ MIC | 0.05 + 0.1125 | 0.05 + 0.05 | 0.2 + 0.1125 |
| ¼ MIC + ½ MIC | 0.025 + 0.45 | 0.025 + 0.2 | 0.1 + 0.45 |
| ¼ MIC + ¼ MIC | 0.025 + 0.225 | 0.025 + 0.1 | 0.1 + 0.225 |
| ¼ MIC + ⅛ MIC | 0.025 + 0.1125 | 0.025 + 0.05 | 0.1 + 0.1125 |
| ⅛ MIC + ½ MIC | 0.0125 + 0.45 | 0.0125 + 0.2 | 0.05 + 0.45 |
| ⅛ MIC + ¼ MIC | 0.0125 + 0.225 | 0.0125 + 0.1 | 0.05 + 0.225 |
| ⅛ MIC + ⅛ MIC | 0.0125 + 0.1125 | 0.0125 + 0.05 | 0.05 + 0.1125 |
| No | SOO | CO | SMO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | RI | % | Compound | RI | % | Compound | RI | % | |
| 1 | α-Pinene | 1028 | 0.54 ± 0.15 | α-Pinene | 1028 | 6.66 ± 0.16 | α-Pinene | 1028 | 3.08 ± 0.04 |
| 2 | β-Myrcene | 1168 | 1.11± 0.00 | Camphene | 1075 | 1.17 ± 0.02 | Camphene | 1075 | 1.35 ± 0.01 |
| 3 | α-Terpinene | 1182 | 0.97 ± 0.01 | β-Pinene | 1113 | 0.45 ± 0.00 | β-Pinene | 1113 | 4.73 ± 0.02 |
| 4 | γ-Terpinene | 1249 | 3.44 ± 0.01 | Limonene | 1203 | 2.62 ± 0.04 | Sabinene | 1124 | 3.95 ± 0.01 |
| 5 | p-Cymene | 1272 | 4.01 ± 0.01 | γ-Terpinene | 1248 | 0.84 ± 0.01 | Limonene | 1203 | 4.09 ± 0.01 |
| 6 | Linalool | 1541 | 4.14 ± 0.00 | p-Cymene | 1272 | 6.14 ± 0.09 | 1,8-Cineole | 1209 | 69.15 ± 0.17 |
| 7 | Bornyl acetate | 1580 | 0.87 ± 0.03 | Geraniol | 1436 | 1.13 ± 0.01 | p-Cymene | 1272 | 3.34 ± 0.02 |
| 8 | β-caryophyllene | 1593 | 1.10 ± 0.01 | Citronellal | 1482 | 0.96 ± 0.03 | Camphor | 1509 | 1.21 ± 0.01 |
| 9 | α-Terpineol | 1696 | 0.77 ± 0.06 | unidentified | 0.81 ± 0.01 | Linalool | 1541 | 1.70 ± 0.01 | |
| 10 | Geranial | 1730 | 2.79 ± 0.01 | unidentified | 4.79 ± 0.02 | Terpinen-4-ol | 1584 | 0.41 ± 0.008 | |
| 11 | Thymol | 2165 | 0.69 ± 0.10 | Linalool | 1541 | 68.45 ± 0.27 | β-Caryophyllene | 1593 | 0.63 ± 0.008 |
| 12 | Carvacrol | 2213 | 79.11 ± 0.64 | unidentified | 0.41 ± 0.01 | α-Terpineol | 1696 | 3.81 ± 0.08 | |
| 13 | Geranyl acetate | 1751 | 2.77 ± 0.06 | Borneol | 1699 | 1.39 ± 0.01 | |||
| 14 | Cirtronellol | 1767 | 0.48 ± 0.02 | Caryophyllene oxide | 1955 | 0.67 ± 0.02 | |||
| 15 | unidentified | 1.13 ± 0.04 | |||||||
| Total identified | 99.54 ± 0.56 | Total identified | 98.82 ± 0.58 | Total identified | 99.52 ± 0.28 | ||||
| Mixture of Essential Oil | ½ MIC + ½ MIC | ½ MIC + ¼ MIC | ½ MIC + ⅛ MIC | ¼ MIC + ½ MIC | ¼ MIC + ¼ MIC |
|---|---|---|---|---|---|
| SOO + SMO | 1(I) | 0.75(A) | 0.625(A) | 0.75(A) | 0.5(S) |
| SOO + CO | 1(I) | 0.75(A) | - | 0.75(A) | 0.5(S) |
| CO + SMO | 1(I) | - | - | 0.75(A) | - |
| Time [h] | Control | SOO 1MIC: 0.1% | CO 1MIC: 0.4% | SMO 1MIC: 0.9% | ¼ MIC SOO + ¼ MIC CO | ¼ MIC SOO + ¼ MIC SMO |
|---|---|---|---|---|---|---|
| Vegetable filtrate (log10 CFU × mL−1) | ||||||
| 0 | 5.8 ± 0.4 a | 5.9 ± 0.3 a | 5.9 ± 0.1 a | 6.1 ± 0.1 a | 5.9 ± 0.1 a | 6.1 ± 0.2 a |
| 4 | 6.0 ± 0.1 e | <1.00 a | 2.4 ± 0.3 c | 3.8 ± 0.2 d | 1.8 ± 0.1 b | 2.7 ± 0.3 c |
| 6 | 6.5 ± 0.3 e | <1.00 a | 2.2 ± 0.2 c | 3.6 ± 0.1 d | 1.5 ± 0.1 b | 2.1 ± 0.1 c |
| 12 | 6.7 ± 0.1 c | <1.00 a | 2.0 ± 0.1 b | 2.3 ± 0.1 b | < 1.00 a | 1.9 ±0.1 b |
| 24 | 8.7 ± 0.2 b | <1.00 a | <1.00 a | <1.00 a | <1.00 a | <1.00 a |
| Fresh vegetable (log10 CFU × g) | ||||||
| 0.5 | 6.3 ± 0.2 e | <1.00 a | 3.8 ± 0.2 c | 4.5 ± 0.2 d | 3.0 ± 0.2 b | 3.7 ± 0.2 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraśniewska, K.; Kosakowska, O.; Pobiega, K.; Gniewosz, M. The Influence of Two-Component Mixtures from Spanish Origanum Oil with Spanish Marjoram Oil or Coriander Oil on Antilisterial Activity and Sensory Quality of a Fresh Cut Vegetable Mixture. Foods 2020, 9, 1740. https://doi.org/10.3390/foods9121740
Kraśniewska K, Kosakowska O, Pobiega K, Gniewosz M. The Influence of Two-Component Mixtures from Spanish Origanum Oil with Spanish Marjoram Oil or Coriander Oil on Antilisterial Activity and Sensory Quality of a Fresh Cut Vegetable Mixture. Foods. 2020; 9(12):1740. https://doi.org/10.3390/foods9121740
Chicago/Turabian StyleKraśniewska, Karolina, Olga Kosakowska, Katarzyna Pobiega, and Małgorzata Gniewosz. 2020. "The Influence of Two-Component Mixtures from Spanish Origanum Oil with Spanish Marjoram Oil or Coriander Oil on Antilisterial Activity and Sensory Quality of a Fresh Cut Vegetable Mixture" Foods 9, no. 12: 1740. https://doi.org/10.3390/foods9121740
APA StyleKraśniewska, K., Kosakowska, O., Pobiega, K., & Gniewosz, M. (2020). The Influence of Two-Component Mixtures from Spanish Origanum Oil with Spanish Marjoram Oil or Coriander Oil on Antilisterial Activity and Sensory Quality of a Fresh Cut Vegetable Mixture. Foods, 9(12), 1740. https://doi.org/10.3390/foods9121740