Improving the Solubility and Digestibility of Potato Protein with an Online Ultrasound-Assisted PH Shifting Treatment at Medium Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Potato Protein
2.3. Ultrasound-Assisted pH Shifting Treatment of Potato Protein
2.4. Solubility
2.5. In Vitro Digestion
2.6. Free and Total Sulfhydryl Contents
2.7. Surface Hydrophobicity
2.8. Intrinsic Fluorescence
2.9. Circular Dichroism (CD) Spectrum
2.10. Electrophoresis
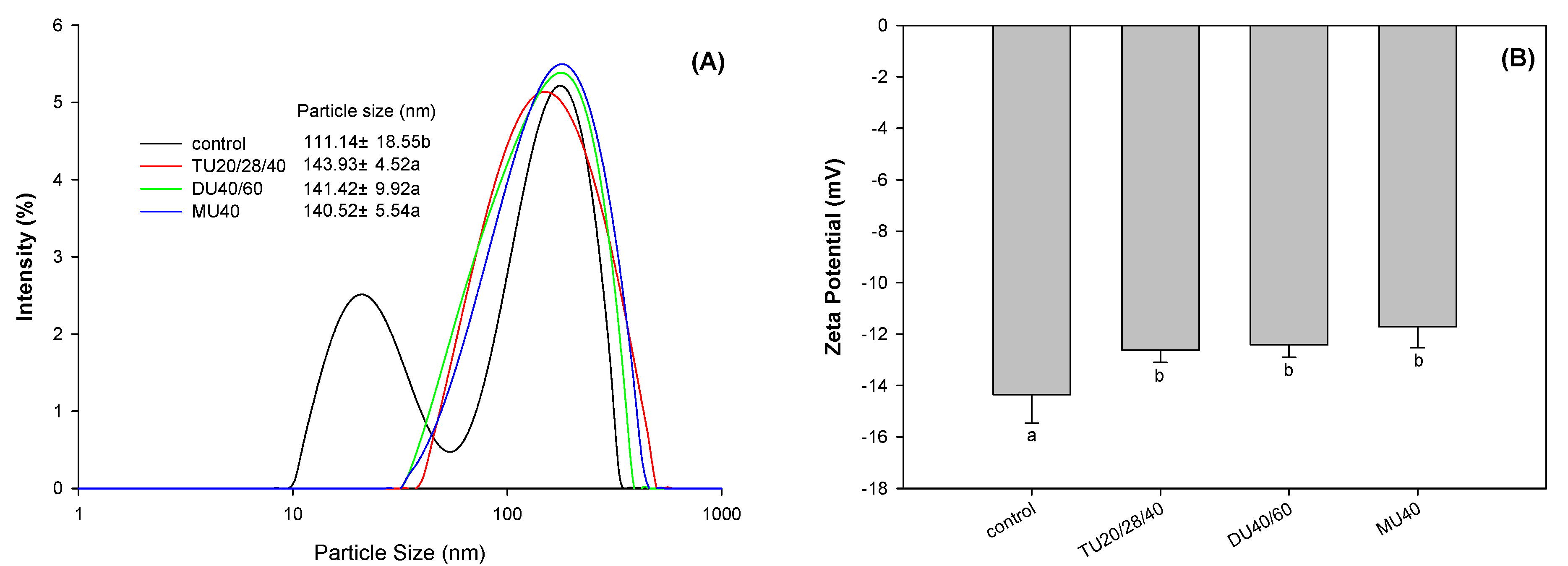
2.11. Particle Size and Zeta Potential
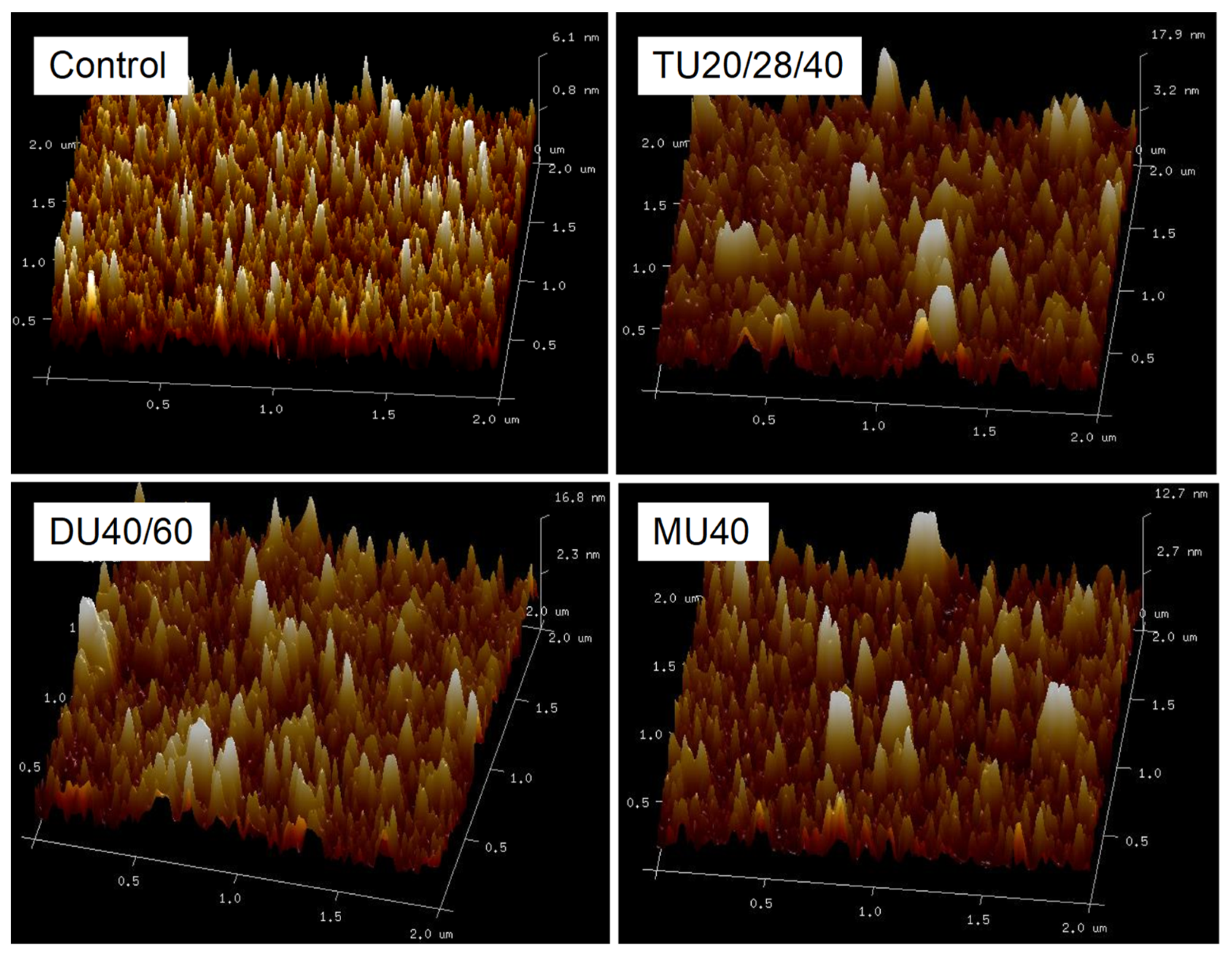
2.12. Atomic Force Microscope (AFM)
2.13. Statistical Analysis
3. Results
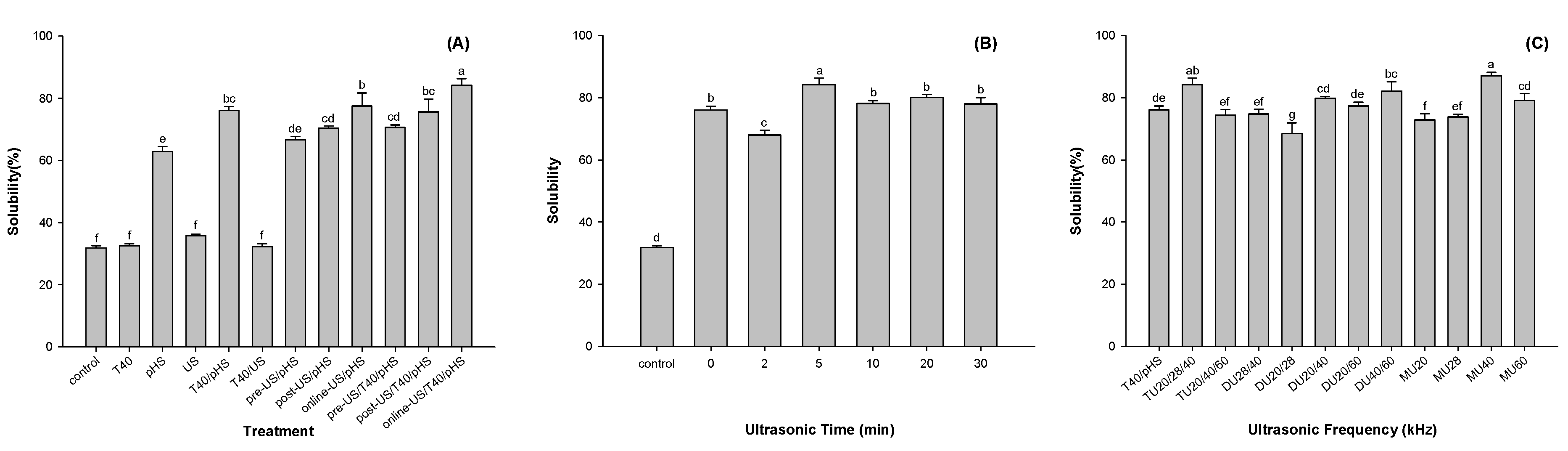
3.1. Solubility
3.1.1. Effect of Different Modification Methods on Potato Protein Solubility
3.1.2. Effect of Ultrasonication Time on the Solubility of Potato Protein
3.1.3. Effect of Ultrasonication Frequency on the Solubility of Potato Protein
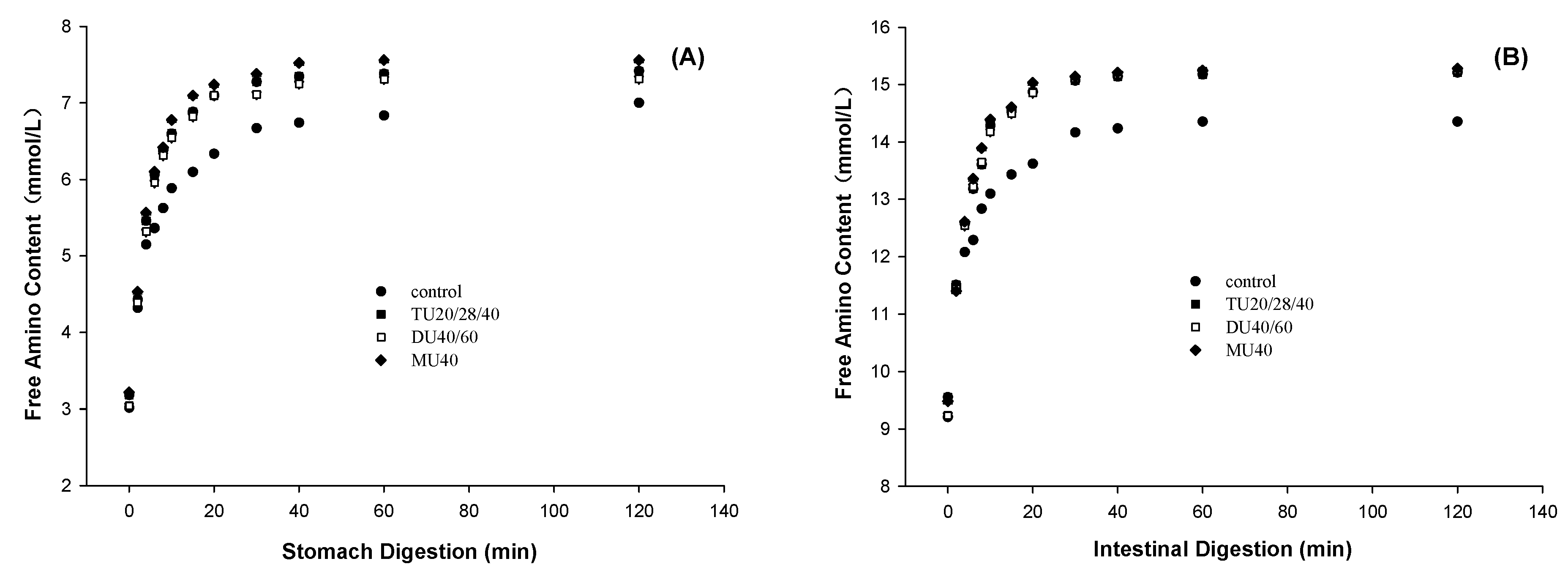
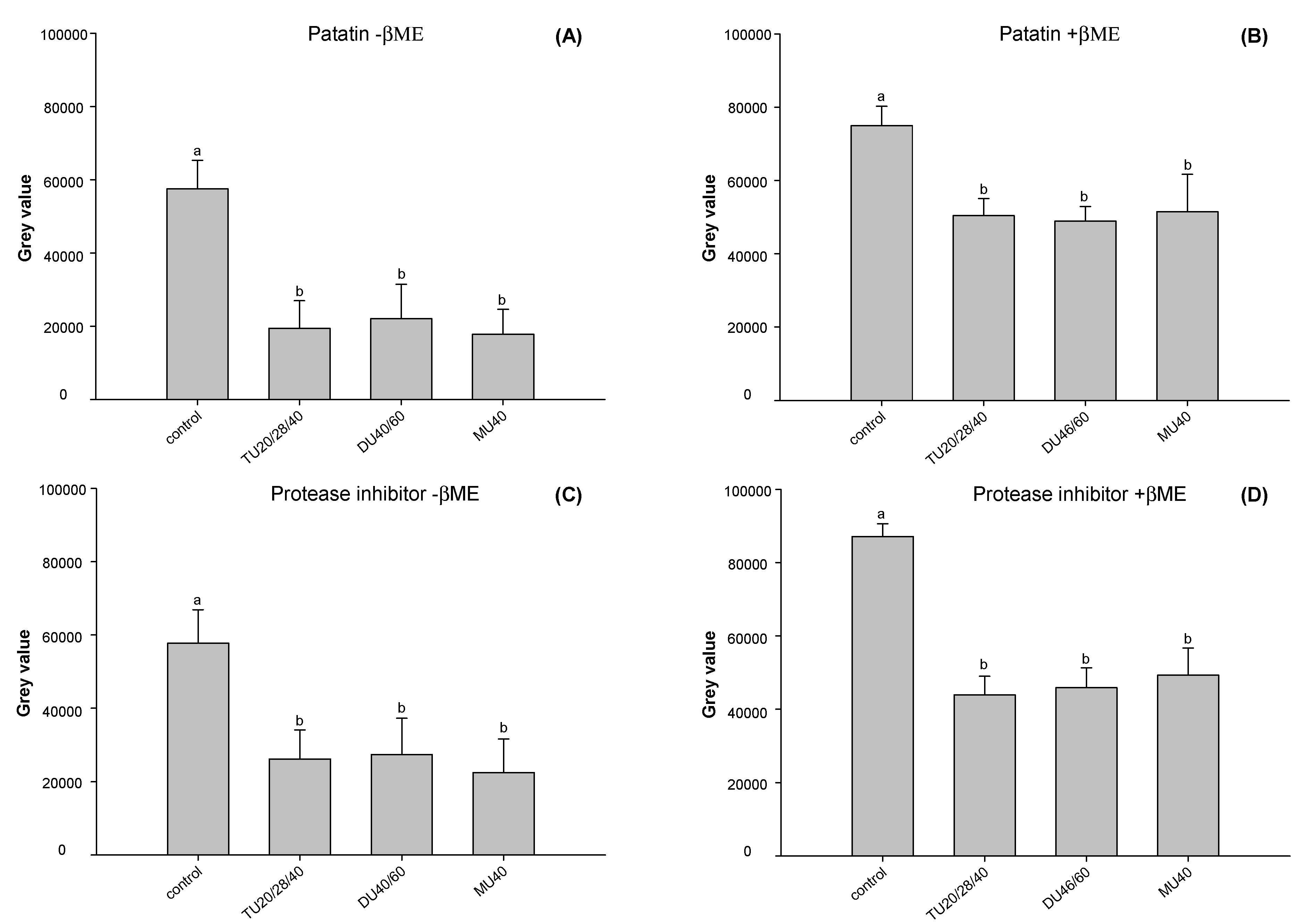
3.2. Effect of Online US/T40/pHS Treatment on Potato Protein Digestion
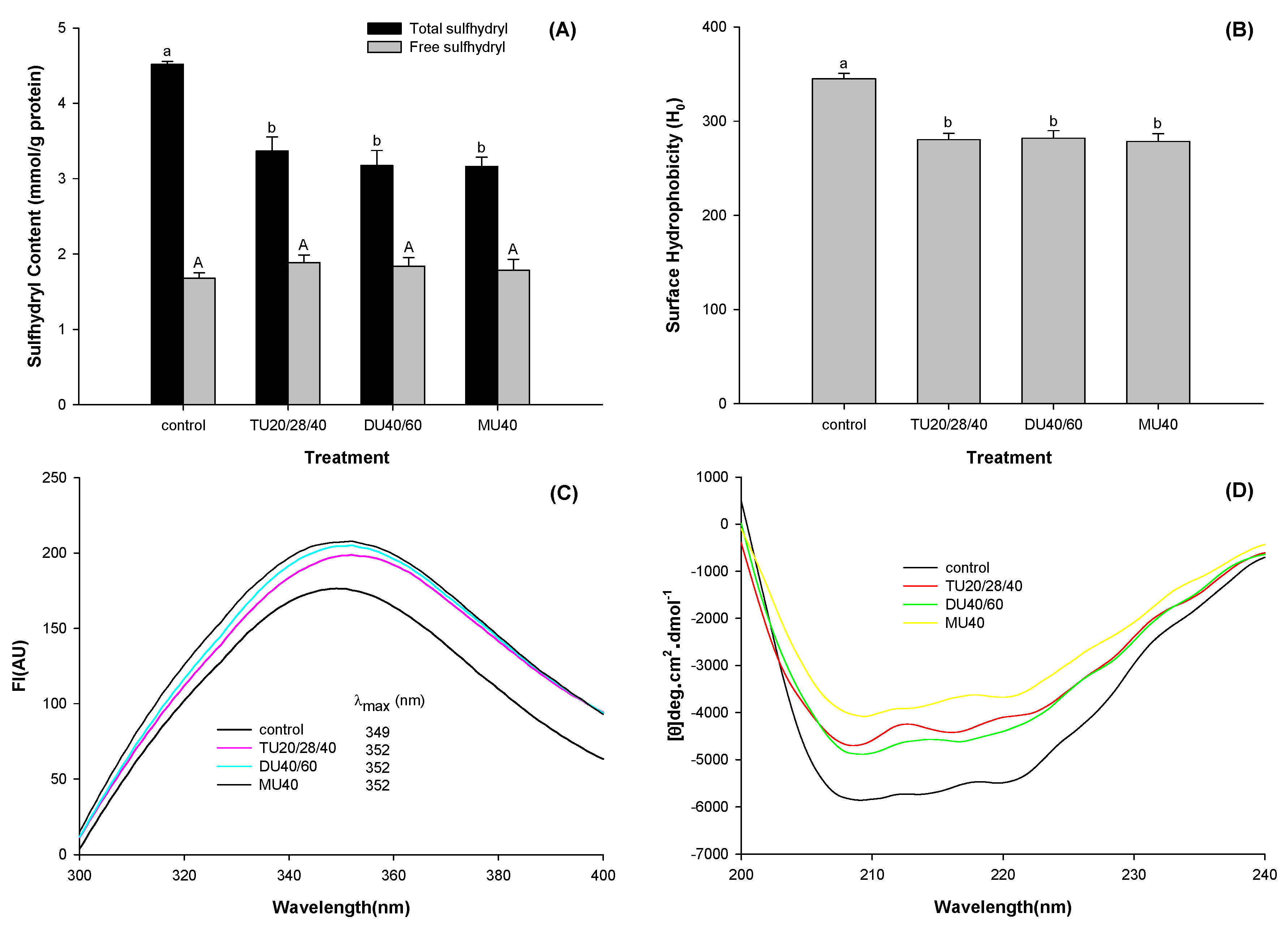
3.3. Effect of Online Us/t40/pHS Treatment on the Structural Characteristics of Potato Protein.
3.3.1. Free and Total Sulfhydryl Contents
3.3.2. Surface Hydrophobicity
3.3.3. Intrinsic Fluorescence
3.3.4. Circular Dichroism Spectra Measurement
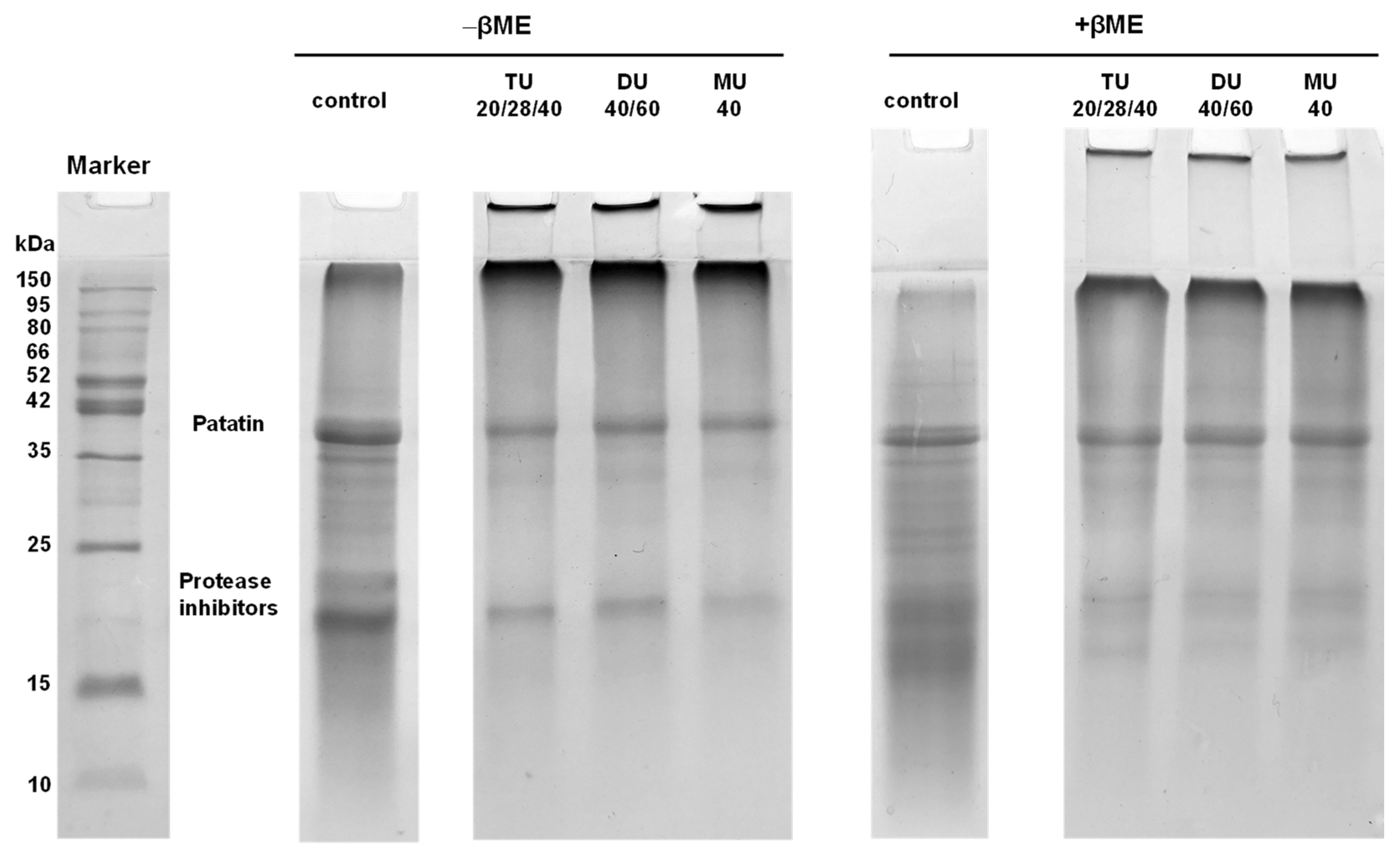
3.3.5. Electrophoresis
3.3.6. Zeta Potential
3.4. Effect of Online Us/t40/pHS Treatment on the Morphology of the Potato Protein
4. Discussion
4.1. The Relationship between the Protein Structure and Solubility of Potato Protein
4.2. The Relationship between the Structure and Digestion of Potato Protein
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Waglay, A.; Karboune, S. Chapter 4—Potato Proteins: Functional Food Ingredients. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 75–104. [Google Scholar]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Pobiega, K. The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry. Open Life Sci. 2020, 15, 787–796. [Google Scholar] [CrossRef]
- Kot, A.M.; Pobiega, K.; Piwowarek, K.; Kieliszek, M.; Błażejak, S.; Gniewosz, M.; Lipińska, E. Biotechnological Methods of Management and Utilization of Potato Industry Waste—A review. Potato Res. 2020, 63, 1–17. [Google Scholar] [CrossRef]
- Mäkinen, S.; Streng, T.; Larsen, L.B.; Laine, A.; Pihlanto, A. Angiotensin I-converting enzyme inhibitory and antihypertensive properties of potato and rapeseed protein-derived peptides. J. Funct. Foods 2016, 25, 160–173. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Q.; Xiong, Y.L. A pH shift approach to the improvement of interfacial properties of plant seed proteins. Curr. Opin. Food Sci. 2018, 19, 50–56. [Google Scholar] [CrossRef]
- Fang, Z.; Cai, X.; Wu, J.; Zhang, L.; Fang, F.; Wang, S. Effect of simultaneous treatment combining ultrasonication and pH-shifting on SPI in the formation of nanoparticles and encapsulating resveratrol. Food Hydrocoll. 2021, 111, 106250. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, J.; Xiong, Y.L. Structural and emulsifying properties of soy protein isolate subjected to acid and alkaline pH-shifting processes. J. Agric. Food Chem. 2009, 57, 7576–7583. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, J.; Andrade, J.; Rababah, T.M.; Almajwal, A.; Abulmeaty, M.M.; Feng, H. Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrason. Sonochemistry 2017, 38, 835–842. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, B.; Liu, Y.; Xiong, Y.L. Interfacial structural role of pH-shifting processed pea protein in the oxidative stability of oil/water emulsions. J. Agric. Food Chem. 2014, 62, 1683–1691. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, Y.; Xiong, Y.L. Heating-Aided pH Shifting Modifies Hemp Seed Protein Structure, Cross-Linking, and Emulsifying Properties. J. Agric. Food Chem. 2018, 66, 10827–10834. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L.; Chen, J. pH Shifting Alters Solubility Characteristics and Thermal Stability of Soy Protein Isolate and Its Globulin Fractions in Different pH, Salt Concentration, and Temperature Conditions. J. Agric. Food Chem. 2010, 58, 8035–8042. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Wu, M.; Li, J.; Bai, Y.; Xu, W.; Qiu, S. Synergistic effect of pH shifting and mild heating in improving heat induced gel properties of peanut protein isolate. LWT 2020, 131, 109812. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Dai, C.; He, R.; Ma, H. Alkali extraction of rice residue protein isolates: Effects of alkali treatment conditions on lysinoalanine formation and structural characterization of lysinoalanine-containing protein. Food Chem. 2018, 261, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Smith, B. The functional modification of legume proteins by ultrasonication: A review. Trends Food Sci. Technol. 2020, 98, 107–116. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Effects of divergent ultrasound pretreatment on the structure of watermelon seed protein and the antioxidant activity of its hydrolysates. Food Chem. 2019, 299, 125165. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Wang, X.-Y.; Yang, X.-Q.; Li, L. Formation of soluble aggregates from insoluble commercial soy protein isolate by means of ultrasonic treatment and their gelling properties. J. Food Eng. 2009, 92, 432–437. [Google Scholar] [CrossRef]
- Lee, H.; Yildiz, G.; Dos Santos, L.; Jiang, S.; Andrade, J.E.; Engeseth, N.J.; Feng, H. Soy protein nano-aggregates with improved functional properties prepared by sequential pH treatment and ultrasonication. Food Hydrocoll. 2016, 55, 200–209. [Google Scholar] [CrossRef]
- Silventoinen, P.; Sozer, N. Impact of ultrasound treatment and pH-shifting on physicochemical properties of protein-enriched barley fraction and barley protein Isolate. Foods 2020, 9, 1055. [Google Scholar] [CrossRef]
- Van Koningsveld, G.A.; Gruppen, H.; De Jongh, H.H.J.; Wijngaards, G.; Van Boekel, M.A.J.S.; Walstra, P.; Voragen, A.G.J. Effects of pH and Heat Treatments on the Structure and Solubility of Potato Proteins in Different Preparations. J. Agric. Food Chem. 2001, 49, 4889–4897. [Google Scholar] [CrossRef]
- Minekus, M.; Marie, A.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Brodkorb, M. A standardised static in-vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Spellman, D.; McEvoy, E.; O’Cuinn, G.; Fitzgerald, R. Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. Int. Dairy J. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Patrick, P.S.; Swaisgood, H.E. Sulfhydryl and Disulfide Groups in Skim Milk as Affected by Direct Ultra-High-Temperature Heating and Subsequent Storage. J. Dairy Sci. 1976, 59, 594–600. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Zhang, W.; Liu, C.; Jauregi, P.; Huang, M. Modification of heat-induced whey protein gels by basic amino acids. Food Hydrocoll. 2020, 100, 105397. [Google Scholar] [CrossRef]
- Liang, Q.; Ren, X.; Qu, W.; Zhang, X.; Cheng, Y.; Ma, H. The impact of ultrasound duration on the structure of β-lactoglobulin. J. Food Eng. 2020, 292, 110365. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Zhang, Z.; Wang, Y.; Mintah, B.K.; Dabbour, M.; Jiang, H.; He, R.; Ma, H. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrason. Sonochemistry 2020, 69, 105240. [Google Scholar] [CrossRef]
- Ma, H.; Huang, L.; Peng, L.; Wang, Z.; Yang, Q. Pretreatment of garlic powder using sweep frequency ultrasound and single frequency countercurrent ultrasound: Optimization and comparison for ACE inhibitory activities. Ultrason. Sonochemistry 2015, 23, 109–115. [Google Scholar] [CrossRef]
- Hoving, S.; Gerrits, B.; Voshol, H.; Müller, D.; Roberts, R.C.; Van Oostrum, J. Preparative two-dimensional gel electrophoresis at alkaline pH using narrow range immobilized pH gradients. Proteomics 2002, 2, 127–134. [Google Scholar] [CrossRef]
- Shi, T.; Yuan, L.; Mu, J.; Gao, R. The effect of Arginine, Lysine and Histidine in the myosin secondary structure by circular dichroism and Raman spectroscopy. CyTA J. Food 2018, 17, 656–660. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Hultin, H.O. Changes in Conformation and Subunit Assembly of Cod Myosin at Low and High pH and after Subsequent Refolding. J. Agric. Food Chem. 2003, 51, 7187–7196. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Song, X.; Zhou, C.; Fu, F.; Chen, Z.; Wu, Q. Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Ind. Crop. Prod. 2013, 43, 538–544. [Google Scholar] [CrossRef]
- Puppo, C.; Chapleau, N.; Speroni, F.; de Lamballerie-Anton, M.; Michel, F.; Añón, C.; Anton, M. Physicochemical modifications of high-pressure-treated soybean protein isolates. J. Agric. Food Chem. 2004, 52, 1564–1571. [Google Scholar]
- Wang, Z.-X.; Wu, C.; Lei, H.; Duan, Y. Accurate ab Initio Study on the Hydrogen-Bond Pairs in Protein Secondary Structures. J. Chem. Theory Comput. 2007, 3, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Keerati-U-Rai, M.; Miriani, M.; Iametti, S.; Bonomi, F.; Corredig, M. Structural changes of soy proteins at the oil–water interface studied by fluorescence spectroscopy. Colloids Surfaces B 2012, 93, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, Y.; Wu, J.; Donkor, P.O.; Li, T.; Ma, H. Improving the enzymolysis efficiency of potato protein by simultaneous dual-frequency energy-gathered ultrasound pretreatment: Thermodynamics and kinetics. Ultrason. Sonochem. 2017, 37, 351–359. [Google Scholar] [CrossRef] [PubMed]

| Control | TU20/28/40 | DU40/60 | MU40 | ||
|---|---|---|---|---|---|
| Gastric digestion | y0 (mmol/L) | 0.24 ± 0.08 | 0.21 ± 0.08 | 0.18 ± 0.09 | 0.22 ± 0.09 |
| A (mmol/L) | 6.2 ± 0.52 b | 6.96 ± 0.39 a | 6.88 ± 1.32 a | 7.07 ± 0.27 a | |
| K (min−1) | 0.39 ± 0.06 b | 0.47 ± 0.02 a | 0.48 ± 0.13 a | 0.45 ± 0.02 a | |
| R2 | 0.95 ± 0.01 | 0.98 ± 0.02 | 0.98 ± 0.01 | 0.97 ± 0.01 | |
| Intestinal digestion | y0 (mmol/L) | 9.44 ± 1.12 | 9.7 ± 0.45 | 9.42 ± 0.04 | 9.42 ± 0.04 |
| A (mmol/L) | 4.76 ± 0.88 b | 5.56 ± 0.13 a | 5.68 ± 0.19 a | 5.68 ± 0.19 a | |
| K (min−1) | 0.14 ± 0.02 b | 0.178 ± 0.02 a | 0.18 ± 0.01 a | 0.18 ± 0.01 a | |
| R2 | 0.97 ± 0.03 | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.99 ± 0.01 |
| α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) | |
|---|---|---|---|---|
| control | 18.35 ± 0.85 a | 27.15 ± 1.05 b | 22.60 ± 0.40 a | 31.45 ± 1.25 a |
| TU20/28/40 | 8.35 ± 0.75 b | 38.05 ± 0.95 a | 21.95 ± 0.15 a,b | 31.70 ± 0.10 a |
| DU40/60 | 8.55 ± 0.05 b | 37.90 ± 0.20 a | 21.25 ± 0.75 a,b | 32.00 ± 0.20 a |
| MU40 | 7.95 ± 1.25 b | 36.60 ± 1.40 a | 22.05 ± 0.95 a,b | 33.45 ± 1.65 a |
| Solubility | Digestibility | α-Helix | β-Sheet | β-Turn | Random | Total Sulfhydryl | Free Sulfhydryl | H0 | λmax | FI | Particle Size | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| digestibility | 0.951 ** | |||||||||||
| α-helix | −0.985 ** | −0.927 ** | ||||||||||
| β-sheet | 0.957 ** | 0.915 ** | −0.957 ** | |||||||||
| β-turn | −0.713 ** | −0.713 ** | 0.692 * | −0.723 ** | ||||||||
| random | 0.402 | 0.387 | −0.401 | 0.154 | −0.125 | |||||||
| total sulfhydryl | −0.959 ** | −0.930 ** | 0.960 ** | −0.912 ** | 0.721 ** | −0.459 | ||||||
| free sulfhydryl | 0.523 | 0.476 | −0.537 | 0.677 * | −0.288 | −0.305 | −0.523 | |||||
| H0 | −0.989 ** | −0.976 ** | 0.976 ** | −0.960 ** | 0.724 ** | −0.384 | 0.964 ** | −0.507 | ||||
| λmax | 0.995 ** | 0.959 ** | −0.987 ** | 0.976 ** | −0.705 * | 0.351 | −0.956 ** | 0.571 | −0.993 ** | |||
| FI | 0.980 ** | 0.950 ** | −0.957 ** | 0.925 ** | −0.769 ** | 0.469 | −0.957 ** | 0.461 | −0.978 ** | 0.974 ** | ||
| particle size | 0.941 ** | 0.889 ** | −0.915 ** | 0.915 ** | −0.640 ** | 0.319 | −0.853 ** | 0.524 | −0.921 ** | 0.939 ** | 0.907 ** | |
| zeta potential | 0.777 ** | 0.723 ** | −0.756 ** | 0.756 ** | −0.698 ** | 0.204 | −0.776 ** | 0.567 | −0.752 ** | 0.769 ** | 0.789 ** | 0.744 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, C.; Wu, J.; Zhang, X.; Ma, F.; Cheng, Y. Improving the Solubility and Digestibility of Potato Protein with an Online Ultrasound-Assisted PH Shifting Treatment at Medium Temperature. Foods 2020, 9, 1908. https://doi.org/10.3390/foods9121908
Mao C, Wu J, Zhang X, Ma F, Cheng Y. Improving the Solubility and Digestibility of Potato Protein with an Online Ultrasound-Assisted PH Shifting Treatment at Medium Temperature. Foods. 2020; 9(12):1908. https://doi.org/10.3390/foods9121908
Chicago/Turabian StyleMao, Chao, Juan Wu, Xiangzhi Zhang, Fengping Ma, and Yu Cheng. 2020. "Improving the Solubility and Digestibility of Potato Protein with an Online Ultrasound-Assisted PH Shifting Treatment at Medium Temperature" Foods 9, no. 12: 1908. https://doi.org/10.3390/foods9121908
APA StyleMao, C., Wu, J., Zhang, X., Ma, F., & Cheng, Y. (2020). Improving the Solubility and Digestibility of Potato Protein with an Online Ultrasound-Assisted PH Shifting Treatment at Medium Temperature. Foods, 9(12), 1908. https://doi.org/10.3390/foods9121908





