Development of a Non-Destructive Method for Detection of the Juiciness of Pear via VIS/NIR Spectroscopy Combined with Chemometric Methods
Abstract
:1. Introduction
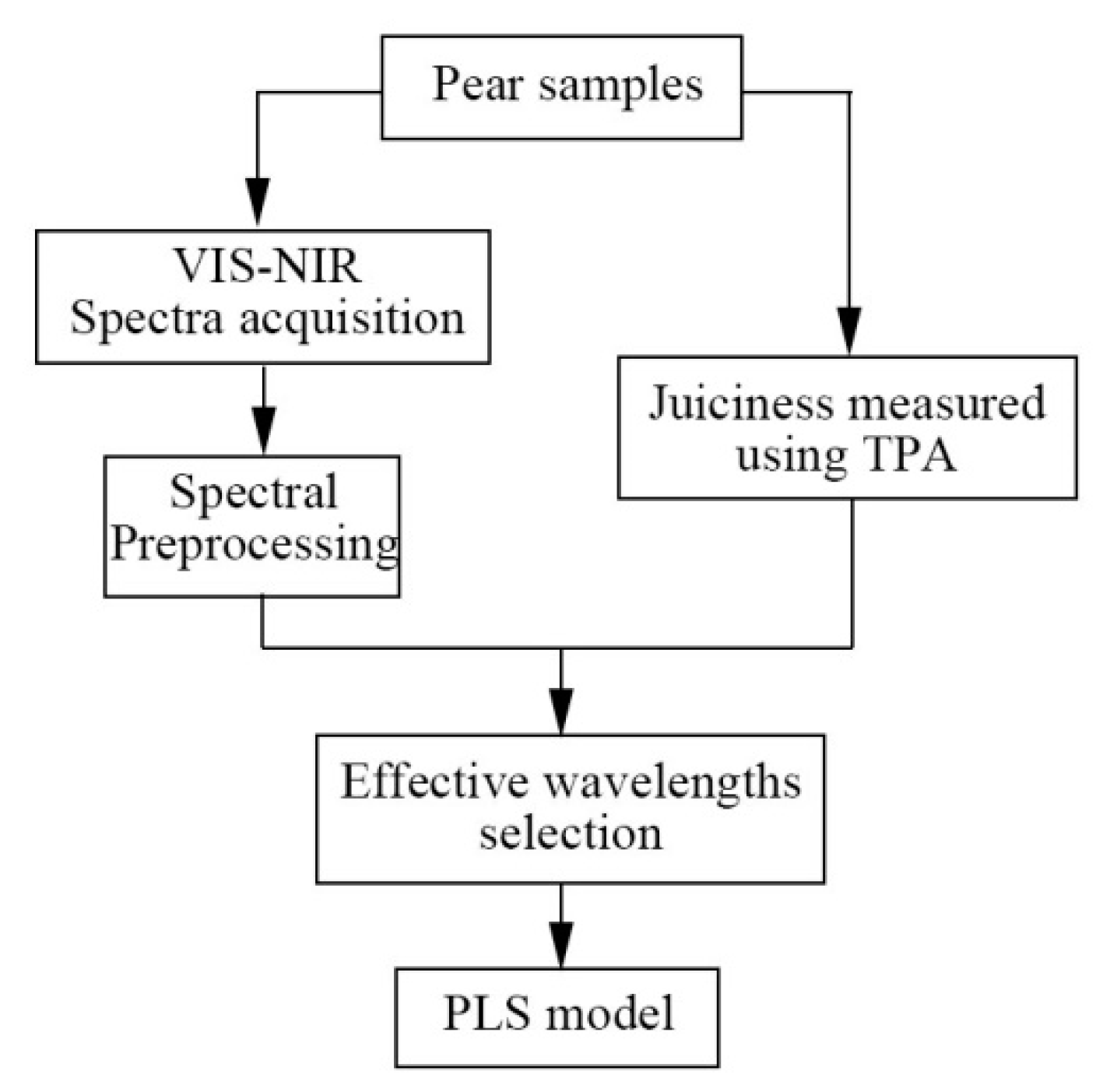
2. Materials and Methods
2.1. Sample Collection
2.2. Spectra Acquisition
2.3. Reference Measurements of Juiciness
2.4. Chemometrics and Data Analysis
3. Results
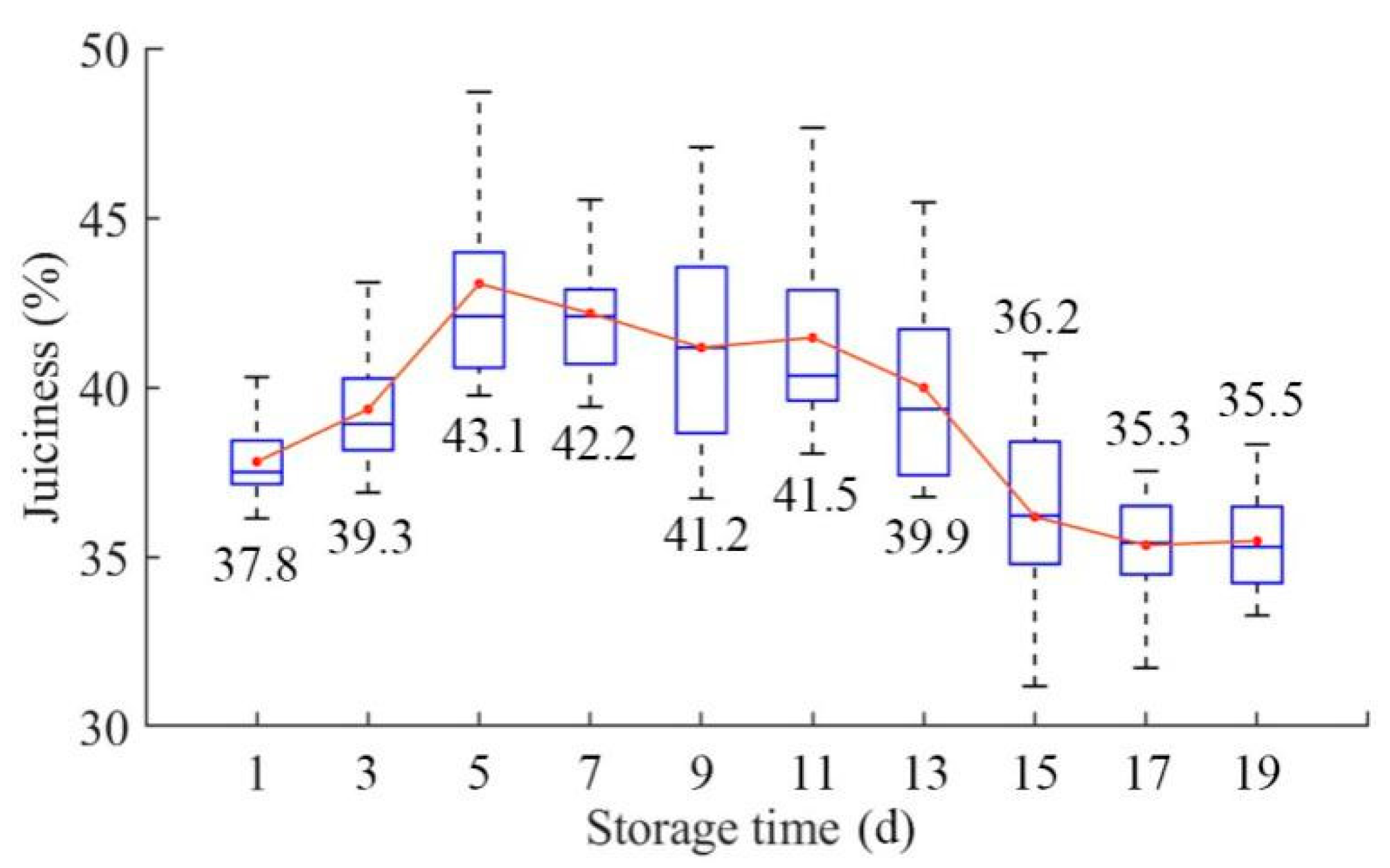
3.1. Juiciness Parameter Distributions
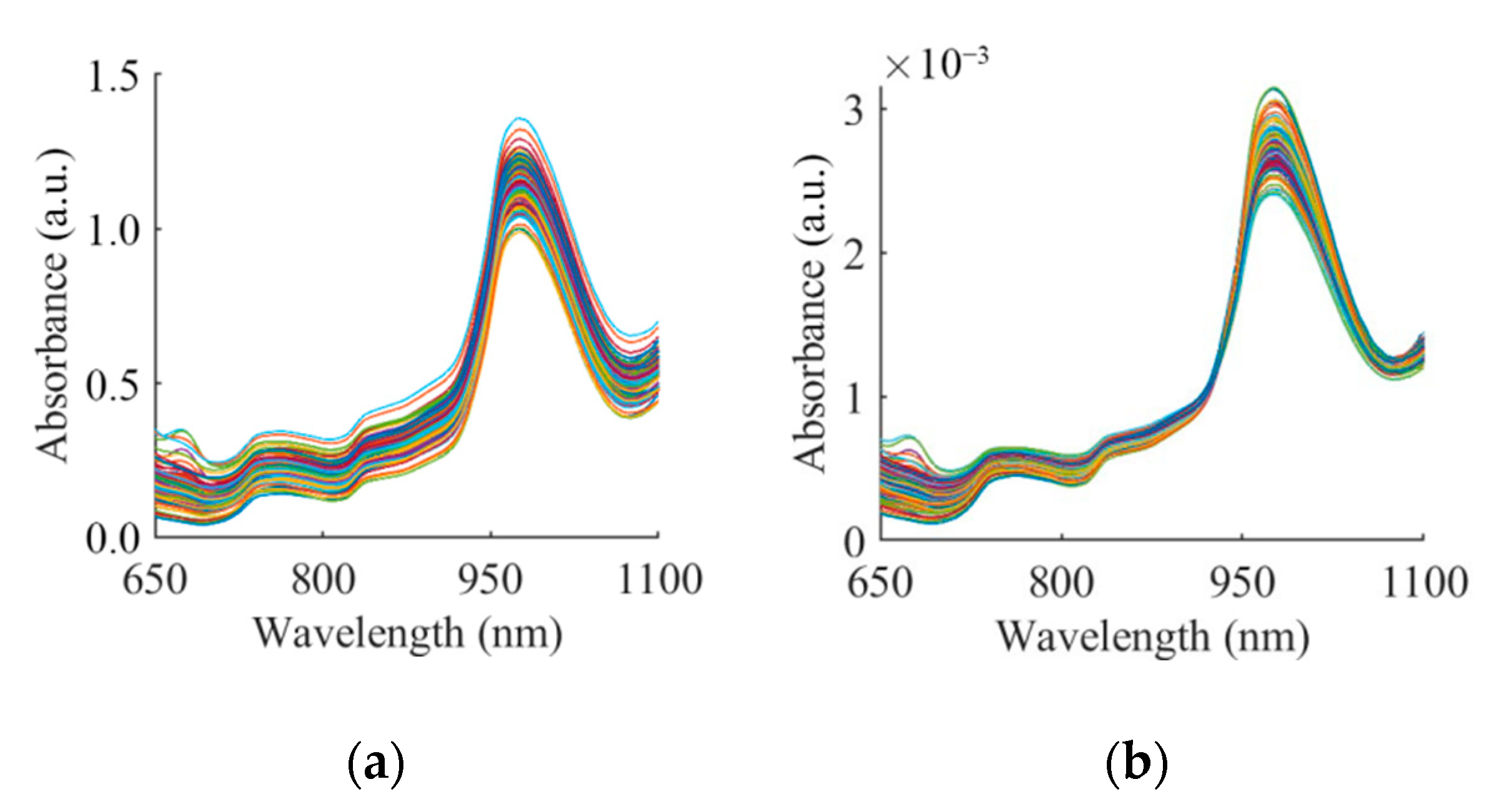
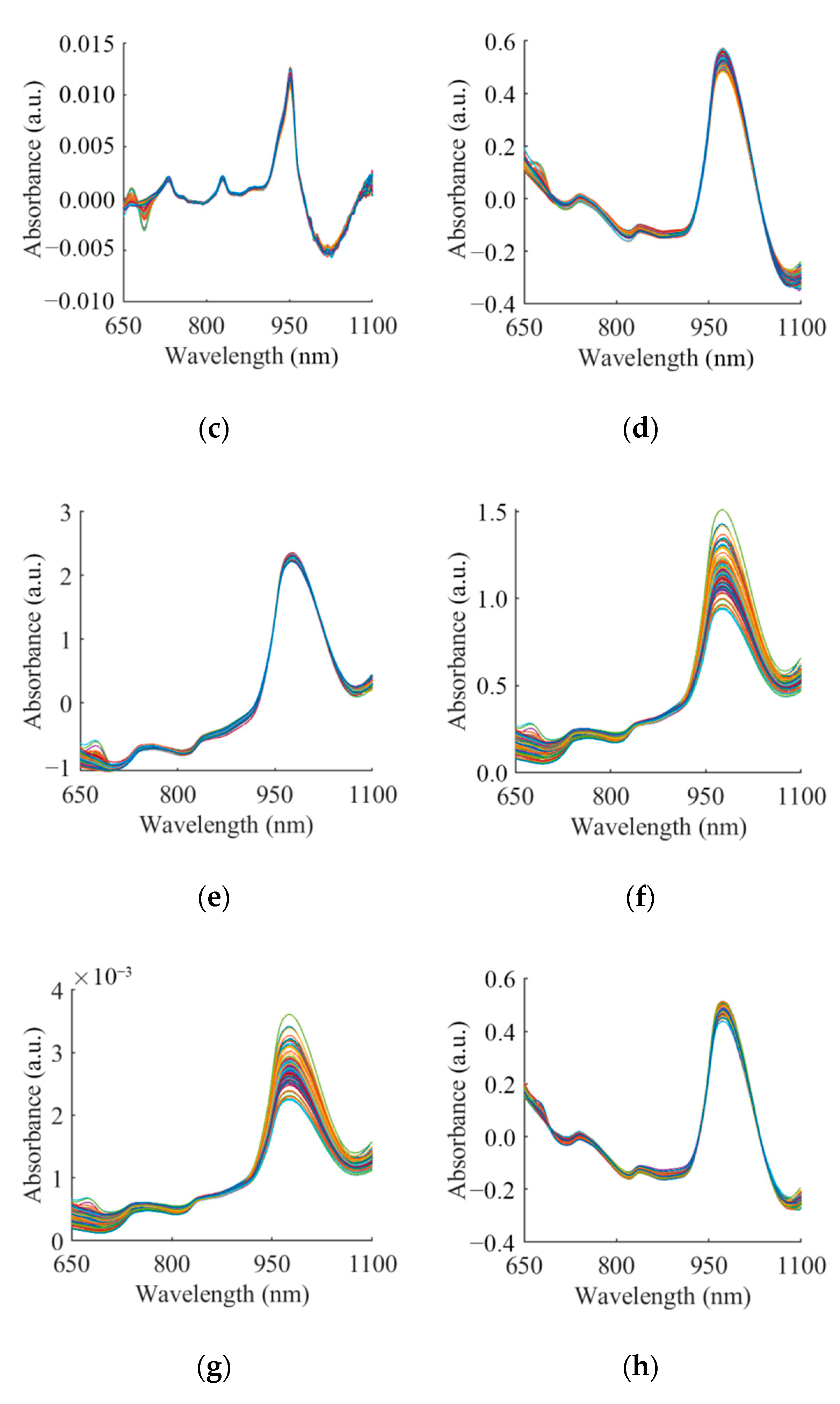
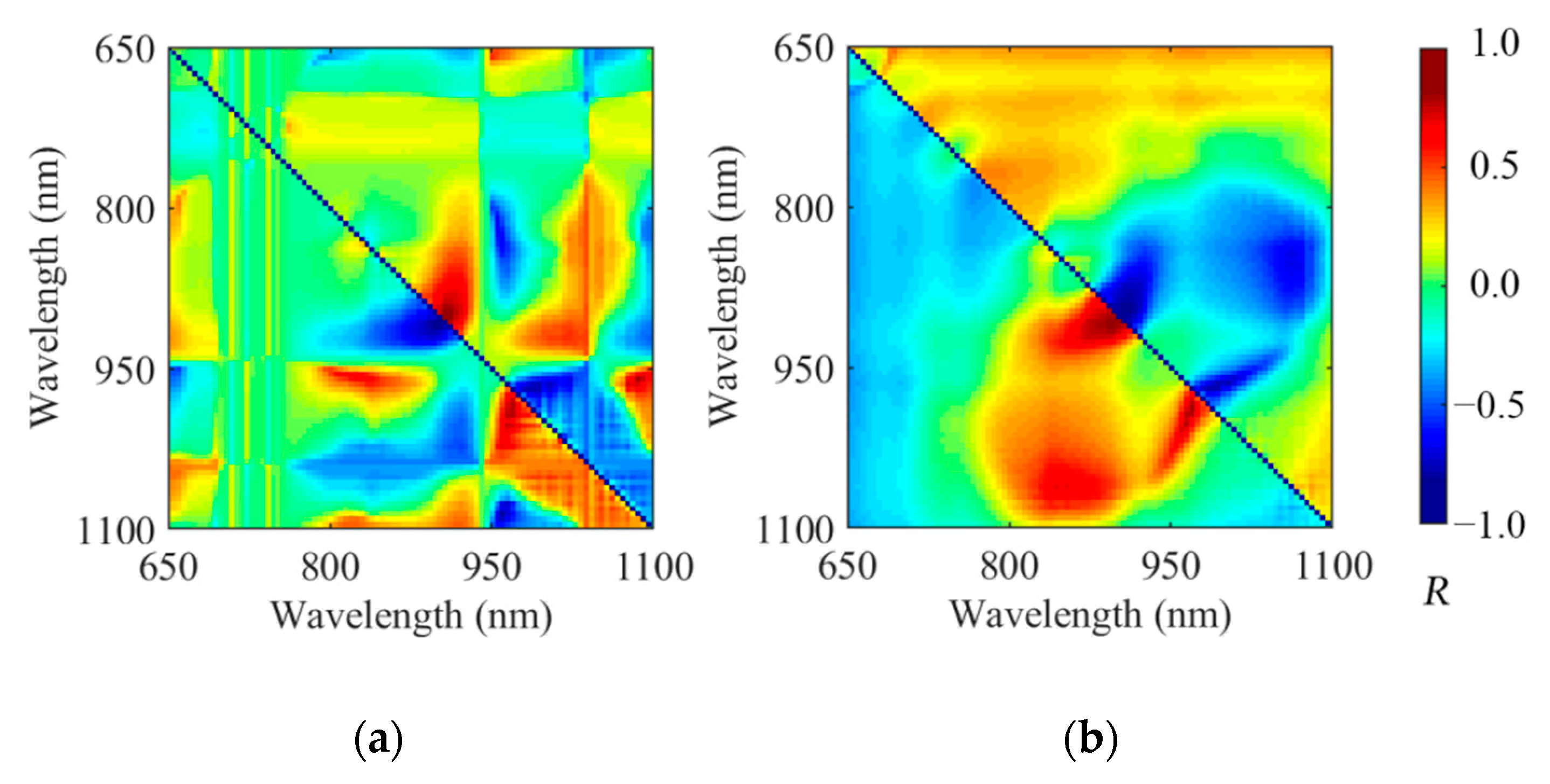
3.2. Spectra and Spectra Analysis
3.3. PLSR Models Based on the Characteristic Wavelength
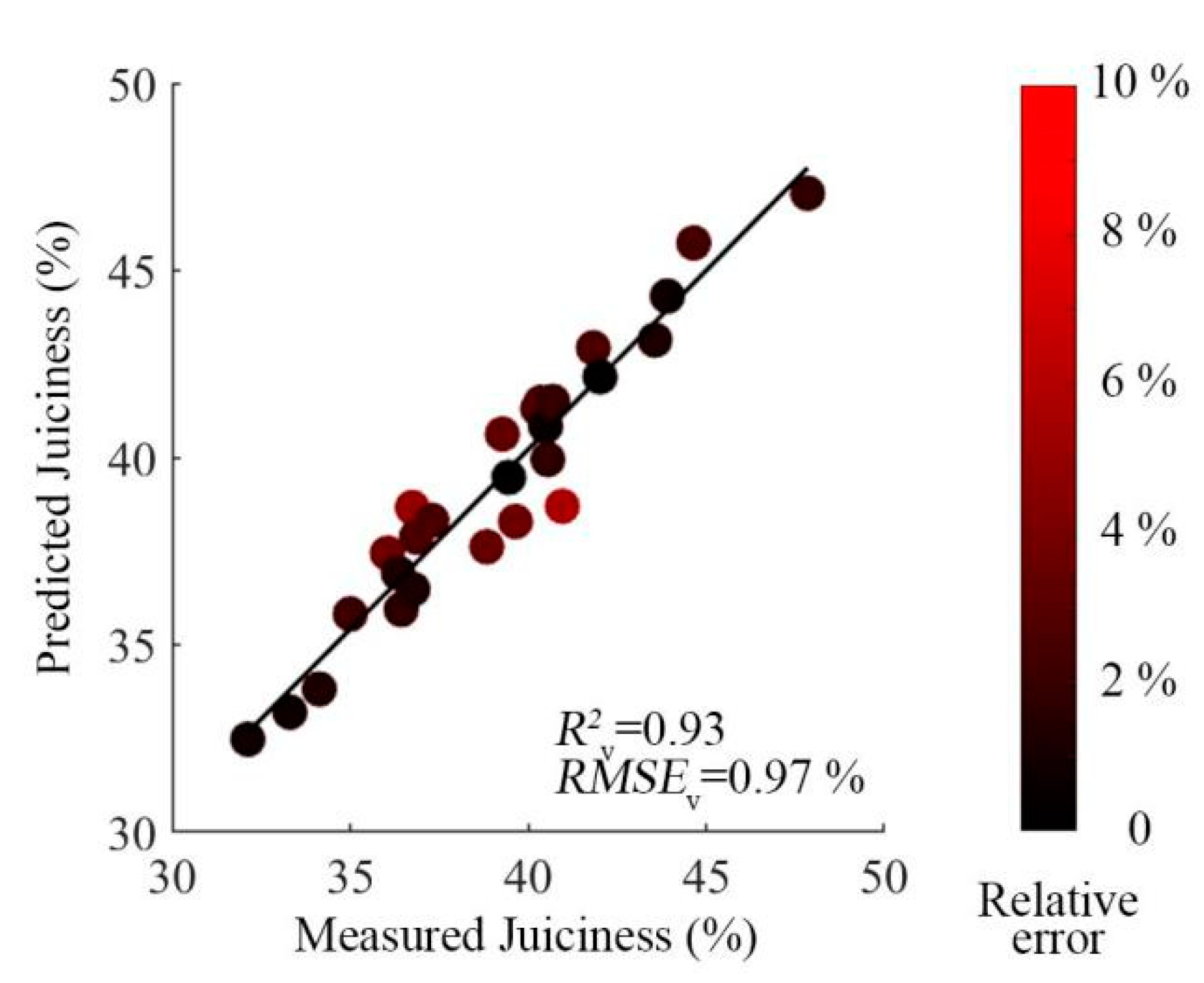
3.4. External Verification of the Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cliff, M.A.; Dever, M.C.; Hall, J.W.; Girard, B. Development and evaluation of multiple regression models for prediction of sweet cherry liking. Food Res. Int. 1995, 28, 583–589. [Google Scholar] [CrossRef]
- Mendoza, F.; Lu, R.; Cen, H. Grading of apples based on firmness and soluble solids content using Vis/SWNIR spectroscopy and spectral scattering techniques. J. Food Eng. 2014, 125, 59–68. [Google Scholar] [CrossRef]
- Kappel, F.; Fisherfleming, R.; Hogue, E.J. Ideal pear sensory attributes and fruit characteristic. HortScience 1995, 30, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Crouch, I.; Bergman, H. Consumer acceptance study of early marketed Forelle pears in the United Kingdom and Germany. SA Fruit J. 2012, 11, 64–71. [Google Scholar]
- Gallardo, R.K. Choice Experiments’ Findings: A Tool for Fruit Agribusiness Managers’ Decision Making. Int. Food Agribus. Manag. Rev. 2011, 14, 95–109. [Google Scholar]
- Steyn, W.J.; Manning, N.; Muller, M.; Human, J.P. Physical, Sensory and Consumer Analysis of Eating Quality and Appearance of Pear Genotypes among South African Consumers. Acta Hortic. 2011, 909, 579–586. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Lund, C.M.; Lau, K.; Harker, F.R. In search of the “Ideal” Pear (Pyrus spp.): Results of a multidisciplinary exploration. J. Food Sci. 2003, 68, 1108–1117. [Google Scholar] [CrossRef]
- Turner, J.; Bai, J.H.; Marin, A.; Colonna, A. Consumer sensory evaluation of pear cultivars in the Pacific Northwest, USA. Acta Hortic. 2005, 671, 355–360. [Google Scholar] [CrossRef]
- Blanckenberg, A.; Muller, M.; Theron, K.I.; Crouch, E.M.; Steyn, W.J. Harvest maturity and ripeness differentially affects consumer preference of ‘Forelle’, Packham’s Triumph’ and ‘Abate Fetel’ pears (Pyrus communis L.). Sci. Hortic. 2016, 207, 131–139. [Google Scholar] [CrossRef]
- Cano-Salazar, J.; Lopez, L.; Crisosto, C.H.; Echeverria, G. Cold storage of six nectarine cultivars: Consequences for volatile compounds emissions, physicochemical parameters, and consumer acceptance. Eur. Food Res. Technol. 2013, 237, 571–589. [Google Scholar] [CrossRef]
- Delgado, C.; Crisosto, G.M.; Heymann, H.; Crisosto, C.H. Determining the Primary Drivers of Liking to Predict Consumers’ Acceptance of Fresh Nectarines and Peaches. J. Food Sci. 2013, 78, S605–S614. [Google Scholar] [CrossRef] [PubMed]
- Iwanami, H.; Moriya, S.; Okada, K.; Abe, K.; Kawamorita, M.; Sasaki, M.; Moriya-Tanaka, Y.; Honda, C.; Hanada, T.; Wada, M. Instrumental measurements of juiciness and freshness to sell apples with a premium value. Sci. Hortic. 2017, 214, 66–75. [Google Scholar] [CrossRef]
- Juhnevica, K.; Skudra, L.; Skrivele, M.; Radenkovs, V.; Seglina, D.; Stepanovs, A. Effect of 1-methylcyclopropene treatment on sensory characteristics of apple fruit. Environ. Exp. Bot. 2013, 11, 99–105. [Google Scholar]
- Raffo, M.D.; Candan, A.P.; Angelis, V.d.; Manueco, L.; Miranda, M.J.; Barda, N. Sensory evaluation of pears: A useful tool to detect changes in eating quality during ripening. Acta Hortic. 2011, 909, 651–656. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Fu, M. Postharvest low temperature conditioning reduces peel browning and improves fruit quality in storage and subsequent shelf life of Huangguan pear. Food Sci. Nutr. 2015, 6, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Makkumrai, W.; Anthon, G.E.; Sivertsen, H.; Ebeler, S.E.; Negre-Zakharov, F.; Barrett, D.M.; Mitcham, E.J. Effect of ethylene and temperature conditioning on sensory attributes and chemical composition of ‘Bartlett’ pears. Postharvest Biol. Technol. 2014, 97, 44–61. [Google Scholar] [CrossRef]
- Vanoli, M.; Rizzolo, A.; Grassi, M. Fruit Quality and Sensory Characteristics of 1-MCP Treated ‘Abbe Fetel’ Pears after Storage under Dynamic Controlled Atmosphere at Different Temperatures. Acta Hortic. 2015, 1071, 437–445. [Google Scholar] [CrossRef]
- Cano-Salazar, J.; Lopez, M.L.; Echeverria, G. Relationships between the instrumental and sensory characteristics of four peach and nectarine cultivars stored under air and CA atmospheres. Postharvest Biol. Technol. 2013, 75, 58–67. [Google Scholar] [CrossRef]
- Harker, F.R.; Amos, R.L.; Echeverria, G.; Gunson, F.A. Influence of texture on taste: Insights gained during studies of hardness, juiciness, and sweetness of apple fruit. J. Food Sci. 2006, 71, S77–S82. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Rega, P.; Migliozzi, T.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Effect of cold storage and shelf life on physiological and quality traits of early ripening pear cultivars. Sci. Hortic. 2013, 162, 341–350. [Google Scholar] [CrossRef]
- ISO 11036:1994. Sensory Analysis—Methodology—Texture Profile; International Organization for Standardization: Geneva, Switzerland, 1994. [Google Scholar]
- Contador, L.; Diaz, M.; Hernandez, E.; Shinya, P.; Infante, R. The relationship between instrumental tests and sensory determinations of peach and nectarine texture. Eur. J. Hortic. Sci. 2016, 81, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Harker, F.R.; Maindonald, J.; Murray, S.H.; Gunson, F.A.; Hallett, I.C.; Walker, S.B. Sensory interpretation of instrumental measurements 1: Texture of apple fruit. Postharvest Biol. Technol. 2002, 24, 225–239. [Google Scholar] [CrossRef]
- Peleg, M.; Brito, L.G.; Malevski, Y. Compressive failure patterns of some juicy fruits. J. Food Sci. 1976, 41, 1320–1324. [Google Scholar] [CrossRef]
- Abasi, S.; Minaei, S.; Jamshidi, B.; Fathi, D. Dedicated non-destructive devices for food quality measurement: A review. Trends Food Sci. Technol. 2018, 78, 197–205. [Google Scholar] [CrossRef]
- Fan, S.; Huang, W.; Guo, Z.; Zhang, B.; Zhao, C. Prediction of Soluble Solids Content and Firmness of Pears Using Hyperspectral Reflectance Imaging. Food Anal. Methods 2015, 8, 1936–1946. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xu, L.; Tian, X.; Xia, Y.; Fan, S. Comparison and Optimization of Models for Determination of Sugar Content in Pear by Portable Vis-NIR Spectroscopy Coupled with Wavelength Selection Algorithm. Food Anal. Methods 2019, 12, 12–22. [Google Scholar] [CrossRef]
- Arefi, A.; Moghaddam, P.A.; Hassanpour, A.; Mollazade, K.; Motlagh, A.M. Non-destructive identification of mealy apples using biospeckle imaging. Postharvest Biol. Technol. 2016, 112, 266–276. [Google Scholar] [CrossRef]
- Mehinagic, E.; Royer, G.; Bertrand, D.; Symoneaux, R.; Laurens, F.; Jourjon, F. Relationship between sensory analysis, penetrometry and visible-NIR spectroscopy of apples belonging to different cultivars. Food Qual. Prefer. 2003, 14, 473–484. [Google Scholar] [CrossRef]
- Watada, A.E.; Massie, D.R.; Abbott, J.A. Relationship between sensory evaluations and nondestructive optical measurements or apple quality. J. Food Qual. 1985, 7, 219–226. [Google Scholar] [CrossRef]
- Baltazar, P.; Cristina Correa, E.; Diezma, B. Instrumental Procedures for the Evaluation of Juiciness in Peach and Nectarine Cultivars for Fresh Consumption. Agronomy 2020, 10, 152. [Google Scholar] [CrossRef] [Green Version]
- Chauvin, M.A.; Ross, C.F.; Pitts, M.; Kupferman, E.; Swanson, B. Relationship between instrumental and sensory determination of apple and pear texture. J. Food Qual. 2010, 33, 181–198. [Google Scholar] [CrossRef]
- Kaur, K.; Dhillon, W.S.; Mahajan, B.V.C. Changes in pectin methyl esterase activity with different packaging materials and stages of fruit harvesting during cold storage of pear cv. Punjab beauty. J. Food Sci. Technol. 2014, 51, 2867–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulme, A.C. The Biochemistry of Fruits and Their Products; Academic Press: London, UK; New York, NY, USA, 1970. [Google Scholar]
- McGlone, V.A.; Jordan, R.B.; Martinsen, P.J. Vis/NIR estimation at harvest of pre- and post-storage quality indices for ‘Royal Gala’ apple. Postharvest Biol. Technol. 2002, 25, 135–144. [Google Scholar] [CrossRef]
- Van Beers, R.; Aernouts, B.; Gutierrez, L.L.; Erkinbaev, C.; Rutten, K.; Schenk, A.; Nicolai, B.; Saeys, W. Optimal Illumination-Detection Distance and Detector Size for Predicting Braeburn Apple Maturity from Vis/NIR Laser Reflectance Measurements. Food Bioproc. Tech. 2015, 8, 2123–2136. [Google Scholar] [CrossRef] [Green Version]
- Infante, R. Harvest maturity indicators in the stone fruit industry. Stewart Postharvest Rev. 2012, 8, 1–6. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Li, Y.; Wang, F. A new scattering correction method of different spectroscopic analysis for assessing complex mixtures. Anal. Chim. Acta 2019, 1087, 20–28. [Google Scholar] [CrossRef]
- Geladi, P.; Macdougall, D.; Martens, H. Linearization and scatter-correction for near-infrared reflectance spectra of meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in H-1 NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Chen, H.; Tang, G.; Song, Q.; Ai, W. Combination of Modified Optical Path Length Estimation and Correction and Moving Window Partial Least Squares to Waveband Selection for the Fourier Transform Near-Infrared Determination of Pectin in Shaddock Peel. Anal. Lett. 2013, 46, 2060–2074. [Google Scholar] [CrossRef]
- Balabin, R.M.; Smirnov, S.V. Variable selection in near-infrared spectroscopy: Benchmarking of feature selection methods on biodiesel data. Anal. Chim. Acta 2011, 692, 63–72. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal. Chim. Acta 2009, 648, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Cheng, F.; Shi, M. Rapid Identification and Visualization of Jowl Meat Adulteration in Pork Using Hyperspectral Imaging. Foods 2020, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Jiang, X.; Ru, Y.; Wang, J.; Zhou, H. Application of hyperspectral imaging for detecting and visualizing leaf lard adulteration in minced pork. Infrared Phys. Technol. 2020, 103467. [Google Scholar] [CrossRef]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemometr. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Dhakal, S.; Schmidt, W.F.; Kim, M.; Tang, X.; Peng, Y.; Chao, K. Detection of Additives and Chemical Contaminants in Turmeric Powder Using FT-IR Spectroscopy. Foods 2019, 8, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohn, E.; Gasser, F.; Datwyler, D.; Jampen, M. Harvest, storage and ripening of pears. Agrarforschung 1996, 3, 369–372. [Google Scholar]
- Benarie, R.; Sonego, L. Pectolytic enzyme-activity involved in woolly breakdown of stored peaches. Phytochem 1980, 19, 2553–2555. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Yang, C.; Li, Y. Optical sensing system for detection of the internal and external quality attributes of apples. Postharvest Biol. Technol. 2020, 162. [Google Scholar] [CrossRef]
- Tian, X.; Li, J.; Wang, Q.; Fan, S.; Huang, W. A bi-layer model for nondestructive prediction of soluble solids content in apple based on reflectance spectra and peel pigments. Food Chem. 2018, 239, 1055–1063. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, J.; Povey, M.J.W.; Holmes, M.; Mao, H. Variables selection methods in near-infrared spectroscopy. Anal. Chim. Acta 2010, 667, 14–32. [Google Scholar] [CrossRef]
- Yu, X.; Lu, H.; Wu, D. Development of deep learning method for predicting firmness and soluble solid content of postharvest Korla fragrant pear using Vis/NIR hyperspectral reflectance imaging. Postharvest Biol. Technol. 2018, 141, 39–49. [Google Scholar] [CrossRef]
- Vangdal, E. Eating quality of pears. Acta Agric. Scand. 1982, 32, 135–139. [Google Scholar] [CrossRef]
- Ignat, T.; Lurie, S.; Nyasordzi, J.; Ostrovsky, V.; Egozi, H.; Hoffman, A.; Friedman, H.; Weksler, A.; Schmilovitch, Z. Forecast of Apple Internal Quality Indices at Harvest and During Storage by VIS-NIR Spectroscopy. Food Bioproc. Tech. 2014, 7, 2951–2961. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhan, B.; Wang, Z.; Jiang, Y. Determination of SSC in pears by establishing the multi-cultivar models based on visible-NIR spectroscopy. Infrared Phys. Technol. 2019, 102, 103066. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.; Xie, L. Influence of the peel on predicting soluble solids content of navel oranges using visible and near-infrared spectroscopy. Trans. ASABE 2016, 59, 31–37. [Google Scholar]
- Vaudelle, F.; L’Huillier, J.P. Influence of the size and skin thickness of apple varieties on the retrieval of internal optical properties using Vis/NIR spectroscopy: A Monte Carlo-based study. Comput. Electron. Agric. 2015, 116, 137–149. [Google Scholar] [CrossRef] [Green Version]



| Sets | Number of the Samples | Juiciness (%) | ||
|---|---|---|---|---|
| Maximum | Minimum | Average | ||
| Calibration set | 100 | 48.5 | 31.2 | 39.2 |
| External verification set | 27 | 47.9 | 32.2 | 39.1 |
| Preprocessing Methods | Latent Variables | Number of the Wavelength Variables | R2cv | RMSEcv (%) |
|---|---|---|---|---|
| RAW | 9 | 21 | 0.88 | 1.24 |
| NOR | 9 | 23 | 0.91 | 1.18 |
| FD | 5 | 14 | 0.86 | 1.37 |
| DET | 10 | 35 | 0.89 | 1.20 |
| SNV | 9 | 28 | 0.91 | 1.02 |
| MSC | 8 | 54 | 0.91 | 1.06 |
| PQN | 9 | 20 | 0.90 | 1.09 |
| OPLECm | 10 | 48 | 0.92 | 1.03 |
| OPS-SR | 10 | 48 | 0.91 | 1.06 |
| LRC-SR | 8 | 19 | 0.93 | 0.94 |
| Preprocessing Methods | R2v | RMSEv (%) | Max Relative Error (%) | Mean Relative Error (%) |
|---|---|---|---|---|
| RAW | 0.88 | 1.30 | 7.5 | 2.7 |
| NOR | 0.83 | 1.53 | 6.7 | 3.3 |
| FD | 0.86 | 1.49 | 8.0 | 3.2 |
| DET | 0.87 | 1.30 | 7.9 | 2.8 |
| SNV | 0.88 | 1.28 | 8.1 | 1.8 |
| MSC | 0.88 | 1.26 | 8.1 | 2.7 |
| PQN | 0.86 | 1.48 | 8.1 | 3.2 |
| OPLECm | 0.87 | 1.33 | 7.8 | 2.8 |
| OPS-SR | 0.91 | 1.06 | 5.6 | 2.3 |
| LRC-SR | 0.93 | 0.97 | 5.7 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhao, C.; Yang, G. Development of a Non-Destructive Method for Detection of the Juiciness of Pear via VIS/NIR Spectroscopy Combined with Chemometric Methods. Foods 2020, 9, 1778. https://doi.org/10.3390/foods9121778
Wang F, Zhao C, Yang G. Development of a Non-Destructive Method for Detection of the Juiciness of Pear via VIS/NIR Spectroscopy Combined with Chemometric Methods. Foods. 2020; 9(12):1778. https://doi.org/10.3390/foods9121778
Chicago/Turabian StyleWang, Fan, Chunjiang Zhao, and Guijun Yang. 2020. "Development of a Non-Destructive Method for Detection of the Juiciness of Pear via VIS/NIR Spectroscopy Combined with Chemometric Methods" Foods 9, no. 12: 1778. https://doi.org/10.3390/foods9121778
APA StyleWang, F., Zhao, C., & Yang, G. (2020). Development of a Non-Destructive Method for Detection of the Juiciness of Pear via VIS/NIR Spectroscopy Combined with Chemometric Methods. Foods, 9(12), 1778. https://doi.org/10.3390/foods9121778




