Influence of Stabilizing and Encapsulating Polymers on Antioxidant Capacity, Stability, and Kinetic Release of Thyme Essential Oil Nanocapsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanocapsule Preparation
2.3. Dynamic Light Scattering and Electrophoretic Movement
2.4. Nanocapsule Morphology
2.5. Encapsulation Efficiency (E.E.)
2.6. Release Kinetics
2.7. Infrared Spectroscopy
2.8. Thermal Behavior
2.9. Diffuse Reflectance
2.10. Antioxidant Capacity
2.10.1. ABTS
2.10.2. DPPH
2.10.3. FRAP
2.11. Statistical Analyses
3. Results
3.1. Dynamic Light Scattering, Electrophoretic Movement, and Charging Efficiency
3.2. Nanocapsule Morphology
3.3. Release Kinetics of Nanocapsules
3.4. Infrared Spectra of the Nanocapsules
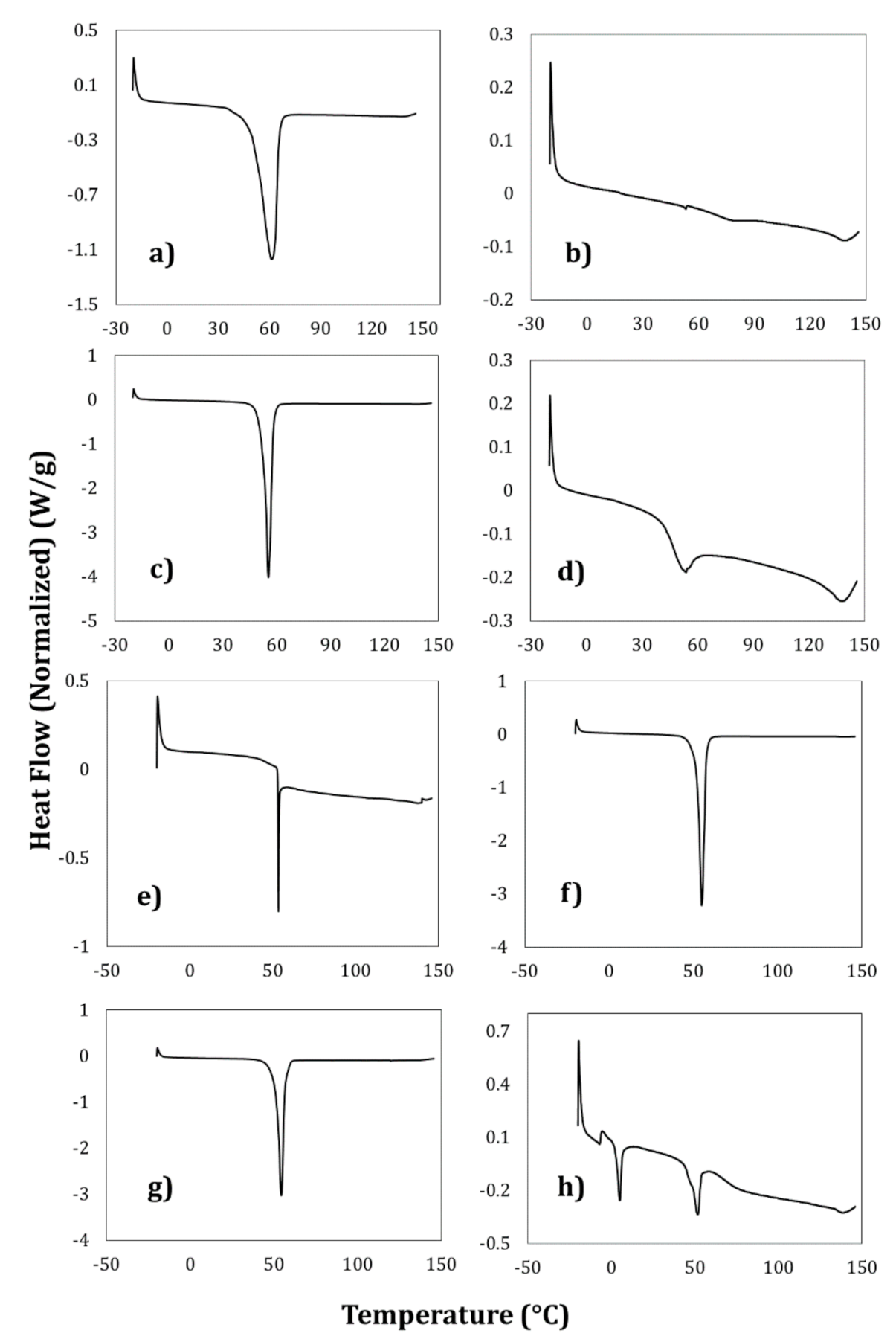
3.5. Thermal Analysis of the Nanocapsules
3.6. Stability of the Nanocapsules
3.7. Antioxidant Capacity of the Nanocapsules
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asprea, M.; Leto, I.; Bergonzi, M.C.; Bilia, A.R. Thyme essential oil loaded in nanocochleates: Encapsulation efficiency, in vitro release study and antioxidant activity. LWT Food Sci. Technol. 2017, 77, 497–502. [Google Scholar] [CrossRef]
- Hussein, M.; Roby, H.; Atef, M.; Selim, K.A.; Ibrahim, K. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crop. Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Pokorný, J.; Yanishlieva, N.; Gordon, M. Antioxidants in Food: Practical Applications; Woodhead Publishing Ltd. and CRC Press LLC: Cambridge, UK, 2001. [Google Scholar]
- González-Reza, R.M.; Quintanar-Guerrero, D.; Del Real-López, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, M.L. Effect of sucrose concentration and pH onto the physical stability of β-carotene nanocapsules. LWT Food Sci. Technol. 2018, 90, 354–361. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Hernández-Sánchez, H. Polymeric Nanoparticles in Foods. In Plant Nanobionics. Nanotechnology in the Life Sciences; Springer: Berlin/Heidelberg, Germany, 2019; pp. 217–233. [Google Scholar]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Gutiérrez-Cortez, E.; Castaño-Tostado, E.; Quintanar-Guerrero, D. Optimization of nanocapsules preparation by the emulsion-diffusion method for food applications. LWT Food Sci. Technol. 2011, 44, 1362–1368. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Allémann, E.; Doelker, E.; Fessi, H. Preparation and characterization of nanocapsnles from preformed polymers by a new process based on emulsification-diffusion technique. Pharm. Res. 1998, 15, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, L.; Luo, B.; Ramakrishna, S.; Kai, D.; Loh, X.J.; Yang, I.H.; Deen, G.R.; Mo, X. Engineering PCL/lignin nanofibers as an antioxidant scaffold for the growth of neuron and Schwann cell. Colloids Surf. B Biointerfaces 2018, 169, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Paulo, F.; Santos, L. Inclusion of hydroxytyrosol in ethyl cellulose microparticles: In vitro release studies under digestion conditions. Food Hydrocoll. 2018, 84, 104–116. [Google Scholar] [CrossRef]
- Rebia, R.A.; Rozet, S.; Tamada, Y.; Tanaka, T. Biodegradable PHBH/PVA blend nanofibers: Fabrication, characterization, in vitro degradation, and in vitro biocompatibility. Polym. Degrad. Stab. 2018, 154, 124–136. [Google Scholar] [CrossRef]
- Adeli, E. The use of supercritical anti-solvent (SAS) technique for preparation of Irbesartan-Pluronic® F-127 nanoparticles to improve the drug dissolution. Powder Technol. 2016, 298, 65–72. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, J.F. The release kinetics of β-carotene nanocapsules/xanthan gum coating and quality changes in fresh-cut melon (cantaloupe). Carbohydr. Polym. 2017, 157, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Del Real, L.A.; Gutiérrez-Cortez, E.; Cornejo-Villegas, M.A.; Quintanar-Guerrero, D. The effect of nano-coatings with α-tocopherol and xanthan gum on shelf-life and browning index of fresh-cut “red Delicious” apples. Innov. Food Sci. Emerg. Technol. 2014, 22, 188–196. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Quintanar-Guerrero, D.; Flores-Minutti, J.J.; Gutiérrez-Cortez, E.; Zambrano-Zaragoza, M.L. Nanocapsules of β-carotene: Thermal degradation kinetics in a scraped surface heat exchanger (SSHE). LWT Food Sci. Technol. 2015, 60, 124–130. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying An Improved Abts Radical. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Galindo-Pérez, M.J.; Quintanar-Guerrero, D.; de los Ángeles Cornejo-Villegas, M.; de la Luz Zambrano-Zaragoza, M. Optimization of the emulsification-diffusion method using ultrasound to prepare nanocapsules of different food-core oils. LWT Food Sci. Technol. 2018, 87, 333–341. [Google Scholar] [CrossRef]
- Gupta, S.S.; Ghosh, M. Preparation and characterisation of protein based nanocapsules of bioactive lipids. J. Food Eng. 2014, 121, 64–72. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Elbasuney, S. Sustainable steric stabilization of colloidal titania nanoparticles. Appl. Surf. Sci. 2017, 409, 438–447. [Google Scholar] [CrossRef]
- Noronha, C.M.; Granada, A.F.; de Carvalho, S.M.; Lino, R.C.; Matheus, M.V.; Barreto, P.L.M. Optimization of α-tocopherol loaded nanocapsules by the nanoprecipitation method. Ind. Crops Prod. 2013, 50, 896–903. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT Food Sci. Technol. 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Cui, H. LWT-Food Science and Technology Electrospun thyme essential oil/gelatin nano fi bers for active packaging against Campylobacter jejuni in chicken. LWT Food Sci. Technol. 2018, 97, 711–718. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Carvajal-Moreno, M.; Correa, B.; Rojo-Callejas, F. Cellular, physiological and molecular approaches to investigate the antifungal and anti-aflatoxigenic effects of thyme essential oil on Aspergillus flavus. Food Chem. 2020, 315, 126096. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.B. ABTS/TEAC (2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)/Trolox®-Equivalent Antioxidant Capacity) radical scavenging mixed-mode assay. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; John Wiley & Sons Ltd.: Pondicherry, India, 2018; pp. 117–139. [Google Scholar]
- Milos, M.; Makota, D. Investigation of antioxidant synergisms and antagonisms among thymol, carvacrol, thymoquinone and p-cymene in a model system using the Briggs-Rauscher oscillating reaction. Food Chem. 2012, 131, 296–299. [Google Scholar] [CrossRef]
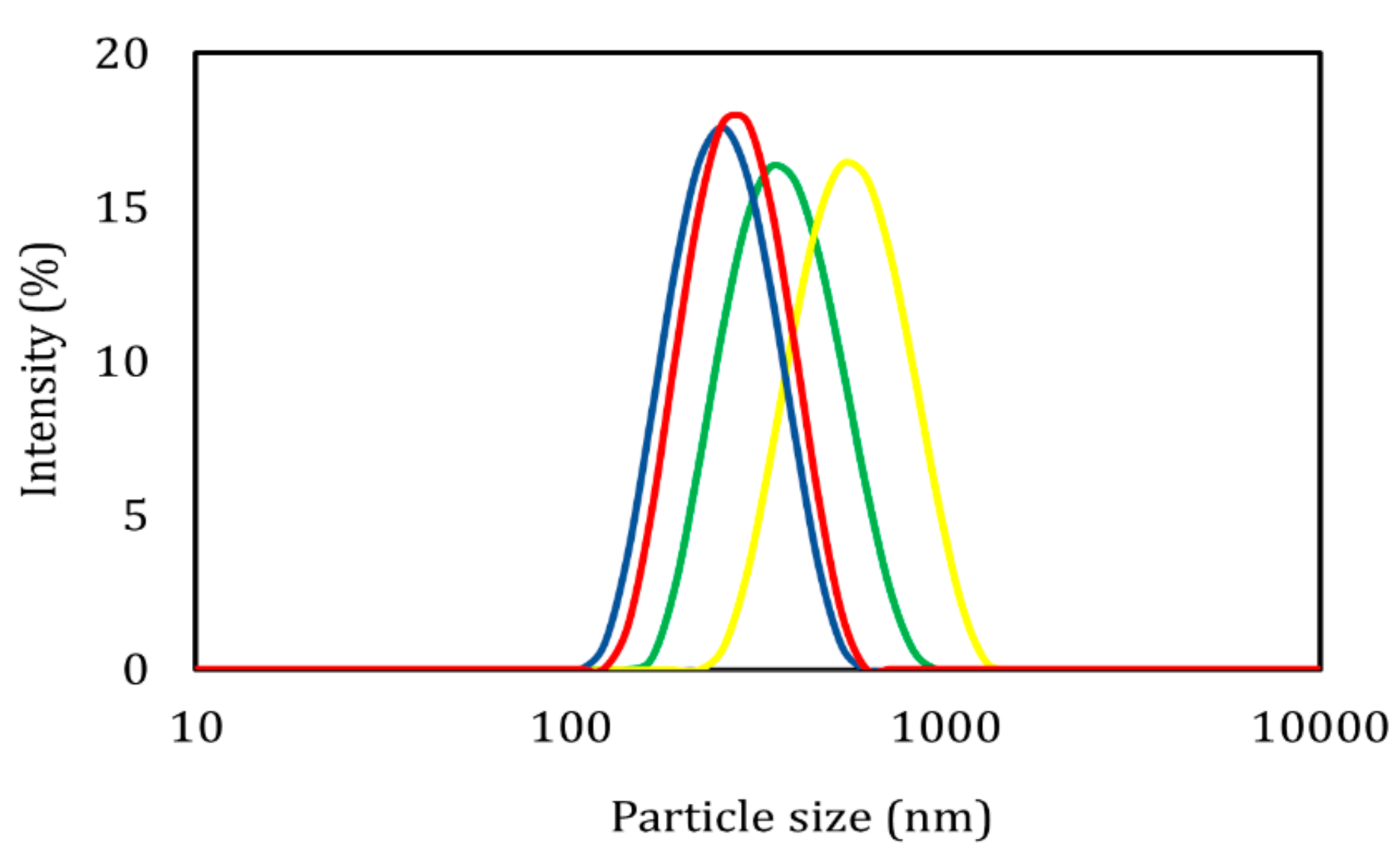




| Sample | P.S. (nm) | PDI (-) | ζ (mV) | E.E. (%) |
|---|---|---|---|---|
| ETC-PVA | 346.97 ± 0.85 | 0.09 ± 0.01 | –7.16 ± 0.28 | 71.28 ± 0.68 |
| ETC-Pluronic® | 266.13 ± 3.53 | 0.11 ± 0.03 | –19.77 ± 0.76 | 69.79 ± 2.92 |
| PCL-PVA | 489.20 ± 3.93 | 0.12 ± 0.01 | –5.89 ± 0.36 | 60.87 ± 0.84 |
| PCL-Pluronic® | 241.37 ± 2.06 | 0.12 ± 0.05 | –16.97 ± 0.83 | 67.43 ± 0.91 |
| Sample | Zero-Order | First-Order | Higuchi | Hixon- Crowel | Korsmeyer-Peppas | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | n | |
| ETC-PVA | 0.5938 | 0.5116 | 0.8259 | 0.5395 | 0.9830 | 0.1363 |
| ETC-Pluronic® | 0.5797 | 0.5015 | 0.8259 | 0.5281 | 0.9808 | 0.1357 |
| PCL-PVA | 0.5925 | 0.5117 | 0.8259 | 0.5385 | 0.9830 | 0.1360 |
| PCL-Pluronic® | 0.5840 | 0.5049 | 0.8259 | 0.5316 | 0.9815 | 0.1357 |
| Sample | ABTS | FRAP | DPPH |
|---|---|---|---|
| μmolascorbic acid equivalents/gnanostructured oil | |||
| Thyme oil | 2010.74 ± 43.94 | 946.04 ± 12.87 | 274.74 ± 37.71 |
| ETC-PVA | 1924.03 ± 74.52 | 966.38 ± 22.18 | 272.79 ± 41.46 |
| ETC-Pluronic® | 2135.58 ± 33.72 | 1071.75 ± 19.59 | 285.13 ± 14.28 |
| PCL-PVA | 1973.69 ± 59.01 | 1108.92 ± 56.37 | 280.46 ± 20.02 |
| PCL-Pluronic® | 2034.40 ± 55.28 | 1133.06 ± 18.97 | 269.30 ± 12.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Reza, R.M.; Hernández-Sánchez, H.; Zambrano-Zaragoza, M.L.; Gutiérrez-López, G.F.; Del-Real, A.; Quintanar-Guerrero, D.; Velasco-Bejarano, B. Influence of Stabilizing and Encapsulating Polymers on Antioxidant Capacity, Stability, and Kinetic Release of Thyme Essential Oil Nanocapsules. Foods 2020, 9, 1884. https://doi.org/10.3390/foods9121884
González-Reza RM, Hernández-Sánchez H, Zambrano-Zaragoza ML, Gutiérrez-López GF, Del-Real A, Quintanar-Guerrero D, Velasco-Bejarano B. Influence of Stabilizing and Encapsulating Polymers on Antioxidant Capacity, Stability, and Kinetic Release of Thyme Essential Oil Nanocapsules. Foods. 2020; 9(12):1884. https://doi.org/10.3390/foods9121884
Chicago/Turabian StyleGonzález-Reza, Ricardo M., Humberto Hernández-Sánchez, Maria L. Zambrano-Zaragoza, Gustavo F. Gutiérrez-López, Alicia Del-Real, David Quintanar-Guerrero, and Benjamín Velasco-Bejarano. 2020. "Influence of Stabilizing and Encapsulating Polymers on Antioxidant Capacity, Stability, and Kinetic Release of Thyme Essential Oil Nanocapsules" Foods 9, no. 12: 1884. https://doi.org/10.3390/foods9121884
APA StyleGonzález-Reza, R. M., Hernández-Sánchez, H., Zambrano-Zaragoza, M. L., Gutiérrez-López, G. F., Del-Real, A., Quintanar-Guerrero, D., & Velasco-Bejarano, B. (2020). Influence of Stabilizing and Encapsulating Polymers on Antioxidant Capacity, Stability, and Kinetic Release of Thyme Essential Oil Nanocapsules. Foods, 9(12), 1884. https://doi.org/10.3390/foods9121884