Systematic Investigation of Co-Crystallization Properties in Binary and Ternary Mixtures of Triacylglycerols Containing Palmitic and Oleic Acids in Relation with Palm Oil Dry Fractionation
Abstract
:1. Introduction
2. Experimental Conditions
2.1. Material
2.2. Reverse Phase High Performance Liquid Chromatography (RP-HPLC)
2.3. Iodine Value (IV)
2.4. Differential Scanning Calorimetry (DSC)
2.4.1. Direct Mode
2.4.2. Tempered Mode
2.5. Variable Temperature Powder X-ray Diffraction (XRD)
2.6. Phase Diagrams
3. Results
3.1. Triacylglycerol Composition of Palm Oil and Fractions
3.2. Polymorphic and Melting Properties of Pure Selected Triacylglycerols: PPP, POP, OPP and POO
3.2.1. Direct Mode
3.2.2. Tempered Mode
3.3. Binary Phase Diagrams
3.3.1. Direct Mode
PPP/POP
PPP/OPP
PPP/POO
POP/OPP
POP/POO
OPP/POO
3.3.2. Tempered Mode
PPP/POP
PPP/OPP
PPP/POO
POP/OPP
POP/POO
OPP/POO
3.4. Ternary Phase Diagrams
3.4.1. Direct Mode
PPP/POP/POO
PPP/OPP/POO
3.4.2. Tempered Mode
PPP/POP/POO
PPP/OPP/POO
4. Discussion
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Willems, M.G.A.; Padley, F.B. Palm oil: Quality requirements from a customer’s point of view. J. Am. Oil Chem. Soc. 1985, 62, 454–462. [Google Scholar] [CrossRef]
- Siew, W.L.; Mohammad, Y. Effects of refining on chemical and physical properties of palm oil products. J. Am. Oil Chem. Soc. 1989, 66, 1116–1119. [Google Scholar] [CrossRef]
- Gibon, V.; De Greyt, W.; Kellens, M. Palm oil refining. Eur. J. Lipid Sci. Technol. 2007, 109, 315–335. [Google Scholar] [CrossRef]
- De Clercq, N.; Danthine, S.; Nguyen, M.T.; Gibon, V.; Dewettinck, K. Enzymatic Interesterification of Palm Oil and Fractions: Monitoring the Degree of Interesterification using Different Methods. J. Am. Oil Chem. Soc. 2012, 89, 219–229. [Google Scholar] [CrossRef]
- Danthine, S.; De Clercq, N.; Dewettinck, K.; Gibon, V. Monitoring batch lipase catalyzed interesterification of palm oil and fractions by differential scanning calorimetry. J. Therm. Anal. Calorim. 2014, 115, 2219–2229. [Google Scholar] [CrossRef]
- Maes, J.; Kodali, S.; Danthine, S.; Gibon, V. Influence of Enzymatic Remediation on Compositional and Thermal Properties of Palm Oil and Palm Oleins from Dry Fractionation. J. Am. Oil Chem. Soc. 2015, 92, 821–831. [Google Scholar] [CrossRef]
- Gibon, V. Fractionation of lipids. In Modifying Lipids for Use in Food; Gunstone, F.D., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 201–229. [Google Scholar]
- Calliauw, G.H.; Gibon, V.; De Greyt, W.F. Principles of palm olein fractionation: A bit of science behind the technology. Lipid Technol. 2007, 19, 152–155. [Google Scholar] [CrossRef]
- Kellens, M.; Gibon, V.; Hendrix, M.; De Greyt, W. Palm oil fractionation. Eur. J. Lipid Sci. Technol. 2007, 109, 336–349. [Google Scholar] [CrossRef]
- Gibon, V. Palm Oil and Palm Kernel Oil Refining and Fractionation Technology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 329–375. [Google Scholar]
- Gibon, V.; Kellens, M. From Specialty to Commodity OFI Magazine, September/October 2018 Issue, pp. 26–30. Available online: https://www.ofimagazine.com/issues/ofi-september-october-2018 (accessed on 4 November 2020).
- Gee, T. Analytical characteristics of crude and refined palm oil and fractions. Eur. J. Lipid Sci. Technol. 2007, 109, 373–379. [Google Scholar] [CrossRef]
- Danthine, S.; Lefébure, E.; Blecker, C.; Dijckmans, P.; Gibon, V. Correlations Between Cloud Point and Compositional Properties of Palm Oil and Liquid Fractions from Dry Fractionation. J. Am. Oil Chem. Soc. 2017, 94, 841–853. [Google Scholar] [CrossRef]
- Braipson-Danthine, S.; Gibon, V. Comparative analysis of triacylglycerol composition, melting properties and polymorphic behavior of palm oil and fractions. Eur. J. Lipid Sci. Technol. 2007, 109, 359–372. [Google Scholar] [CrossRef]
- Gibon, V. Use of DSC in the Oils and Fats Processing Industry. In Proceedings of the Mettler-Toledo Seminar—Thermal Analysis for Food Applications, Brussels, Belgium, 13 June 2018. [Google Scholar]
- Moorthy, S. Melting and Solidification of Fats. In Structure-Function Analysis of Edible Fats, 2nd ed.; Marangoni, A.G., Ed.; AOCS Press: Urbana, IL, USA, 2018. [Google Scholar]
- West, R.; Rousseau, D. Tripalmitin-Driven Crystallization of Palm Oil: The Role of Shear and Dispersed Particles. J. Am. Oil Chem. Soc. 2020, 97, 989–999. [Google Scholar] [CrossRef]
- Macridachis-González, J.; Bayés-García, L.; Calvet, A.T. An Insight into the Solid-State Miscibility of Triacylglycerol Crystals. Molecules 2020, 25, 4562. [Google Scholar] [CrossRef] [PubMed]
- Gibon, V.; Blanpain, P.; Durant, F.; Deroanne, C. Application de la diffraction des rayons X, de la résonance magnétique nucléaire et de l’analyse calorimétrique différentielle à l’etude du polymorphisme et de l’intersolubilité des triglycérides PPP, PSP et POP. Belg. J. Food Chem. Biotechnol. 1985, 40, 119–134. [Google Scholar]
- Gibon, V.; Durant, F. Etude du polymorphisme et de l’intersolubilité de triglycérides palmito-oléiques par diffraction Rx de poudres et analyse calorimétrique différentielle. Bull. Soc. Chim. Belg. 1985, 94, 1009–1020. [Google Scholar] [CrossRef]
- Gibon, V.; Durant, F.; Deroanne, C. Polymorphism and intersolubility of some palmitic, stearic and oleic triglycerides: PPP, PSP and POP. J. Am. Oil Chem. Soc. 1986, 63, 1047–1055. [Google Scholar] [CrossRef]
- Minato, A.; Ueno, S.; Yano, J.; Wang, Z.H.; Seto, H.; Amemiya, Y.; Sato, K. Synchrotron radiation X-ray diffraction study on phase behavior of PPP-POP binary mixtures. J. Am. Oil Chem. Soc. 1996, 73, 1567–1572. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, B.; Zhang, H.; Guo, Y.; Dang, L.; Liu, Z.; Shu, Q.; Wang, Z. Solid–Liquid Phase Equilibrium and Phase Behaviors for Binary Mixtures Composed of Tripalmitoylglycerol (PPP), 1,3-Dipalmitoyl-2-oleoyl-glycerol (POP), and 1,2-Dioleoyl-3-palmitoyl-glycerol (POO). Ind. Eng. Chem. Res. 2019, 58, 10044–10052. [Google Scholar] [CrossRef]
- Minato, A.; Ueno, S.; Smith, K.W.; Amemiya, Y.; Sato, K. Thermodynamic and Kinetic Study on Phase Behavior of Binary Mixtures of POP and PPO Forming Molecular Compound Systems. J. Phys. Chem. B 1997, 101, 3498–3505. [Google Scholar] [CrossRef]
- Bayés-García, L.; Calvet, A.T.; Cuevas-Diarte, M.À.; Ueno, S.; Sato, K. Phase Behavior of Binary Mixture Systems of Saturated-Unsaturated Mixed-Acid Triacylglycerols: Effects of Glycerol Structures and Chain–Chain Interactions. J. Phys. Chem. B 2015, 119, 4417–4427. [Google Scholar] [CrossRef]
- Moran, D.P.J. Phase behaviour of some palmito-oleo triglyceride systems. J. Appl. Chem. 2007, 13, 91–100. [Google Scholar] [CrossRef]
- Minato, A.; Ueno, S.; Yano, J.; Smith, K.; Seto, H.; Amemiya, Y.; Sato, K. Thermal and structural properties ofsn-1,3-dipalmitoyl-2-oleoylglycerol andsn-1,3-dioleoyl-2-palmitoylglycerol binary mixtures examined with synchrotron radiation X-ray diffraction. J. Am. Oil Chem. Soc. 1997, 74, 1213–1220. [Google Scholar] [CrossRef]
- Ikeda, E.; Ueno, S.; Miyamoto, R.; Sato, K. Phase Behavior of a Binary Mixture of 1,3-Dipalmitoyl-2-oleoyl-sn-glycerol and 1,3-Dioleoyl-2-palmitoyl-sn-glycerol inn-Dodecane Solution. J. Phys. Chem. B 2010, 114, 10961–10969. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Konishi, H. Crystallization behavior of 1, 3-dipalmitoyl-2-oleoyl-glycerol and 1-palmitoyl-2, 3-dioleoyl-glycerol. Eur. J. Lipid Sci. Technol. 2001, 103, 804–809. [Google Scholar] [CrossRef]
- Zhang, L.; Ueno, S.; Miura, S.; Sato, K. Binary Phase Behavior of 1,3-Dipalmitoyl-2-oleoyl-sn-glycerol and 1,2-Dioleoyl-3-palmitoyl-rac-glycerol. J. Am. Oil Chem. Soc. 2007, 84, 219–227. [Google Scholar] [CrossRef]
- Mizobe, H.; Tanaka, T.; Hatakeyama, N.; Nagai, T.; Ichioka, K.; Hondoh, H.; Ueno, S.; Sato, K. Structures and Binary Mixing Characteristics of Enantiomers of 1-Oleoyl-2,3-dipalmitoyl-sn-glycerol (S-OPP) and 1,2-Dipalmitoyl-3-oleoyl-sn-glycerol (R-PPO). J. Am. Oil Chem. Soc. 2013, 90, 1809–1817. [Google Scholar] [CrossRef]
- Elisabettini, P.; Lognay, G.; Desmedt, A.; Culot, C.; Istasse, N.; Deffense, E.; Durant, F. Synthesis and physicochemical characterization of mixed diacid triglycerides that contain elaidic acid. J. Am. Oil Chem. Soc. 1998, 75, 285–291. [Google Scholar] [CrossRef]
- AOCS. Method Ce 5b-89: Triglycerides in vegetable oils by HPLC. In Official Methods and Recommended Practices of the AOCS, 7th ed.; AOCS: Urbana, IL, USA, 2017. [Google Scholar]
- AOCS. Method Ce 1h-05: Cis-, trans-, saturated, monounsaturated and polyunsaturated fatty acids in vegetable or non-ruminant animal oils and fats by capillary GLC. In Official Methods and Recommended Practices of the AOCS, 7th ed.; AOCS: Urbana, IL, USA, 2017. [Google Scholar]
- AOCS. Method Ce 2-66: Preparation of methyl esters of fatty acids. In Official Methods and Recommended Practices of the AOCS, 7th ed.; AOCS: Urbana, IL, USA, 2017. [Google Scholar]
- AOCS. Method Cd 1c-85: Calculated Iodine Value. In Official Methods and Recommended Practices of the AOCS, 7th ed.; AOCS: Urbana, IL, USA, 2017. [Google Scholar]
- Smith, K.W. Crystallization of Palm Oil and Its Fractions. In Crystallization Processes in Fats and Lipid Systems; Informa UK Limited: London, UK, 2001; pp. 371–394. [Google Scholar]
- Sato, K.; Ueno, S.; Yano, J. Molecular interactions and kinetic properties of fats. Prog. Lipid Res. 1999, 38, 91–116. [Google Scholar] [CrossRef]
- Idziak, S. Powder X-ray Diffraction of Triglycerides in the Study of Polymorphism. In Crystallization Processes in Fats and Lipid Systems; Garti, N., Sato, K., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001. [Google Scholar]
- Anihouvi, P.P.; Blecker, C.; Dombree, A.; Danthine, S. Comparative Study of Thermal and Structural Behavior of Four Industrial Lauric Fats. Food Bioprocess Technol. 2012, 6, 3381–3391. [Google Scholar] [CrossRef]
- Nelis, V.; Declerck, A.; De Neve, L.; Moens, K.; Dewettinck, K.; Van Der Meeren, P. Fat crystallization and melting in W/O/W double emulsions: Comparison between bulk and emulsified state. Colloids Surf. A 2019, 566, 196–206. [Google Scholar] [CrossRef]
- Campos, R.; Ollivon, M.; Marangoni, A.G. Molecular Composition Dynamics and Structure of Cocoa Butter. Cryst. Growth Des. 2010, 10, 205–217. [Google Scholar] [CrossRef]
- Calliauw, G.; Gibon, V.; De Greyt, W.; Plees, L.; Foubert, I.; Dewettinck, K. Phase Composition During Palm Olein Fractionation and its Effect on Soft PMF and Superolein Quality. J. Am. Oil Chem. Soc. 2007, 84, 885–891. [Google Scholar] [CrossRef]
- Gibon, V. Dry Fractionation for Designing Specialty Fats. In Proceedings of the 12th Practical Short Course: Nutritional & Functional Lipids, Characteristics and Applications in Food System, Franfurt, Germany, 29–30 November 2018. [Google Scholar]
- Bhaggan, K.; Blecker, C.; Danthine, S.; Smith, K.W. Binary Mixtures of Tripalmitoylglycerol (PPP) and 1,3-Dipalmitoyl-2-stearoyl-sn -glycerol (PSP): Polymorphism and Kinetic Phase Behavior. Eur. J. Lipid Sci. Technol. 2018, 120, 1700306. [Google Scholar] [CrossRef]
- Bhaggan, K.; Smith, K.W.; Blecker, C.; Danthine, S. Polymorphism and Kinetic Behavior of Binary Mixtures of Trisaturated Triacylglycerols Containing Palmitic and Stearic Acid Under Non-Isothermal Conditions. Eur. J. Lipid Sci. Technol. 2018, 120. [Google Scholar] [CrossRef]
- Lutton, E.S.; Jackson, F.L. The Polymorphism of Synthetic and Natural 2-Oleyldipalmitin. J. Am. Chem. Soc. 1950, 72, 3254–3257. [Google Scholar] [CrossRef]
- Sato, K.; Arishima, T.; Wang, Z.H.; Ojima, K.; Sagi, N.; Mori, H. Polymorphism of POP and SOS. I. occurrence and polymorphic transformation. J. Am. Oil Chem. Soc. 1989, 66, 664–674. [Google Scholar] [CrossRef]
- Bayés-García, L.; Calvet, T.; Cuevas-Diarte, M.; Ueno, S. In situ crystallization and transformation kinetics of polymorphic forms of saturated-unsaturated-unsaturated triacylglycerols: 1-palmitoyl-2,3-dioleoyl glycerol, 1-stearoyl-2,3-dioleoyl glycerol, and 1-palmitoyl-2-oleoyl-3-linoleoyl glycerol. Food Res. Int. 2016, 85, 244–258. [Google Scholar] [CrossRef]










| Triacylglycerol (%) | Palm Oil IV 51–53 | Olein IV 56–57 | Super-Olein IV 64–65 | Top-Olein IV 71–73 | Stearin IV 30–32 | Super-Stearin IV 9–14 | Soft PMF IV 44–46 | Hard PMF IV 32–34 |
|---|---|---|---|---|---|---|---|---|
| PPP | 4–6 | N.D. | N.D. | N.D. | 25–27 | 65–70 | <1 | <2 |
| P2O | 27–29 | 28–30 | 17–18 | 7–9 | 27–29 | 8–12 | 47–48 | 65–67 |
| PO2 | 20–22 | 22–24 | 29–30 | 34–36 | 10–11 | 2–4 | 12–13 | 2–3 |
| Direct Mode | Tempered Mode | ||||
|---|---|---|---|---|---|
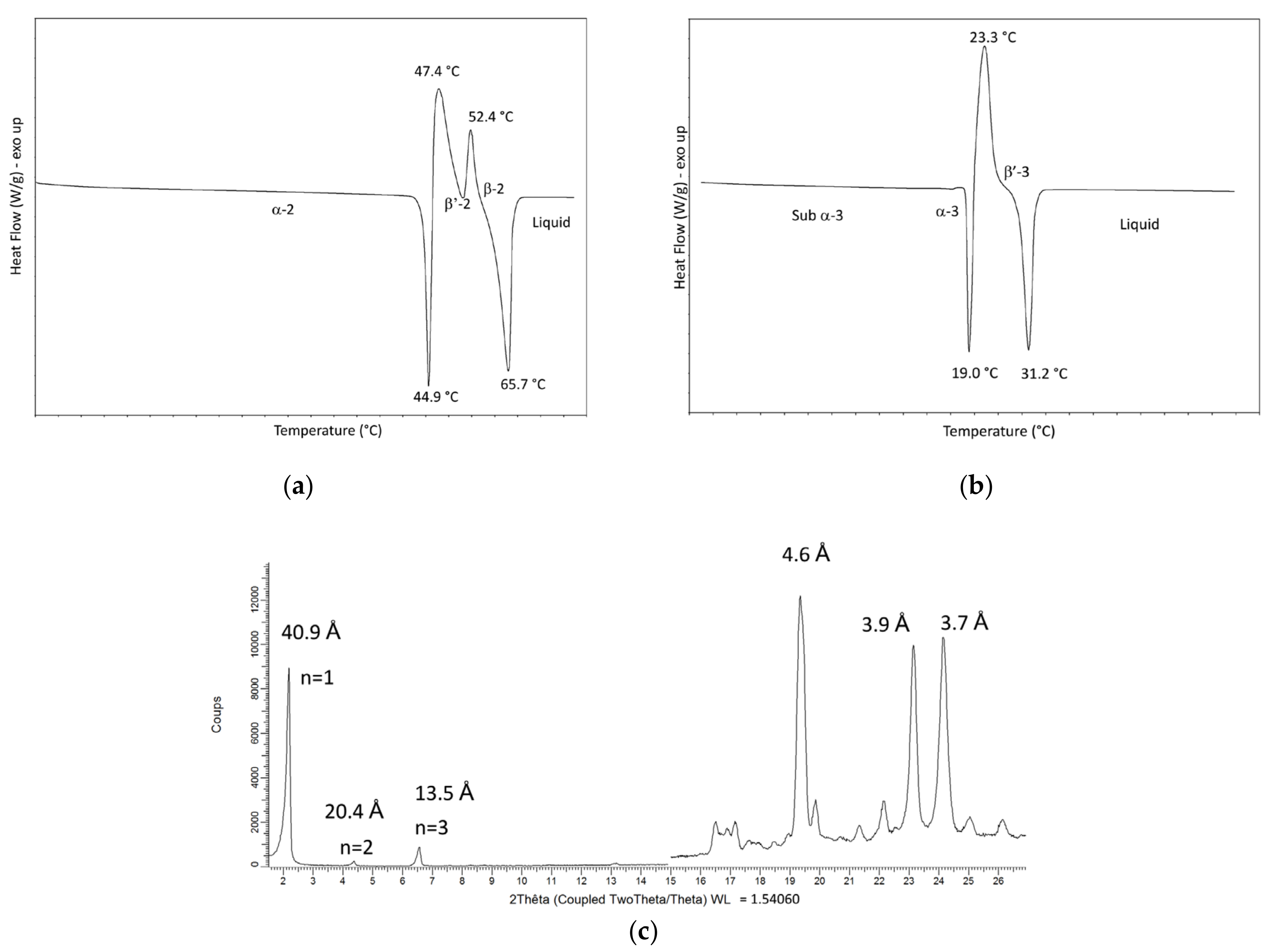
| PPP | 44.9 °C | 47.4 °C | 52.4 °C | 65.7 °C | 65.8 °C |
| endotherm | exotherm | exotherm | endotherm | endotherm | |
| α-2 form | β′-2 form | β-2 form | β-2 form | ||
| POP | 14.1 °C | 27.5 °C | 34.2 °C | ||
| exotherm | endotherm | endotherm | |||
| α-2 form | β′-2 form | β-3 form | |||
| OPP | - | 19.0 °C | 23.3 °C | 31.2 °C | 32.6 °C |
| - | endotherm | exotherm | endotherm | endotherm | |
| sub-α-3 form | α-3 form | β-3 form | β′-3form | ||
| POO | −14.3 °C | 3.1 °C | 18.6 °C | Same as in direct mode | |
| exotherm | exotherm | endotherm | |||
| sub-α-3 form | α-3 form | β′-3 form | |||
| POP | α-2 | sub–β′-2 | β′-2 |
| Short spacings (Å) | 4.2 (VS) | 4.4 (S) 4.2 (S) 4.9 (m) | 5.0 (m) 4.5 (m) 4.4 (S) 4.2 (m) 4.0 (VS) |
| Long spacings (Å) | 50.1 | 45.1 | 43.2 |
| OPP | sub-α-3 | α-3 | β′-3 |
| Short spacings (Å) | 4.2 (S) 3.8 (m) | 4.2 (VS) | 4.7 (m) 4.4 (m) 4.1 (VS) 3.8 (S) |
| Long spacings (Å) | 82.9 | 81.1 | 71.3 |
| POO | sub-α-3 | α-3 | β′-3 |
| Short spacings (Å) | 4.3 (S) 3.9 (m) | 4.2 (VS) | 4.6 (m) 4.4 (m) 3.9 (S) |
| Long spacings (Å) | 68.3 | 67.0 | 66.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibon, V.; Danthine, S. Systematic Investigation of Co-Crystallization Properties in Binary and Ternary Mixtures of Triacylglycerols Containing Palmitic and Oleic Acids in Relation with Palm Oil Dry Fractionation. Foods 2020, 9, 1891. https://doi.org/10.3390/foods9121891
Gibon V, Danthine S. Systematic Investigation of Co-Crystallization Properties in Binary and Ternary Mixtures of Triacylglycerols Containing Palmitic and Oleic Acids in Relation with Palm Oil Dry Fractionation. Foods. 2020; 9(12):1891. https://doi.org/10.3390/foods9121891
Chicago/Turabian StyleGibon, Veronique, and Sabine Danthine. 2020. "Systematic Investigation of Co-Crystallization Properties in Binary and Ternary Mixtures of Triacylglycerols Containing Palmitic and Oleic Acids in Relation with Palm Oil Dry Fractionation" Foods 9, no. 12: 1891. https://doi.org/10.3390/foods9121891
APA StyleGibon, V., & Danthine, S. (2020). Systematic Investigation of Co-Crystallization Properties in Binary and Ternary Mixtures of Triacylglycerols Containing Palmitic and Oleic Acids in Relation with Palm Oil Dry Fractionation. Foods, 9(12), 1891. https://doi.org/10.3390/foods9121891







