Effect of Roasting Time and Cryogenic Milling on the Physicochemical Characteristics of Dried Ginseng Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ginseng Preparation and Pretreatment
2.2. Preparation of Roasted Ginseng Powder
2.3. Treatments
2.4. Particle Size
2.5. Color
2.6. WSI
2.7. DPPH Free Radical Scavenging Activity
2.8. Polyphenolic and Polysaccharide Extract Preparation
2.8.1. TPC
2.8.2. Total Polysaccharide Content
2.8.3. Acidic Polysaccharide Content
2.9. Ginsenoside Content
2.10. Statistical Analysis
3. Results and Discussion
3.1. Particle Size
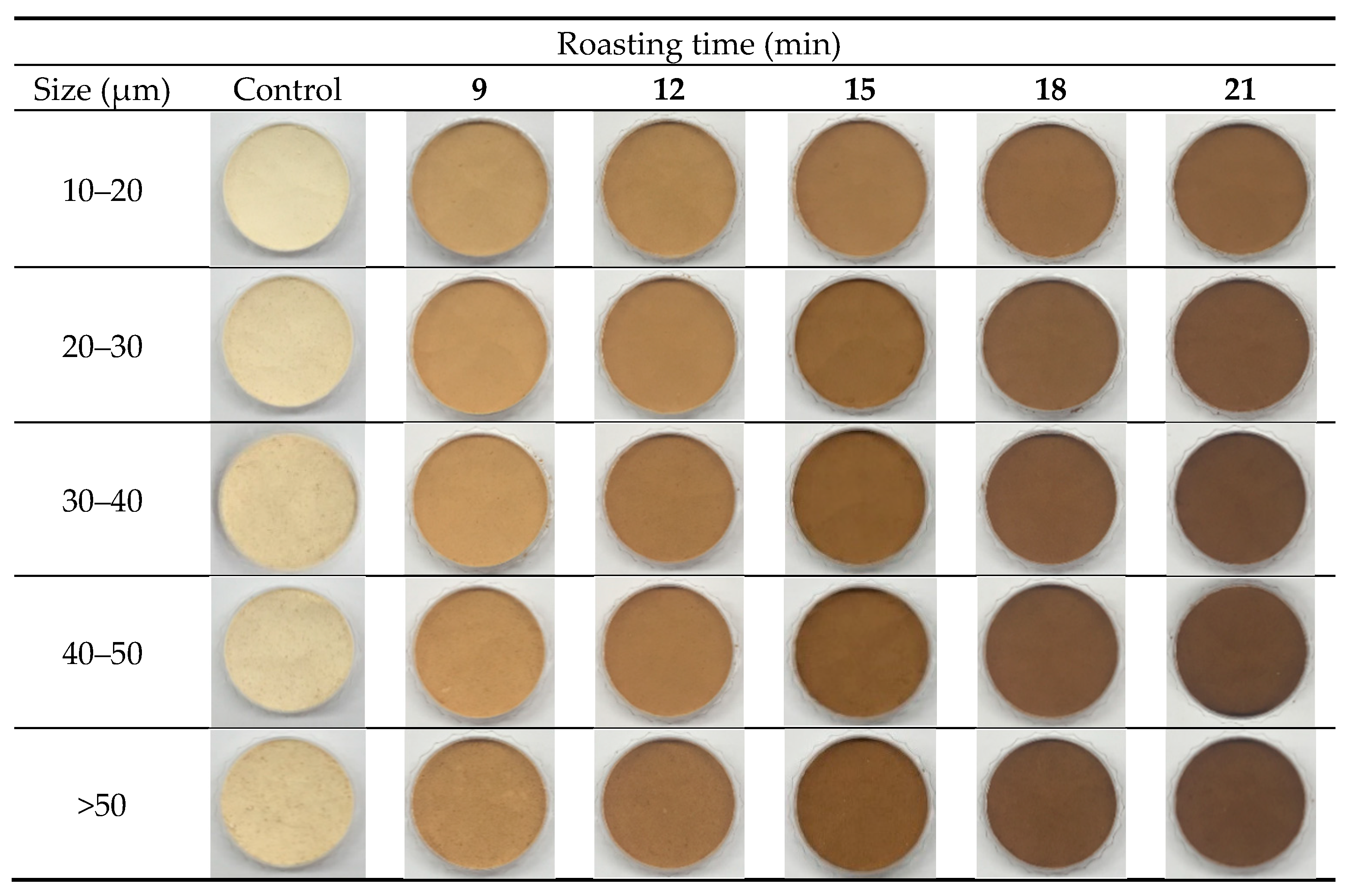
3.2. Appearance
3.3. Color
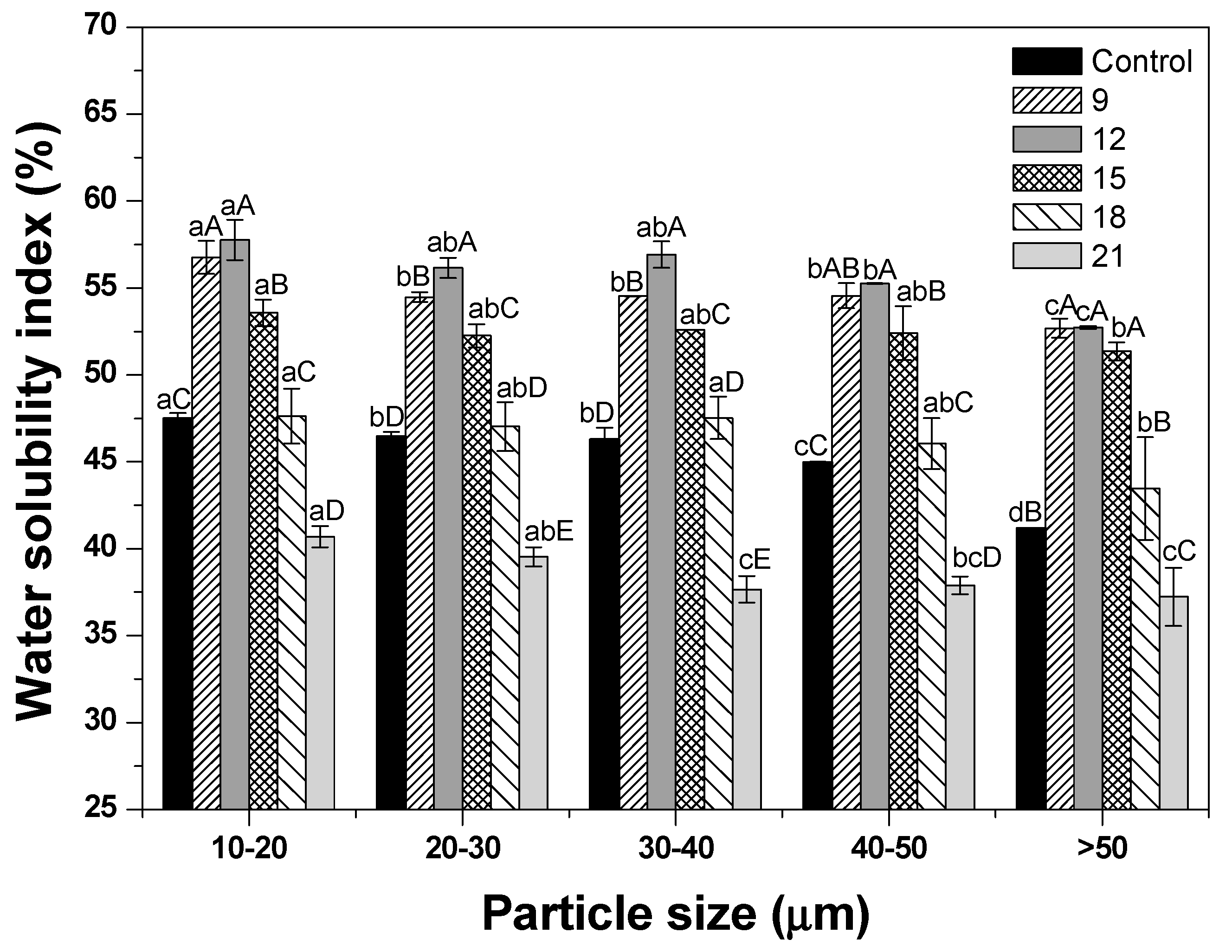
3.4. WSI
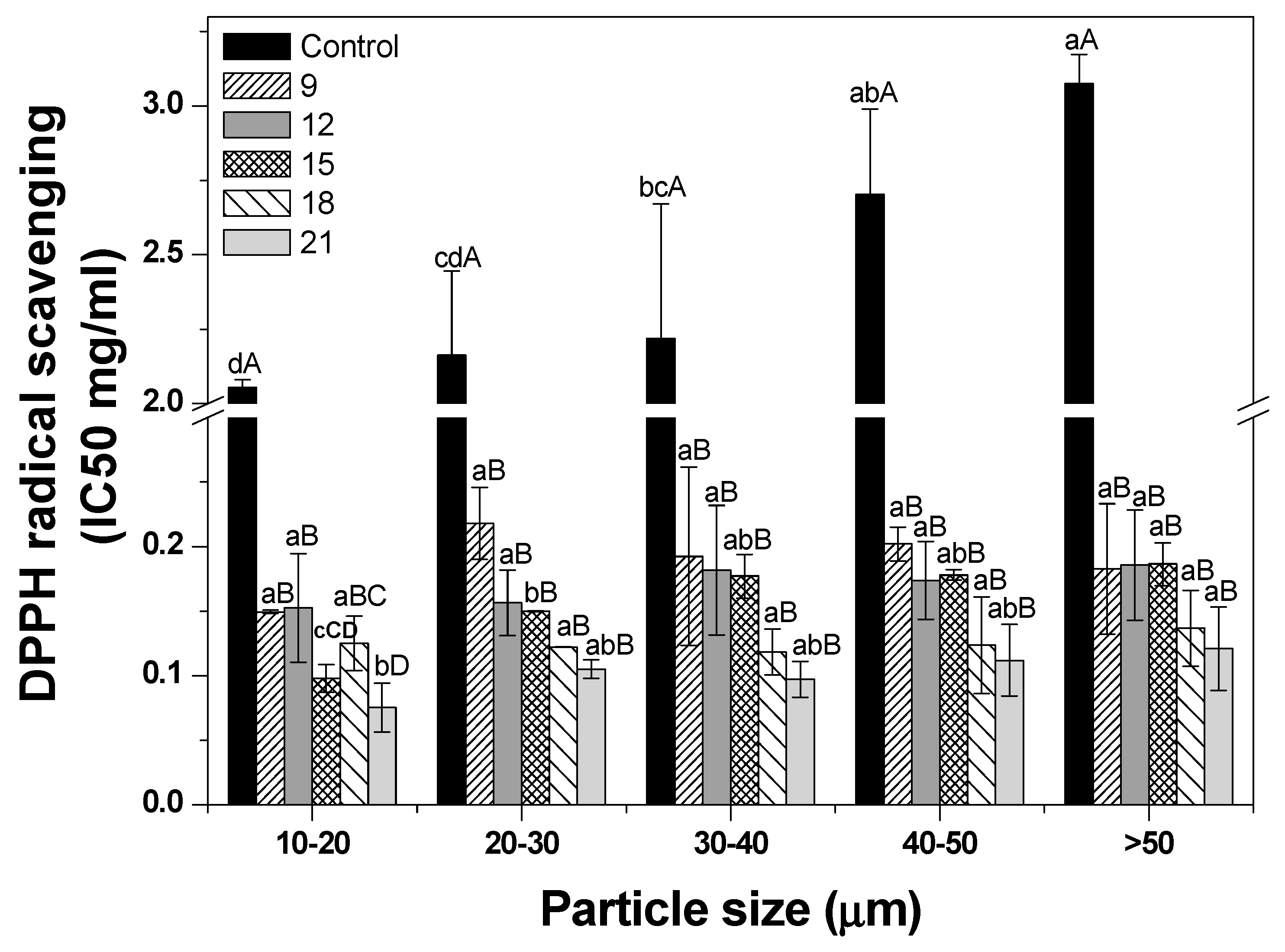
3.5. Antioxidant Activity
3.6. TPC
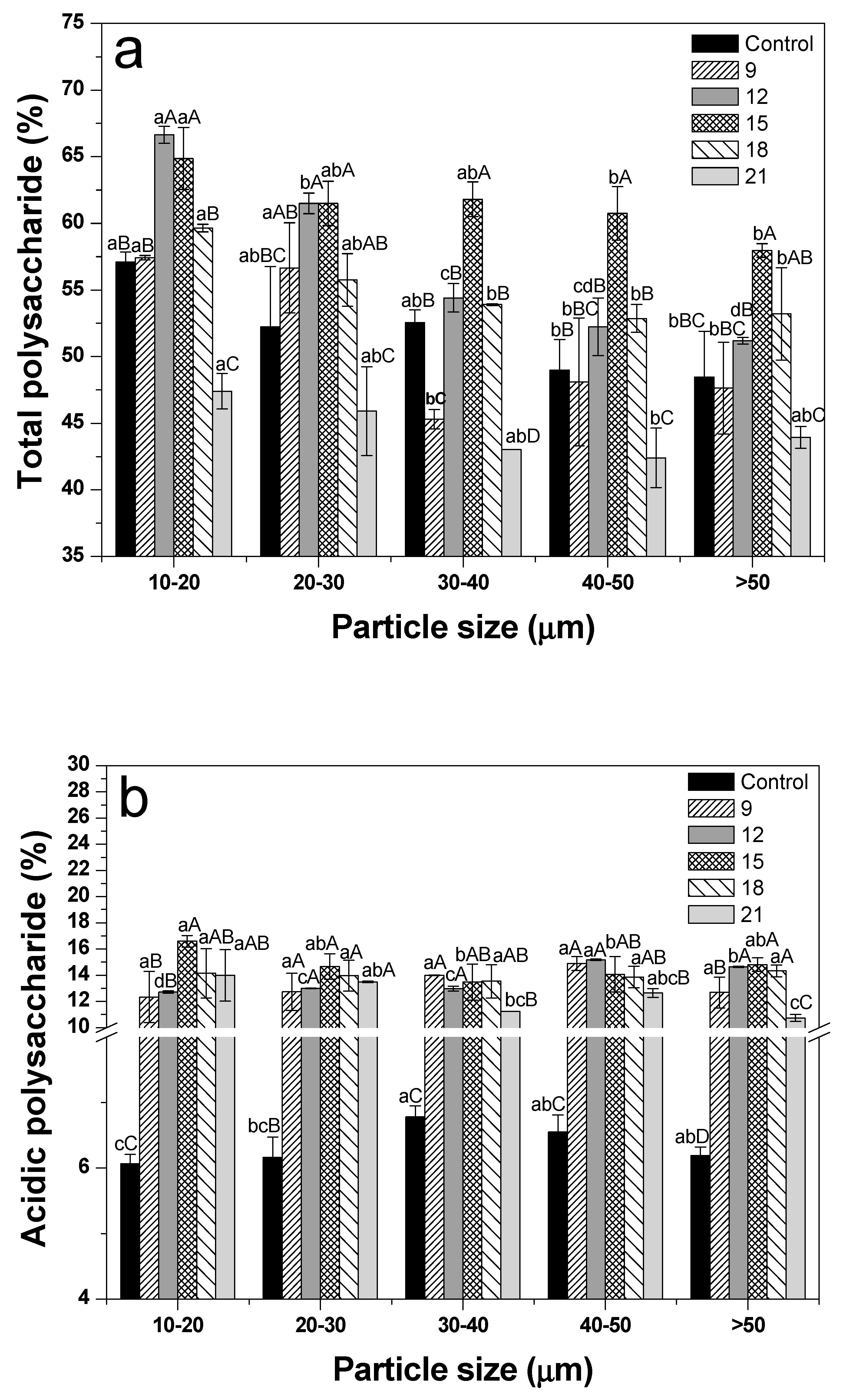
3.7. Polysaccharide Content
3.8. Ginsenoside Content
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yun, T.K. Brief introduction of Panax ginseng CA Meyer. J. Korean Med. Sci. 2001, 16, S3–S5. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Lee, K.Y.; Cho, Y.J.; Park, B.K.; Kim, K.J.; Lee, N.R.; Kim, D.G.; Kim, Y.H.; Hahn, T.W. Efficacy of orally administered ginseng stem and leaf in chickens. Korean J. Vet. Res. 2015, 55, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, X.G. Studies on the transforming mechanism of amino acid components in ginseng in the course of ginseng process. J. Ginseng Res. 1992, 16, 64–67. [Google Scholar]
- Kang, K.S.; Yokozawa, T.; Yamabe, N.; Kim, H.Y.; Park, J.H. ESR study on the structure and hydroxyl radical-scavenging activity relationships of ginsenosides isolate from Panax ginseng CA Meyer. Biol. Pharm. Bull. 2007, 30, 917–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.K.; Jeon, B.S.; Yang, J.W. The chemical components of Korean ginseng. Food Ind. Nutr. 2003, 8, 10–23. [Google Scholar]
- Lee, J.H.; Choi, K.H.; Sohn, E.H.; Jang, K.H. Quality characteristics and ginsenosides composition of ginseng-Yakju according to the particle size of ginseng powder. Nutr. Food Sci. Prev. 2013, 18, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.T. Botanical characteristics, pharmacological effects and medicinal compo-nents of Korean Panax ginseng CA Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.M.; Yao, Q.; Chen, C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.O.; Lee, I.; Park, S.Y.R.; Kim, D.E.; Lim, J.D.; Kang, W.S.; Ko, S.H. Ultrafine Angelica gigas powder normalizes ovarian hormone levels and has antiosteoporosis properties in ovariectomized rats: Particle size effect. J. Med. Food 2012, 15, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Suh, C.S.; Chun, J.K. Relationships among the roasting conditions, colors and extractable solid content of roasted barley. Korean J. Food Sci. Technol. 1981, 13, 334–339. [Google Scholar]
- Redgwell, R.J.; Trovato, V.; Curti, D. Cocoa bean carbohydrates: Roasting induced changes and polymer interactions. Food Chem. 2003, 80, 511–516. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Trovato, V.; Curti, D.; Fischer, M. Effect of roasting on degradation and structural features of polysaccharides in Arabica coffee beans. Carbohydr. Res. 2002, 337, 421–431. [Google Scholar] [CrossRef]
- Saklara, S.; Unganb, S.; Katnasc, S. Microstructural changes in hazelnuts during roasting. Food Res. Int. 2003, 36, 19–23. [Google Scholar] [CrossRef]
- Jeon, E.J.; Kim, K.Y.; Lee, J.E.; Catherine, W.; Kwon, J.H. Monitoring of Roasting Conditions for the Functional Properties of Lateral Root of Red Ginseng. Korean J. Food Preserv. 2008, 15, 396–404. [Google Scholar]
- Park, M.H.; Kim, K.C. Changes in Physicochemical Components of Ginseng Marc by Roasting Process. Korean J. Ginseng Sci. 1995, 19, 144–152. [Google Scholar]
- Sivetz, M.; Desrosier, N.W. Coffee technology. United States Department of Agriculture National Agricultural Library; Westport, CT, Avi: Chicago, IL, USA, 1979; p. 716. [Google Scholar]
- Yoon, S.K.; Kim, W.J. Effects of roasting conditions on quality and yields of barley tea. Korean J. Food Sci. Technol. 1989, 21, 575–582. [Google Scholar]
- Lee, B.G.; Lee, K.Y.; Jorge, S.; Jorge, R.; Baek, H.; Min, J.H.; Kang, W.S. Ultrafine powderization using low temperature turbo mill to improve water solubility of red ginseng powder. In Proceedings of the 2012 12th IEEE International Conference on Nanotechnology (IEEE-NANO), Birmingham, UK, 20–23 August 2012; pp. 1–4. [Google Scholar] [CrossRef]
- Manohar, B.; Sridhar, B.S. Size and shape characterization of conventionally and cryogenically ground turmeric (Curcuma domestica) particles. Powder Technol. 2001, 120, 292–297. [Google Scholar] [CrossRef]
- Meghwal, M.; Goswami, T.K. Evaluation of size reduction and power requirement in ambient and cryogenically ground fenugreek powder. Adv. Powder Technol. 2013, 24, 427–435. [Google Scholar] [CrossRef]
- Lee, S.B.; Yoo, S.H.; Ganesan, P.; Kwak, H.S. Physicochemical and antioxidative properties of Korean nanopowdered white ginseng. Food Sci. Technol. 2013, 48, 2159–2165. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Ough, C.S.; Amerine, M.A. Methods for Analysis of Musts and Wine; Wiley & Sons: New York, NY, USA, 1988; pp. 176–180. [Google Scholar]
- Dubois, M.; Gillers, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, J. Colorimetric method for determination of sugar and related substance. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Do, J.H.; Lee, H.O.; Lee, S.K.; Jang, J.K.; Lee, S.D.; Sung, H.S. Colorimetric Determination of Acidic Polysaccharide from Panax ginseng, its Extraction Condition and Stability. J. Ginseng Res. 1993, 17, 139–144. [Google Scholar]
- Kim, W.; Choi, S.; Kerr, W.; Johnson, J.; Gaines, C. Effect of heating temperature on particle size distribution in hard and soft wheat flour. J. Cereal Sci. 2004, 40, 9–16. [Google Scholar] [CrossRef]
- Im, G.Y.; Jang, S.Y.; Jeong, Y.J. Quality characteristics of Panax ginseng CA Meyer with steaming heat and wet grinding conditions. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1005–1010. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.S. Effect of grinding methods on particle size and crystalline structure of copper phthalocyanine. J. Ind. Eng. Chem. 2007, 18, 41–47. [Google Scholar]
- Jo, G.S.; Sin, J.S.; Kim, J.H. Measurement of particle size and particle size distribution. Polym. Sci. Technol. 2004, 15, 198–208. [Google Scholar]
- Uhm, Y.R.; Kim, J.W.; Jung, J.W.; Rhee, C.K. The fabrication of PVA polymer coated on the surface of B4C nanocomposite by high energy ball mill. J. Korean Powder Metall. 2009, 16, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Seo, H.I.; Ko, J.Y.; Kim, J.I.; Lee, J.S.; Song, S.B.; Jung, T.W.; Kim, K.Y.; Kwak, D.Y.; Oh, I.S.; et al. Physicochemical characteristics of the sorghum (Sorghum bicolor L. Moench) powder following low temperature-microparticulation. Koran J. Food Nutr. 2012, 25, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.K.; Jeong, Y.H.; Gu, S.Y.; Song, K.Y.; Kim, I.Y.; Kim, K.Y. Physicochemical characteristics of Brazilian coffea arabica cv. Catuai coffee extracts with different roasting conditions. J. Korean Soc. Food Sci. Nutr. 2019, 48, 748–756. [Google Scholar] [CrossRef]
- Hemansson, A. Gel characteristics-structure as related to texture and water binding of blood plasma gels. J. Food Sci. 1982, 47, 1965–1972. [Google Scholar] [CrossRef]
- Guha, M.; Ali, S.Z.; Bhattacharya, S. Twin-screw extrusion of rice flour without a die: Effect of barrel temperature and screw speed on extrusion and extrudate characteristics. J. Food Eng. 1997, 32, 251–267. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Cho, Y.J. Antioxidant, angiotensinconverting enzyme and xanthin oxidase inhibitory activity of extracts from Saururus chinensis leaves by ultrafine grinding. Korean J. Food Preserv. 2014, 21, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Lee, I.H.; Lee, H.S.; Park, J.K. R & D trend and information analysis of nanoparticles. Pros. Ind. Chem. 2013, 6, 46–61. [Google Scholar]
- Kim, C.S.; Kim, H.I. Physicochemical properties of non-waxy rice flour affected by grinding methods and steeping times. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1076–1083. [Google Scholar] [CrossRef]
- Song, Y.B.; Lee, K.S.; Lee, M.S.; Kim, A.J. Bioactivity changes in mung beans according to the roasting time. Korean J. Food Nutr. 2013, 26, 502–507. [Google Scholar] [CrossRef]
- Seong, B.J.; Kim, S.I.; Jee, M.G.; Kim, S.D.; Kwon, A.R.; Kim, H.H.; Hwang, Y.G.; Lee, K.S. Physicochemical characteristics according to the roasting conditions and grinding grade for the development of drip type red ginseng. J. Korean Soc. Food Sci. Nutr. 2018, 47, 309–319. [Google Scholar] [CrossRef]
- Cha, S.M.; Son, B.Y.; Lee, J.S.; Baek, S.B.; Kim, S.L.; Ku, J.H.; Hwang, J.J.; Song, B.H.; Woo, S.H.; Kwon, Y.U.; et al. Effect of particle size on physico-chemical properties and antioxidant activity of corn silk powder. Korean J. Crop. Sci. 2012, 57, 41–50. [Google Scholar] [CrossRef]
- Durmaz, G.; Alpaslan, M. Antioxidant properties of roasted apricot (Prunus armeniaca L.) Kernel. Food Chem. 2007, 100, 1177–1181. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, O.J.; Gweon, O.C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Chung, H.S.; Chung, S.K.; Youn, K.S. Effects of roasting temperature and time on bulk density, soluble solids, browning index and phenolic compounds of corn kernels. J. Food Process Pres. 2011, 35, 832–839. [Google Scholar] [CrossRef]
- Cho, K.L.; Woo, H.J.; Lee, I.S.; Lee, J.W.; Cho, Y.C.; Lee, I.N.; Chae, H.J. Optimization of Enzymatic Pretreatment for the Production of Fermented Ginseng using Leaves, Stems and Roots of Ginseng. J. Ginseng Res. 2010, 34, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.W.; Kim, S.W.; Rho, J.H.; Rhee, Y.K. Extraction characteristics of saponin and acidic polysaccharide based on the red ginseng particle size. J. Ginseng Res. 2008, 32, 179–186. [Google Scholar] [CrossRef]
- Lee, J.W.; Do, J.H. Extraction condition of acidic polysaccharide from Korean red ginseng marc. J. Ginseng Res. 2002, 26, 202–205. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Bi, H.; Li, X.; Ni, W.; Han, H.; Li, N.; Wang, B.; Zhou, Y.; Tai, G. Total fractionation and characterization of the water soluble polysaccharides iso-lated from panax ginsen C.A. Meyer. Carbohydr. Polym. 2009, 77, 544–552. [Google Scholar] [CrossRef]
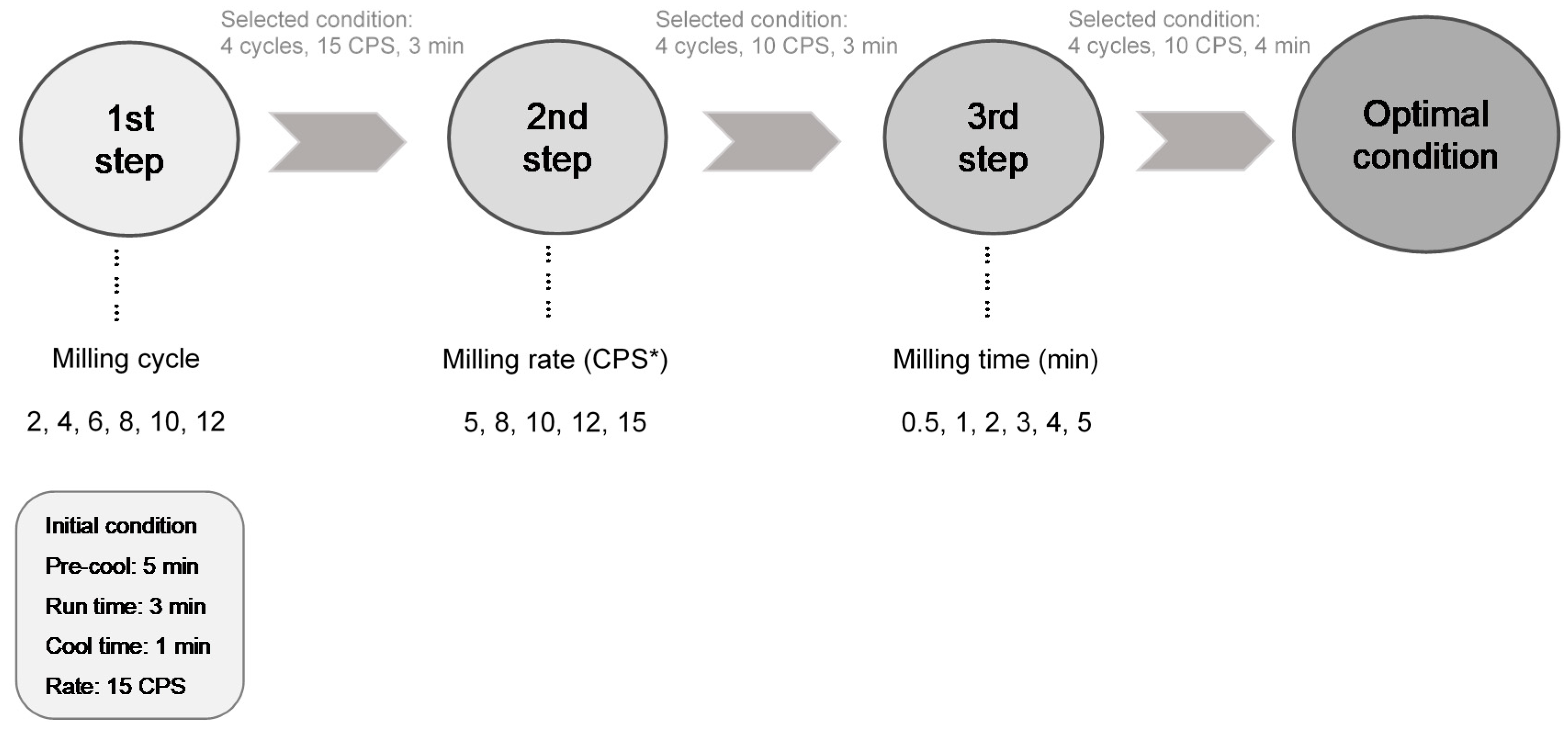


| Treatment Conditions | Particle Size (μm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Size (μm) | Treatment | Cycle | CPS | Time | D [3,2] | D [4,3] | Dx (10) | Dx (50) | Dx (90) |
| 10–20 | 1 | 4 | 10 | 4 | 14.05 ± 0.21 jC | 68.24 ± 7.08 iB | 5.78 ± 0.15 jB | 28.49 ± 0.93 gC | 145.88 ± 8.50 iB |
| 2 | 4 | 10 | 3 | 14.97 ± 0.70 jB | 63.82 ± 4.49 iB | 6.13 ± 0.31 jA | 30.75 ± 1.24 gB | 150.93 ± 8.15 iB | |
| 3 | 4 | 10 | 5 | 15.52 ± 0.61 jA | 89.43 ± 10.93 hA | 6.20 ± 0.16 jA | 32.15 ± 1.74 gA | 193.54 ± 16.51 hA | |
| 20–30 | 4 | 4 | 10 | 2 | 21.04 ± 2.20 iE | 114.43 ± 5.47 fgBC | 8.07 ± 0.88 iE | 47.55 ± 1.42 fC | 308.43 ± 15.75 fC |
| 5 | 4 | 8 | 3 | 22.54 ± 0.43 hD | 105.13 ± 3.72 gC | 8.91 ± 0.26 hD | 47.83 ± 0.76 fC | 263.75 ± 11.08 gD | |
| 6 | 4 | 12 | 3 | 24.93 ± 0.35 gC | 113.71 ± 4.23 fgBC | 9.58 ± 0.13 gC | 57.15 ± 0.77 eB | 299.17 ± 4.71 fCD | |
| 7 | 6 | 15 | 3 | 28.74 ± 1.02 eA | 131.47 ± 13.30 eA | 11.82 ± 0.29 eB | 53.69 ± 2.79 eA | 377.87 ± 58.51 dA | |
| 8 | 4 | 15 | 3 | 29.57 ± 0.64 fB | 124.00 ± 8.16 fB | 13.78 ± 0.15 dA | 60.53 ± 2.56 eA | 345.50 ± 40.43 eB | |
| 30–40 | 9 | 8 | 15 | 3 | 31.67 ± 1.16 eC | 162.80 ± 17.15 eC | 12.92 ± 0.31 eB | 60.79 ± 4.72 eC | 522.33 ± 54.00 dC |
| 10 | 10 | 15 | 3 | 31.90 ± 1.64 eC | 177.00 ± 14.38 dB | 11.90 ± 0.27 fD | 61.05 ± 4.34 eC | 567.27 ± 45.37 cB | |
| 11 | 4 | 10 | 1 | 34.48 ± 0.82 dB | 215.55 ± 11.16 bA | 12.44 ± 0.28 eC | 113.82 ± 9.08 bA | 591.73 ± 24.49 bcB | |
| 12 | 12 | 15 | 3 | 40.02 ± 2.77 cA | 226.00 ± 3.00 bA | 15.20 ± 0.54 cA | 86.65 ± 6.28 dB | 664.20 ± 33.04 aA | |
| 13 | 4 | 5 | 3 | 40.63 ± 1.01 cA | 217.63 ± 6.44 bA | 15.01 ± 0.33 cA | 111.25 ± 4.43 bA | 602.38 ± 15.85 bB | |
| 40–50 | 14 | 2 | 15 | 3 | 46.83 ± 0.99 b | 198.80 ± 6.98 c | 19.03 ± 0.38 b | 104.00 ± 1.41 c | 529.40 ± 22.83 d |
| >50 | 15 | 4 | 10 | 0.5 | 64.07 ± 5.31 a | 289.00 ± 5.00 a | 24.95 ± 2.06 a | 207.40 ± 6.27 a | 690.40 ± 10.60 a |
| Roasting (1) | Particle Size (μm) | |||||
|---|---|---|---|---|---|---|
| Size (μm) | Time (min) | D [3,2] | D [4,3] | Dx (10) | Dx (50) | Dx (90) |
| 10–20 | Control | 14.00 ± 0.20 eA | 68.20 ± 7.10 eA | 5.80 ± 0.20 eA | 28.50 ± 0.90 eA | 145.90 ± 8.50 eA |
| 9 | 8.22 ± 0.33 eC | 45.78 ± 4.61 eB | 3.28 ± 0.11 eC | 18.67 ± 1.70 eB | 110.00 ± 3.92 dB | |
| 12 | 7.58 ± 0.27 dD | 30.83 ± 2.86 eC | 3.04 ± 0.10 eD | 15.87 ± 1.13 eC | 77.60 ± 8.60 dC | |
| 15 | 5.60 ± 0.12 eF | 20.94 ± 0.88 dD | 2.19 ± 0.05 eE | 10.29 ± 0.63 eE | 55.83 ± 1.18 eD | |
| 18 | 7.06 ± 0.10 eE | 26.03 ± 2.02 eCD | 2.90 ± 0.07 eD | 13.93 ± 0.30 eD | 68.48 ± 6.00 dC | |
| 21 | 9.27 ± 0.10 dB | 29.38 ± 3.99 dC | 3.89 ± 0.05 dB | 17.77 ± 1.67 bB | 70.55 ± 11.40 dC | |
| 20–30 | Control | 24.90 ± 0.40 dA | 113.70 ± 4.20 dA | 9.60 ± 0.10 dA | 57.20 ± 0.80 dA | 299.20 ± 4.70 dA |
| 9 | 20.34 ± 1.52 dB | 112.50 ± 5.45 dA | 7.87 ± 0.15 dB | 48.94 ± 1.14 dB | 288.33 ± 5.03 cAB | |
| 12 | 16.92 ± 0.63 cD | 92.02 ± 13.90 dB | 7.10 ± 0.34 cC | 35.08 ± 0.30 dC | 274.50 ± 19.94 cB | |
| 15 | 9.80 ± 0.44 dE | 52.40 ± 11.92 cD | 3.87 ± 0.13 dE | 22.56 ± 2.38 dD | 118.35 ± 16.78 dE | |
| 18 | 17.38 ± 0.39 dCD | 68.33 ± 5.95 dC | 6.51 ± 0.69 dD | 35.58 ± 6.31 dC | 181.33 ± 14.64 cD | |
| 21 | 17.62 ± 0.48 cC | 97.50 ± 8.48 cB | 7.07 ± 0.24 cC | 37.62 ± 1.48 bC | 252.67 ± 4.16 cC | |
| 30–40 | Control | 34.50 ± 0.80 cA | 215.50 ± 11.20 bA | 12.40 ± 0.30 cA | 113.80 ± 9.10 bA | 591.70 ± 24.50 bA |
| 9 | 30.60 ± 0.84 cB | 181.00 ± 10.86 cB | 11.88 ± 0.44 cB | 87.08 ± 1.99 cB | 503.00 ± 46.90 bB | |
| 12 | 16.45 ± 0.13 cD | 137.40 ± 9.10 cD | 6.38 ± 0.12 dD | 48.70 ± 1.21 cD | 403.17 ± 57.68 bC | |
| 15 | 12.66 ± 0.59 cE | 62.40 ± 2.49 cE | 4.92 ± 0.16 cE | 31.21 ± 2.28 cE | 180.80 ± 6.30 cD | |
| 18 | 23.58 ± 0.99 cC | 143.75 ± 3.86 cCD | 9.26 ± 0.48 cC | 57.28 ± 2.61 cC | 403.20 ± 28.90 bC | |
| 21 | 24.22 ± 0.79 bC | 155.00 ± 3.56 bC | 9.27 ± 0.46 bcC | 62.08 ± 1.65 bC | 445.20 ± 34.80 bC | |
| 40–50 | Control | 46.80 ± 1.00 bA | 198.80 ± 7.00 cA | 19.00 ± 0.40 bA | 104.00 ± 1.40 cB | 529.40 ± 22.80 cA |
| 9 | 37.98 ± 2.72 bB | 208.67 ± 6.81 bA | 14.18 ± 1.18 bB | 127.25 ± 11.76 bA | 524.67 ± 15.50 bA | |
| 12 | 28.80 ± 0.73 bC | 156.60 ± 3.78 bB | 11.14 ± 0.46 bC | 75.54 ± 1.84 bC | 423.80 ± 15.91 bB | |
| 15 | 14.88 ± 0.53 bE | 90.56 ± 11.83 bC | 5.98 ± 0.28 bE | 41.40 ± 0.88 bD | 248.50 ± 29.22 bC | |
| 18 | 26.83 ± 0.68 bD | 156.00 ± 3.87 bB | 10.36 ± 0.46 bD | 73.58 ± 3.24 bC | 427.00 ± 8.03 bB | |
| 21 | 26.46 ± 0.50 bD | 157.40 ± 4.28 bB | 10.05 ± 0.32 bD | 70.20 ± 1.90 bC | 442.00 ± 18.75 bB | |
| >50 | Control | 64.10 ± 5.30 aA | 289.00 ± 5.00 aB | 25.00 ± 2.10 aA | 207.40 ± 6.30 aAB | 690.40 ± 10.60 aB |
| 9 | 48.33 ± 0.96 aB | 282.25 ± 10.14 aB | 18.80 ± 0.75 aB | 193.50 ± 7.33 aB | 686.00 ± 26.15 aB | |
| 12 | 45.83 ± 0.51 aB | 287.67 ± 0.58 aB | 16.53 ± 0.38 aB | 197.75 ± 7.14 aAB | 708.67 ± 7.77 aB | |
| 15 | 25.58 ± 0.10 aC | 219.40 ± 4.28 aC | 9.73 ± 0.12 aC | 96.60 ± 3.32 aC | 635.20 ± 10.28 aC | |
| 18 | 43.26 ± 1.31 aB | 293.80 ± 14.82 aB | 15.76 ± 0.64 aB | 192.00 ± 19.7 aB | 731.60 ± 19.40 aB | |
| 21 | 45.96 ± 6.74 aB | 338.57 ± 57.82 aA | 16.97 ± 3.84 aB | 265.71 ± 95.90 aA | 781.57 ± 54.01 aA | |
| Roasting (1) | Color | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (μm) | Time (min) | L* | a* | b* | ΔE | ||||||||
| 10–20 | Control | 82.13 | ± | 0.54 aA | 0.71 | ± | 0.22 dC | 15.40 | ± | 0.14 dC | - | ||
| 9 | 65.65 | ± | 0.04 aB | 5.26 | ± | 0.00 eB | 17.03 | ± | 0.04 cA | 17.18 | ± | 0.02 dE | |
| 12 | 57.65 | ± | 0.05 aC | 6.01 | ± | 0.02 eA | 16.16 | ± | 0.03 aB | 25.06 | ± | 0.05 dD | |
| 15 | 52.35 | ± | 0.08 aD | 5.44 | ± | 0.03 dB | 14.32 | ± | 0.06 aD | 30.17 | ± | 0.08 eC | |
| 18 | 45.95 | ± | 0.07 aE | 5.87 | ± | 0.01 aA | 12.36 | ± | 0.01 aE | 36.67 | ± | 0.07 eB | |
| 21 | 44.53 | ± | 0.05 aF | 5.42 | ± | 0.01 aB | 11.50 | ± | 0.03 aF | 38.11 | ± | 0.03 dA | |
| 20–30 | Control | 80.44 | ± | 0.25 bA | 1.07 | ± | 0.04 cF | 16.03 | ± | 0.36 cB | - | ||
| 9 | 60.99 | ± | 0.06 cB | 5.94 | ± | 0.01 dC | 17.02 | ± | 0.03 cA | 20.08 | ± | 0.06 aE | |
| 12 | 54.27 | ± | 0.13 bC | 6.29 | ± | 0.01 dA | 15.53 | ± | 0.03 bC | 26.65 | ± | 0.10 cD | |
| 15 | 44.59 | ± | 0.09 bD | 6.04 | ± | 0.05 bB | 11.90 | ± | 0.03 cD | 36.42 | ± | 0.09 aC | |
| 18 | 44.03 | ± | 0.02 bE | 5.55 | ± | 0.02 cD | 11.26 | ± | 0.01 bE | 37.00 | ± | 0.02 dB | |
| 21 | 40.31 | ± | 0.01 bF | 5.27 | ± | 0.00 bE | 9.57 | ± | 0.00 bF | 40.86 | ± | 0.01 bA | |
| 30–40 | Control | 78.77 | ± | 0.77 cA | 1.41 | ± | 0.09 bF | 16.64 | ± | 0.26 bB | - | ||
| 9 | 61.23 | ± | 0.05 bB | 6.01 | ± | 0.01 cC | 17.24 | ± | 0.01 aA | 18.15 | ± | 0.05 cE | |
| 12 | 51.71 | ± | 0.14 cC | 6.66 | ± | 0.02 bA | 15.08 | ± | 0.03 cC | 27.61 | ± | 0.14 bD | |
| 15 | 43.83 | ± | 0.16 cD | 6.32 | ± | 0.02 aB | 12.28 | ± | 0.02 bD | 35.51 | ± | 0.14 bC | |
| 18 | 42.03 | ± | 0.02 cE | 5.71 | ± | 0.02 bD | 10.60 | ± | 0.02 cE | 37.48 | ± | 0.02 bB | |
| 21 | 38.19 | ± | 0.13 cF | 4.99 | ± | 0.01 cE | 8.22 | ± | 0.05 cF | 41.60 | ± | 0.12 aA | |
| 40–50 | Control | 77.57 | ± | 0.04 dA | 1.83 | ± | 0.02 aF | 17.21 | ± | 0.01 aA | - | ||
| 9 | 59.63 | ± | 0.08 dB | 6.49 | ± | 0.01 bB | 17.18 | ± | 0.05 bA | 18.54 | ± | 0.08 bE | |
| 12 | 51.65 | ± | 0.14 cC | 6.56 | ± | 0.03 cA | 14.77 | ± | 0.00 dB | 26.56 | ± | 0.04 cD | |
| 15 | 43.36 | ± | 0.03 dD | 5.91 | ± | 0.03 cC | 11.23 | ± | 0.05 dC | 34.97 | ± | 0.03 dC | |
| 18 | 40.78 | ± | 0.14 dE | 5.54 | ± | 0.02 cD | 9.81 | ± | 0.04 dD | 37.71 | ± | 0.13 dB | |
| 21 | 37.72 | ± | 0.07 dF | 4.99 | ± | 0.02 cE | 8.01 | ± | 0.01 dE | 41.00 | ± | 0.06 bA | |
| >50 | Control | 76.31 | ± | 0.10 eA | 1.91 | ± | 0.05 aE | 17.26 | ± | 0.01 aA | - | ||
| 9 | 56.92 | ± | 0.08 eB | 6.83 | ± | 0.03 aA | 16.80 | ± | 0.05 dB | 20.01 | ± | 0.08 aE | |
| 12 | 49.10 | ± | 0.12 eC | 6.86 | ± | 0.00 aA | 14.04 | ± | 0.01 eC | 27.85 | ± | 0.13 aD | |
| 15 | 41.77 | ± | 0.06 eD | 5.95 | ± | 0.04 cB | 10.84 | ± | 0.03 eD | 35.36 | ± | 0.06 cC | |
| 18 | 40.08 | ± | 0.06 eE | 5.34 | ± | 0.00 dC | 9.22 | ± | 0.02 eE | 37.28 | ± | 0.05 cB | |
| 21 | 37.35 | ± | 0.18 eF | 4.97 | ± | 0.01 dD | 7.90 | ± | 0.02 eF | 40.18 | ± | 0.17 cA | |
| Roasting (1) | Ginsenosides Content (mg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Size (μm) | Time (min) | Rg1 | Re | Rf | Rb1 | Rc | Rb2 | Rb3 | Rd |
| 10–20 | Control | 3.68 ± 0.03 C | 3.41 ± 0.02 D | 1.46 ± 0.08 B | 4.26 ± 0.03 C | 1.14 ± 0.04 C | 0.97 ± 0.03 C | 0.19 ± 0.02 C | 0.31 ± 0.01 C |
| 9 | 4.62 ± 0.02 A | 2.82 ± 0.01 E | 1.01 ± 0.01 C | 4.18 ± 0.04 C | 1.01 ± 0.03 D | 0.91 ± 0.01 C | 0.15 ± 0.00 C | 0.35 ± 0.00 C | |
| 12 | 3.91 ± 0.13 B | 7.56 ± 0.21 B | 1.77 ± 0.00 A | 8.34 ± 0.30 B | 3.35 ± 0.13 B | 2.99 ± 0.11 B | 0.51 ± 0.02 B | 0.91 ± 0.03 B | |
| 15 | 2.30 ± 0.14 E | 1.84 ± 0.10 F | 0.73 ± 0.01 D | 3.04 ± 0.03 D | 1.05 ± 0.03 CD | 0.92 ± 0.01 C | 0.17 ± 0.01 C | 0.18 ± 0.01 D | |
| 18 | 4.00 ± 0.09 B | 8.01 ± 0.17 A | 1.85 ± 0.04 A | 9.88 ± 0.82 A | 4.07 ± 0.10 A | 3.60 ± 0.11 A | 0.66 ± 0.03 A | 1.02 ± 0.05 A | |
| 21 | 3.35 ± 0.05 D | 6.88 ± 0.14 C | 0.27 ± 0.08 E | ND E | ND E | ND D | ND D | 0.21 ± 0.02 D | |
| Time (min) | Rg2(S) | Rg2(R) | Rg3(S) | Rg3(R) | Rh1(S) | Rh2(S) | Sum | ||
| Control | 0.17 ± 0.01 D | 0.09 ± 0.01 C | ND B,(2) | ND B | 0.10 ± 0.02 B | ND | 15.77 ± 0.02 bC | ||
| 9 | 0.15 ± 0.01 D | 0.14 ± 0.00 BC | ND B | ND B | 0.03 ± 0.00 D | ND | 15.39 ± 0.09 bC | ||
| 12 | 0.73 ± 0.02 B | 0.48 ± 0.02 A | ND B | ND B | 0.04 ± 0.00 D | ND | 30.64 ± 1.03 cB | ||
| 15 | 0.26 ± 0.04 C | 0.10 ± 0.04 C | 0.19 ± 0.01 A | 0.07 ± 0.03 A | 0.22 ± 0.00 A | ND | 11.06 ± 0.03 cD | ||
| 18 | 0.89 ± 0.02 A | 0.34 ± 0.30 AB | ND B | ND B | 0.07 ± 0.00 C | ND | 34.39 ± 1.10 aA | ||
| 21 | NDE | 0.19 ± 0.04 BC | ND B | ND B | NDE | ND | 10.73 ± 0.00 dD | ||
| Size (μm) | Time (min) | Rg1 | Re | Rf | Rb1 | Rc | Rb2 | Rb3 | Rd |
| 20–30 | Control | 3.28 ± 0.04 B | 2.68 ± 0.02 C | 0.81 ± 0.70 B | 3.60 ± 0.06 D | 0.90 ± 0.02 D | 0.78 ± 0.02 D | 0.19 ± 0.01 C | 0.28 ± 0.02 D |
| 9 | 4.59 ± 0.07 A | 2.37 ± 0.03 CD | 0.96 ± 0.03 B | 4.24 ± 0.06 C | 1.12 ± 0.01 C | 1.03 ± 0.01 D | 0.17 ± 0.00 C | 0.38 ± 0.01 C | |
| 12 | 3.10 ± 0.05 B | 8.88 ± 0.15 A | 1.65 ± 0.06 A | 9.62 ± 0.15 A | 4.14 ± 0.06 A | 3.64 ± 0.07 A | 0.62 ± 0.01 A | 1.14 ± 0.01 A | |
| 15 | 2.17 ± 0.07 C | 2.08 ± 0.08 D | 0.73 ± 0.03 B | 3.08 ± 0.16 E | 1.05 ± 0.00 C | 0.84 ± 0.02 D | 0.14 ± 0.01 C | 0.23 ± 0.02 E | |
| 18 | 2.98 ± 0.37 B | 8.71 ± 0.24 A | 1.67 ± 0.17 A | 9.71 ± 0.34 A | 4.09 ± 0.10 A | 3.29 ± 0.41 B | 0.60 ± 0.09 A | 1.11 ± 0.02 A | |
| 21 | 2.11 ± 0.03 C | 5.47 ± 0.35 B | 1.60 ± 0.10 A | 6.49 ± 0.04 B | 3.14 ± 0.01 B | 2.88 ± 0.00 C | 0.51 ± 0.02 B | 0.87 ± 0.05 B | |
| Time (min) | Rg2(S) | Rg2(R) | Rg3(S) | Rg3(R) | Rh1(S) | Rh2(S) | Sum | ||
| Control | 0.06 ± 0.02 E | 0.06 ± 0.01 E | ND C | ND B | 0.10 ± 0.01 C | ND | 12.75 ± 0.07 cD | ||
| 9 | 0.15 ± 0.00 D | 0.14 ± 0.00 D | ND C | ND B | 0.04 ± 0.00 E | ND | 15.18 ± 0.20 bC | ||
| 12 | 0.93 ± 0.01 B | 0.63 ± 0.01 A | ND C | ND B | 0.04 ± 0.00 E | ND | 34.39 ± 0.55 bA | ||
| 15 | 0.32 ± 0.02 C | 0.16 ± 0.01 D | 0.19 ± 0.02 B | 0.05 ± 0.01 A | 0.22 ± 0.03 B | ND | 11.26 ± 0.03 bcE | ||
| 18 | 0.97 ± 0.02 B | 0.56 ± 0.07 B | ND C | ND B | 0.07 ± 0.01 D | ND | 34.41 ± 0.97 aA | ||
| 21 | 1.21 ± 0.08 A | 0.39 ± 0.03 C | 0.46 ± 0.03 A | ND B | 0.26 ± 0.02 A | ND | 25.80 ± 0.15 aB | ||
| Size (μm) | Time (min) | Rg1 | Re | Rf | Rb1 | Rc | Rb2 | Rb3 | Rd |
| 30–40 | Control | 3.93 ± 0.08 B | 3.24 ± 0.04 D | 1.13 ± 0.31 C | 4.60 ± 0.28 D | 1.57 ± 0.16 D | 1.33 ± 0.17 C | 0.19 ± 0.04 D | 0.42 ± 0.07 E |
| 9 | 5.20 ± 0.08 A | 2.94 ± 0.03 E | 1.04 ± 0.01 C | 5.16 ± 0.09 C | 1.42 ± 0.03 D | 1.29 ± 0.04 C | 0.21 ± 0.01 D | 0.49 ± 0.01 D | |
| 12 | 3.20 ± 0.02 C | 9.94 ± 0.03 A | 1.75 ± 0.07 A | 11.14 ± 0.02 A | 4.54 ± 0.06 A | 4.00 ± 0.04 A | 0.68 ± 0.01 B | 1.30 ± 0.01 A | |
| 15 | 2.14 ± 0.05 F | 2.26 ± 0.07 F | 0.68 ± 0.00 D | 3.39 ± 0.07 E | 1.26 ± 0.09 E | 1.06 ± 0.06 D | 0.15 ± 0.04 E | 0.26 ± 0.03 F | |
| 18 | 3.02 ± 0.10 D | 8.59 ± 0.22 B | 1.81 ± 0.06 A | 10.01 ± 0.37 B | 4.31 ± 0.13 B | 4.03 ± 0.11 A | 0.74 ± 0.03 A | 1.14 ± 0.02 B | |
| 21 | 1.79 ± 0.02 E | 4.70 ± 0.08 C | 1.51 ± 0.03 B | 5.40 ± 0.06 C | 2.53 ± 0.05 C | 2.29 ± 0.03 B | 0.43 ± 0.00 C | 0.74 ± 0.02 C | |
| Time (min) | Rg2(S) | Rg2(R) | Rg3(S) | Rg3(R) | Rh1(S) | Rh2(S) | Sum | ||
| Control | 0.20 ± 0.08 E | 0.11 ± 0.12 E | ND C | ND B | 0.05 ± 0.09 C | ND | 16.77 ± 0.10 aE | ||
| 9 | 0.20 ± 0.00 E | 0.20 ± 0.00 D | ND C | ND B | 0.05 ± 0.00 C | ND | 18.20 ± 0.25 aD | ||
| 12 | 1.04 ± 0.01 C | 0.73 ± 0.01 A | ND C | ND B | 0.05 ± 0.00 C | ND | 38.36 ± 0.21 aA | ||
| 15 | 0.32 ± 0.01 D | 0.09 ± 0.00 E | 0.18 ± 0. 00 B | 0.05 ± 0.00 A | 0.20 ± 0.04 B | ND | 12.05 ± 0.03 aF | ||
| 18 | 1.13 ± 0.04 B | 0.56 ± 0.01 B | 0.02 ± 0.01 C | NDB | 0.10 ± 0.01 C | ND | 35.46 ± 1.01 aB | ||
| 21 | 1.28 ± 0.03 A | 0.38 ± 0.01 C | 0.67 ± 0.01 A | NDB | 0.33 ± 0.01 A | ND | 22.05 ± 0.31 cC | ||
| Size (μm) | Time (min) | Rg1 | Re | Rf | Rb1 | Rc | Rb2 | Rb3 | Rd |
| 40–50 | Control | 3.14 ± 0.02 C | 2.70 ± 0.01 E | 1.05 ± 0.03 C | 3.23 ± 0.14 E | 0.73 ± 0.06 E | 0.66 ± 0.04 F | 0.17 ± 0.01 C | 0.25 ± 0.02 E |
| 9 | 4.93 ± 0.03 A | 3.41 ± 0.05 D | 1.08 ± 0.01 C | 5.38 ± 0.06 D | 1.35 ± 0.02 C | 1.20 ± 0.05 D | 0.19 ± 0.01 C | 0.49 ± 0.01 D | |
| 12 | 3.67 ± 0.06 B | 8.68 ± 0.11 A | 1.61 ± 0.04 B | 10.22 ± 0.14 A | 4.01 ± 0.05 A | 3.55 ± 0.06 A | 0.58 ± 0.00 A | 1.23 ± 0.04 A | |
| 15 | 2.16 ± 0.03 F | 1.85 ± 0.01 F | 0.76 ± 0.01 D | 2.95 ± 0.05 F | 1.04 ± 0.06 D | 0.91 ± 0.03 E | 0.18 ± 0.07 C | 0.19 ± 0.01 F | |
| 18 | 2.94 ± 0.02 D | 8.46 ± 0.06 B | 1.78 ± 0.02 A | 9.53 ± 0.16 B | 4.03 ± 0.02 A | 3.44 ± 0.03 B | 0.62 ± 0.01 A | 1.08 ± 0.02 B | |
| 21 | 2.36 ± 0.03 E | 5.67 ± 0.06 C | 1.64 ± 0.02 B | 6.93 ± 0.06 C | 2.63 ± 0.02 B | 2.29 ± 0.06 C | 0.45 ± 0.01 B | 0.72 ± 0.03 C | |
| Time (min) | Rg2(S) | Rg2(R) | Rg3(S) | Rg3(R) | Rh1(S) | Rh2(S) | Sum | ||
| Control | 0.07 ± 0.01 E | 0.02 ± 0.01 E | ND D | ND B | 0.11 ± 0.01 B | ND | 12.13 ± 0.03 cE | ||
| 9 | 0.21 ± 0.00 D | 0.25 ± 0.00 C | ND D | ND B | 0.05 ± 0.00 C | ND | 18.52 ± 0.22 aD | ||
| 12 | 0.86 ± 0.01 C | 0.67 ± 0.01 A | ND D | ND B | 0.05 ± 0.00 C | ND | 35.14 ± 0.50 bA | ||
| 15 | 0.27 ± 0.08 D | 0.16 ± 0.04 D | 0.19 ± 0.02 B | 0.05 ± 0.05 A | 0.23 ± 0.06 A | ND | 10.93 ± 0.04 cF | ||
| 18 | 1.12 ± 0.03 B | 0.65 ± 0.01 A | 0.03 ± 0.00 C | ND B | 0.12 ± 0.00 B | ND | 33.73 ± 0.42 aB | ||
| 21 | 1.19 ± 0.01 A | 0.47 ± 0.01 B | 0.38 ± 0.01 A | ND B | 0.25 ± 0.00 A | ND | 24.99 ± 0.28 bC | ||
| Size (μm) | Time (min) | Rg1 | Re | Rf | Rb1 | Rc | Rb2 | Rb3 | Rd |
| >50 | Control | 3.81 ± 0.10 B | 3.27 ± 0.05 D | 1.50 ± 0.05 B | 4.20 ± 0.11 E | 1.10 ± 0.05 E | 0.94 ± 0.05 E | 0.20 ± 0.03 C | 0.29 ± 0.03 E |
| 9 | 4.47 ± 0.16 A | 3.53 ± 0.08 D | 1.13 ± 0.04 C | 5.33 ± 0.19 D | 1.53 ± 0.06 D | 1.36 ± 0.05 D | 0.24 ± 0.01 C | 0.47 ± 0.01 D | |
| 12 | 2.91 ± 0.07 C | 8.32 ± 0.32 A | 1.52 ± 0.03 B | 9.65 ± 0.33 A | 4.15 ± 0.18 A | 3.77 ± 0.15 A | 0.62 ± 0.03 A | 1.18 ± 0.07 A | |
| 15 | 2.22 ± 0.01 E | 1.98 ± 0.05 E | 0.81 ± 0.00 D | 3.14 ± 0.03 F | 1.14 ± 0.07 E | 0.96 ± 0.03 E | 0.20 ± 0.06 C | 0.27 ± 0.03 E | |
| 18 | 2.74 ± 0.03 D | 7.55 ± 0.09 B | 1.66 ± 0.02 A | 8.91 ± 0.05 B | 3.73 ± 0.02 B | 3.27 ± 0.03 B | 0.62 ± 0.01 A | 0.97 ± 0.02 B | |
| 21 | 1.96 ± 0.06 F | 4.76 ± 0.22 C | 1.50 ± 0.03 B | 5.86 ± 0.17 C | 2.48 ± 0.06 C | 2.22 ± 0.05 C | 0.42 ± 0.00 B | 0.72 ± 0.02 C | |
| Time (min) | Rg2(S) | Rg2(R) | Rg3(S) | Rg3(R) | Rh1(S) | Rh2(S) | Sum | ||
| Control | 0.16 ± 0.03 E | 0.09 ± 0.01 F | ND D | ND D | 0.11 ± 0.02 C | ND | 15.66 ± 0.04 bE | ||
| 9 | 0.29 ± 0.02 D | 0.25 ± 0.01 D | ND D | ND D | 0.05 ± 0.00 D | ND | 18.66 ± 0.62 aD | ||
| 12 | 0.91 ± 0.03 C | 0.55 ± 0.01 B | 0.01 ± 0.02 D | ND D | 0.04 ± 0.00 D | ND | 33.64 ± 1.22 bA | ||
| 15 | 0.33 ± 0.04 D | 0.17 ± 0.02 E | 0.26 ± 0.02 B | 0.12 ± 0.00 A | 0.21 ± 0.00 B | ND | 11.82 ± 0.02 abF | ||
| 18 | 1.09 ± 0.00 B | 0.57 ± 0.00 A | 0.06 ± 0.00 C | 0.06 ± 0.00 B | 0.11 ± 0.00 C | ND | 31.36 ± 0.22 bB | ||
| 21 | 1.21 ± 0.03 A | 0.40 ± 0.01 C | 0.50 ± 0.01 A | 0.03 ± 0.02 C | 0.27 ± 0.02 A | ND | 22.32 ± 0.61 cC | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.; Park, D.H.; Seo, H.G.; Choi, M.-J.; Cho, Y. Effect of Roasting Time and Cryogenic Milling on the Physicochemical Characteristics of Dried Ginseng Powder. Foods 2020, 9, 223. https://doi.org/10.3390/foods9020223
Jeong H, Park DH, Seo HG, Choi M-J, Cho Y. Effect of Roasting Time and Cryogenic Milling on the Physicochemical Characteristics of Dried Ginseng Powder. Foods. 2020; 9(2):223. https://doi.org/10.3390/foods9020223
Chicago/Turabian StyleJeong, Hayeong, Dong Hyeon Park, Han Geuk Seo, Mi-Jung Choi, and Youngjae Cho. 2020. "Effect of Roasting Time and Cryogenic Milling on the Physicochemical Characteristics of Dried Ginseng Powder" Foods 9, no. 2: 223. https://doi.org/10.3390/foods9020223
APA StyleJeong, H., Park, D. H., Seo, H. G., Choi, M.-J., & Cho, Y. (2020). Effect of Roasting Time and Cryogenic Milling on the Physicochemical Characteristics of Dried Ginseng Powder. Foods, 9(2), 223. https://doi.org/10.3390/foods9020223





