New Evidences of Antibacterial Effects of Cranberry Against Periodontal Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cranberry Extract
2.2. Analysis of Phenolic Compounds in the Cranberry Extract
2.3. Bacteria Strains and Culture Conditions
2.4. Antibacterial Assays
2.4.1. Antibacterial Effect of Cranberry Extract Against Planktonic Bacteria
2.4.2. Antibacterial Effect in an Oral Biofilm Model in Vitro
2.5. Anti-Biofilm Assay
2.6. Microbiological Outcomes
2.7. Confocal Laser Scanning Microscopy (CLSM) Analyses
2.8. Statistical Analyses
3. Results
3.1. Phenolic Composition of the Cranberry Extract
3.2. Antibacterial Assays
3.2.1. Antibacterial Effect of Cranberry Extract Against Planktonic Bacteria
3.2.2. Antibacterial Effects in an in Vitro Biofilm Model: Bacteria Counts
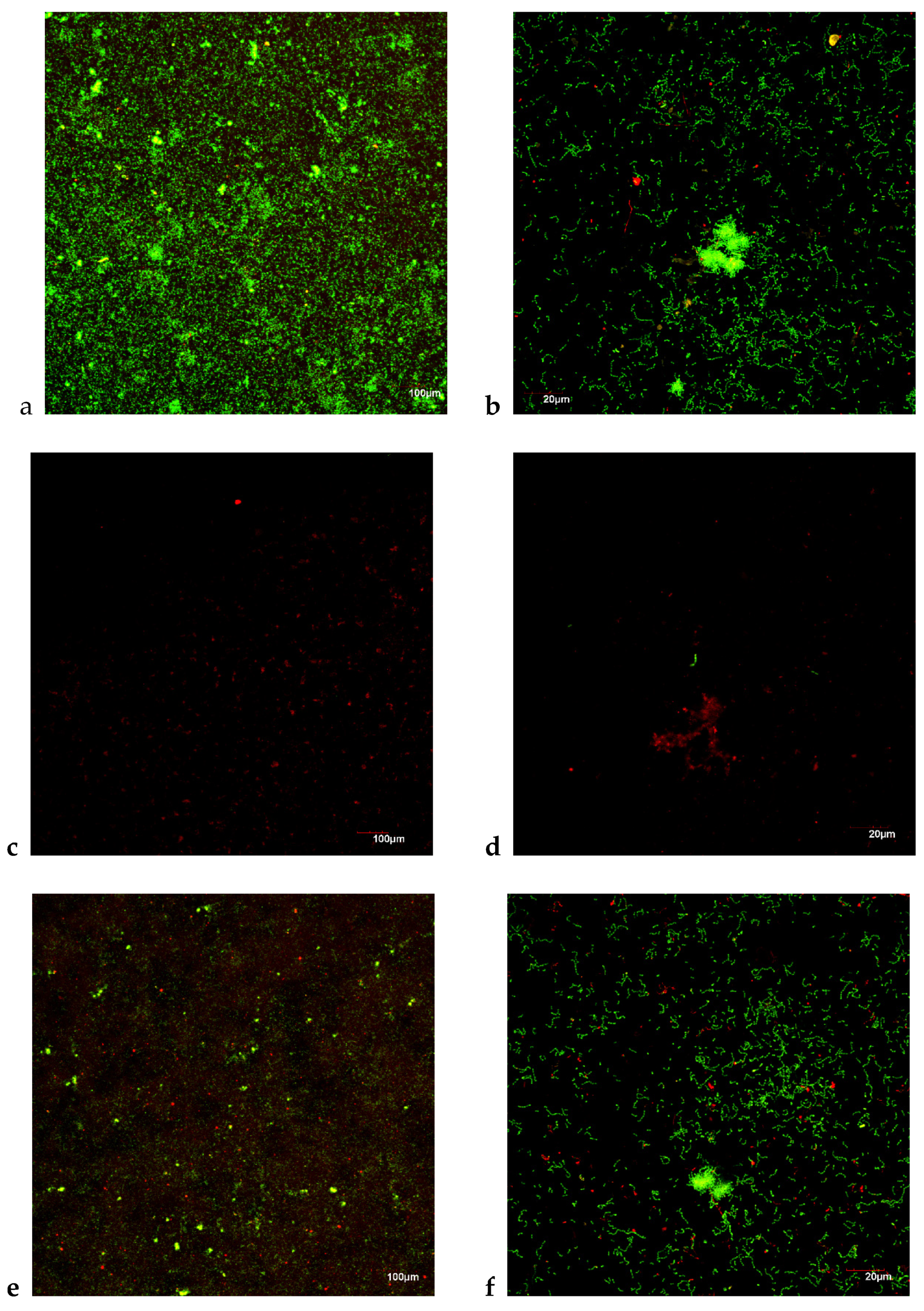
3.2.3. Antibacterial Effects in an in Vitro Biofilm Model: CLSM
3.3. Anti-Biofilm Assay
3.3.1. Anti-Biofilm Assay: Bacteria Counts
3.3.2. Anti-Biofilm Assay: CLSM
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teles, R.; Teles, F.; Frias-Lopez, J.; Paster, B.; Haffajee, A. Lessons learned and unlearned in periodontal microbiology. Periodontol 2013, 62, 95–162. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Van Dyke, T.E. Working group 1 of the joint EFPAAPw. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S24–S29. [Google Scholar] [CrossRef]
- Buset, S.L.; Walter, C.; Friedmann, A.; Weiger, R.; Borgnakke, W.S.; Zitzmann, N.U. Are periodontal diseases really silent? A systematic review of their effect on quality of life. J. Clin. Periodontol. 2016, 43, 333–344. [Google Scholar]
- Herrera, D.; Alonso, B.; Leon, R.; Roldan, S.; Sanz, M. Antimicrobial therapy in periodontitis: The use of systemic antimicrobials against the subgingival biofilm. J. Clin. Periodontol. 2008, 35, 45–66. [Google Scholar] [CrossRef]
- Serrano, J.; Escribano, M.; Roldan, S.; Martin, C.; Herrera, D. Efficacy of adjunctive anti-plaque chemical agents in managing gingivitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, S106–S138. [Google Scholar] [CrossRef]
- van Winkelhoff, A.J.; Herrera, D.; Oteo, A.; Sanz, M. Antimicrobial profiles of periodontal pathogens isolated from periodontitis patients in The Netherlands and Spain. J. Clin. Periodontol. 2005, 32, 893–898. [Google Scholar] [CrossRef]
- van Winkelhoff, A.J. Antibiotics in the treatment of peri-implantitis. Eur. J. Oral Implantol. 2012, 5, S43–S50. [Google Scholar] [PubMed]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human peri-implantitis microbiota. Clin. Oral Implants. Res. 2014, 25, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strydonck, D.A.C.; Slot, D.E.; Van der Velden, U.; Van der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Cozens, D.; Read, R.C. Anti-adhesion methods as novel therapeutics for bacterial infections. Expert. Rev. Anti. Infect. Ther. 2012, 10, 1457–1468. [Google Scholar] [CrossRef]
- Gutierrez, S.; Moran, A.; Martinez-Blanco, H.; Ferrero, M.A.; Rodriguez-Aparicio, L.B. The Usefulness of Non-Toxic Plant Metabolites in the Control of Bacterial Proliferation. Probiotics Antimicrob. Proteins 2017, 9, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Ribeiro-Vidal, H.; Esteban-Fernández, A.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.V.; Sanz, M.; Herrera, D. Antimicrobial activity of red wine and oenological extracts against periodontal pathogens in a validated oral biofilm model. BMC Complement. Altern. Med. 2019, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.; Schaich, K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Impact of plant derivatives on the growth of foodborne pathogens and the functionality of probiotics. Appl. Microbiol. Biotechnol. 2012, 95, 29–45. [Google Scholar] [CrossRef]
- Jensen, H.D.; Struve, C.; Christensen, S.B.; Krogfelt, K.A. Cranberry juice and combinations of its organic acids are effective against experimental urinary tract infection. Front. Microbiol. 2017, 8, 542. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Marais, J.P.; Khoo, C.; LaPlante, K.; Vejborg, R.M.; Givskov, M.; Tolker-Nielsen, T.; Seeram, N.P.; Rowley, D.C. Cranberry (Vaccinium macrocarpon) oligosaccharides decrease biofilm formation by uropathogenic Escherichia coli. J. Funct. Foods 2015, 17, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez de Llano, D.; Liu, H.; Khoo, C.; Moreno-Arribas, M.V.; Bartolome, B. Some new findings regarding the antiadhesive activity of cranberry phenolic compounds and their microbial-derived metabolites against uropathogenic bacteria. J. Agric. Food. Chem. 2019, 67, 2166–2174. [Google Scholar] [CrossRef] [Green Version]
- Maisuria, V.B.; Los Santos, Y.L.; Tufenkji, N.; Deziel, E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 30169. [Google Scholar] [CrossRef] [Green Version]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Murphy, B.T.; Hammond, G.B.; Vinson, J.A.; Neto, C.C. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J. Agric. Food. Chem. 2002, 50, 5844–5849. [Google Scholar] [CrossRef]
- Eydelnant, I.A.; Tufenkji, N. Cranberry derived proanthocyanidins reduce bacterial adhesion to selected biomaterials. Langmuir 2008, 24, 10273–10281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feghali, K.; Feldman, M.; La, V.D.; Santos, J.; Grenier, D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J. Agric. Food Chem. 2012, 60, 5728–5735. [Google Scholar] [CrossRef] [PubMed]
- O’May, C.; Tufenkji, N. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl. Environ. Microbiol. 2011, 77, 3061–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’May, C.; Ciobanu, A.; Lam, H.; Tufenkji, N. Tannin derived materials can block swarming motility and enhance biofilm formation in Pseudomonas aeruginosa. Biofouling 2012, 28, 1063–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: Systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine 2018, 97, e0686. [Google Scholar] [CrossRef]
- Neto, C.C.; Amoroso, J.W.; Liberty, A.M. Anticancer activities of cranberry phytochemicals: An update. Mol. Nutr. Food Res. 2008, 5, S18–S27. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.K.; Kim, K.K.; Satyan, K.S.; Nussbaum, R.; Torres, M.; Brand, L.; Vorsa, N. Cranberry proanthocyanidins are cytotoxic to human cancer cells and sensitize platinum-resistant ovarian cancer cells to paraplatin. Phytother. Res. 2009, 23, 1066–1074. [Google Scholar] [CrossRef] [Green Version]
- Bodet, C.; Grenier, D.; Chandad, F.; Ofek, I.; Steinberg, D.; Weiss, E.I. Potential oral health benefits of cranberry. Crit. Rev. Food. Sci. Nutr. 2008, 48, 672–680. [Google Scholar] [CrossRef]
- Philip, N.; Walsh, L.J. Cranberry polyphenols: Natural weapons against dental caries. Dent. J. 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Bonifait, L.; Grenier, D. Cranberry polyphenols: Potential benefits for dental caries and periodontal disease. J. Can. Dent. Assoc. 2010, 76, a130. [Google Scholar] [PubMed]
- Steinberg, D.; Feldman, M.; Ofek, I.; Weiss, E.I. Cranberry high molecular weight constituents promote Streptococcus sobrinus desorption from artificial biofilm. Int. J. Antimicrob. Agents 2005, 25, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Nino de Guzman, P.; Schobel, B.D.; Vacca Smith, A.V.; Bowen, W.H. Influence of cranberry juice on glucan-mediated processes involved in Streptococcus mutans biofilm development. Caries Res. 2006, 40, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Duarte, S.; Murata, R.M.; Scott-Anne, K.; Gregoire, S.; Watson, G.E.; Singh, A.P.; Vorsa, N. Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Res. 2010, 44, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Gregoire, S.; Singh, A.P.; Vorsa, N.; Koo, H. Influence of cranberry phenolics on glucan synthesis by glucosyltransferases and Streptococcus mutans acidogenicity. J. Appl. Microbiol. 2007, 103, 1960–1968. [Google Scholar] [CrossRef]
- Yamanaka, A.; Kimizuka, R.; Kato, T.; Okuda, K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol. Immunol. 2004, 19, 150–154. [Google Scholar] [CrossRef]
- Labrecque, J.; Bodet, C.; Chandad, F.; Grenier, D. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. J. Antimicrob. Chemother. 2006, 58, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, A.; Kouchi, T.; Kasai, K.; Kato, T.; Ishihara, K.; Okuda, K. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J. Periodontal Res. 2007, 42, 589–592. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Bartolomé, B.; Martín-Álvarez, P.J.; Anderson, M.; Howell, A.; Monagas, M. Comprehensive assessment of the quality of commercial cranberry products. Phenolic characterization and in vitro bioactivity. J. Agric. Food Chem. 2012, 60, 3396–3408. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Llama-Palacios, A.; Blanc, V.; Leon, R.; Herrera, D.; Sanz, M. Structure, viability and bacterial kinetics of an in vitro biofilm model using six bacteria from the subgingival microbiota. J. Periodontal Res. 2011, 46, 252–260. [Google Scholar] [CrossRef]
- Keijser, J.A.; Verkade, H.; Timmerman, M.F.; Van der Weijden, F.A. Comparison of 2 commercially available chlorhexidine mouthrinses. J. Periodontol. 2003, 74, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Roldan, S.; Santacruz, I.; Santos, S.; Masdevall, M.; Sanz, M. Differences in antimicrobial activity of four commercial 0.12% chlorhexidine mouthrinse formulations: An in vitro contact test and salivary bacterial counts study. J. Clin. Periodontol. 2003, 30, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Sekino, S.; Ramberg, P. The effect of a mouth rinse containing phenolic compounds on plaque formation and developing gingivitis. J Clin. Periodontol. 2005, 32, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.C.; Marin, M.J.; Figuero, E.; Llama-Palacios, A.; Leon, R.; Blanc, V.; Herrera, D.; Sanz, M. Quantitative real-time PCR combined with propidium monoazide for the selective quantification of viable periodontal pathogens in an in vitro subgingival biofilm model. J. Periodontal Res. 2014, 49, 20–28. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Slobodnikova, L.; Fialova, S.; Rendekova, K.; Kovac, J.; Mucaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Nogueira, M.C.; Oyarzabal, O.A.; Gombas, D.E. Inactivation of Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella in cranberry, lemon, and lime juice concentrates. J. Food. Prot. 2003, 66, 1637–1641. [Google Scholar] [CrossRef]
- Wojnicz, D.; Tichaczek-Goska, D.; Korzekwa, K.; Kicia, M.; Hendrich, A.B. Study of the impact of cranberry extract on the virulence factors and biofilm formation by Enterococcus faecalis strains isolated from urinary tract infections. Int. J. Food Sci. Nutr. 2016, 67, 1005–1016. [Google Scholar] [CrossRef]
- Cote, J.; Caillet, S.; Doyon, G.; Dussault, D.; Sylvain, J.F.; Lacroix, M. Antimicrobial effect of cranberry juice and extracts. Food Control 2011, 22, 1413–1418. [Google Scholar] [CrossRef]
- La, V.D.; Howell, A.B.; Grenier, D. Anti-Porphyromonas gingivalis and Anti-Inflammatory Activities of A-Type Cranberry Proanthocyanidins. Antimicrob. Agents Chemother. 2010, 54, 1778–1784. [Google Scholar] [CrossRef] [Green Version]
- Chrubasik-Hausmann, S.; Vlachojannis, C.; Zimmermann, B.F. Proanthocyanin Content in Cranberry CE Medicinal Products. Phytother. Res. 2014, 28, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug. Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.; Bandara, H.; Leishman, S.J.; Walsh, L.J. Inhibitory effects of fruit berry extracts on Streptococcus mutans biofilms. Eur. J. Oral Sci. 2019, 127, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka-Okada, A.; Sato, E.; Kouchi, T.; Kimizuka, R.; Kato, T.; Okuda, K. Inhibitory effect of cranberry polyphenol on cariogenic bacteria. Bull Tokyo Dent. Coll. 2008, 49, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Polak, D.; Naddaf, R.; Shapira, L.; Weiss, E.I.; Houri-Haddad, Y. Protective potential of non-dialyzable material fraction of cranberry juice on the virulence of P. gingivalis and F. nucleatum mixed infection. J. Periodontol. 2013, 84, 1019–1025. [Google Scholar] [CrossRef]
- Bodet, C.; Chandad, F.; Grenier, D. Anti-inflammatory activity of a high-molecular-weight cranberry fraction on macrophages stimulated by lipopolysaccharides from periodontopathogens. J. Dent. Res. 2006, 85, 235–239. [Google Scholar] [CrossRef]
- Mi, H.; Wang, D.; Xue, Y.; Zhang, Z.; Niu, J.; Hong, Y.; Drlica, K.; Zhao, X. Dimethyl sulfoxide protects escherichia coli from rapid antimicrobial-mediated killing. Antimicrob. Agents Chemother. 2016, 60, 5054–5058. [Google Scholar] [CrossRef] [Green Version]
- Su, P.W.; Yang, C.H.; Yang, J.F.; Su, P.Y.; Chuang, L.Y. Antibacterial activities and antibacterial mechanism of Polygonum cuspidatum extracts against nosocomial drug-resistant pathogens. Molecules 2015, 20, 11119–11130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, Z.I.; Millar, B.C.; Downey, D.G.; Moore, J.E. Antimicrobial effect of dimethyl sulfoxide and N, N-Dimethylformamide on Mycobacterium abscessus: Implications for antimicrobial susceptibility testing. Int. J. Mycobacteriol. 2018, 7, 134–136. [Google Scholar] [PubMed]
- Weiss, E.I.; Kozlovsky, A.; Steinberg, D.; Lev-Dor, R.; Bar Ness Greenstein, R.; Feldman, M.; Sharon, N.; Ofek, I. A high molecular mass cranberry constituent reduces mutans streptococci level in saliva and inhibits in vitro adhesion to hydroxyapatite. FEMS Microbiol. Lett. 2004, 232, 89–92. [Google Scholar] [CrossRef]


| Compounds Group | Phenolic Compound | Concentration (μg g−1 ± SD) |
|---|---|---|
| Benzoic acids | Benzoic acid | 8317.88 ± 222.31 |
| Protocatechuic acid | 735.12 ± 17.76 | |
| Vanillic acid | 262.54 ± 10.16 | |
| Gallic acid | 136.16 ± 1.50 | |
| 4-Hydroxybenzoic acid | 94.81 ± 2.23 | |
| Salycilic acid | 91.05 ± 2.16 | |
| 4-Hydroxymandelic acid | 30.84 ± 1.14 | |
| 3-O-methylgallic acid | 30.05 ± 0.64 | |
| 4-Hydroxy-3-methoxymandelic acid | 14.33± 0.45 | |
| Syringic acid | 11.80 ± 1.18 | |
| 3-Hydroxybenzoic acid | 11.58 ± 0.01 | |
| 3-(3,4-Dihydroxyphenyl)-propionic acid | 9.61 ± 0.16 | |
| 4-Hydroxy-3-methoxyphenylacetic acid | 6.55 ± 0.66 | |
| 3,4-Dihydroxy mandelic acid | 3.56 ± 0.31 | |
| 4-Hydroxypheny lacetic acid | 3.20 ± 0.41 | |
| Hippuric acid | 1.14 ± 0.10 | |
| 3,4-Dihydroxy phenylacetic acid | 1.05 ± 0.10 | |
| 3,4,5-Trimethoxy benzoic acid | 0.32 ± 0.03 | |
| Cinnamic acids | p-Coumaric acid | 844.16 ± 15.20 |
| trans-Cinnamic acid | 260.55 ± 0.04 | |
| Caffeic acid | 133.67 ± 2.52 | |
| Ferulic acid | 111.92 ± 4.38 | |
| Trimethoxycinnamic acid | 2.72 ± 0.27 | |
| Flavan-3-ols | ∑ A-type trimers | 1579.04 ± 27.31 |
| ∑ A-type dimers | 230.95 ± 18.11 | |
| ∑ B-type dimers | 201.87 ± 17.21 | |
| ∑ Monomers | 65.81 ± 5.20 | |
| ∑ B-type trimers | 34.1 ± 0.91 | |
| Anthocyanins | Peonidin-3-arabinoside | 32.73 ± 3.27 |
| Cyanidin-3-arabinoside | 15.01 ± 0.05 | |
| Peonidin-3-glucoside | 4.84 ± 0.48 | |
| Malvidin-3-arabinoside | 1.16 ± 0.02 | |
| Peonidin-3-galactoside | 1.03 ± 0.09 | |
| Cyanidin-3-glucoside | 0.31 ± 0.02 | |
| Cyanidin-3-galactoside | 0.19 ± 0.01 |
| Exposure Time (seconds) | Viable CFUs mL−1 [mean (SD)] | p-Value When Compared to Negative Control | % of Reduction of viable CFUs mL−1 as Compared with Negative Control | |||||
|---|---|---|---|---|---|---|---|---|
| Negative Control (PBS) | Cranberry Extract | DMSO | Cranberry Extract | DMSO | Cranberry Extract | DMSO | ||
| S. oralis | 30 | 1.2 × 106 ± 1.1 × 106 | 1.3 × 104 ± 1.1 × 104 | 8.3 × 104 ± 1.4 × 105 | 0.000 | 0.000 | 98.9 | 93.1 |
| 60 | 6.8 × 105 ± 4.3 × 105 | 7.3 × 103 ± 4.4 × 103 | 2.8 × 105 ± 2.3 × 105 | 0.017 | 0.282 | 98.9 | 58.8 | |
| A. naeslundii | 30 | 6.7 × 104 ±5.6 × 104 | 2.3 × 104 ± 1.3 × 104 | 3.4 × 104 ± 2.1 × 104 | 0.006 | 0.050 | 65.7 | 49.2 |
| 60 | 2.2 × 104 ±1.3 × 104 | 3.2 × 104 ± 2.4 × 104 | 2.0 × 104 ± 1.4 × 104 | 1.000 | 1.000 | 45.4 | 9.1 | |
| V. parvula | 30 | 3.6 × 106 ±2.8 × 106 | 1.2 × 106 ± 1.4 × 106 | 2.0 × 106 ± 2.1 × 106 | 0.010 | 0.147 | 66.7 | 44.4 |
| 60 | 1.6 × 106 ± 8.6 × 105 | 4.4 × 105 ± 3.6 × 105 | 1.3 × 106 ± 1.3 × 106 | 0.395 | 1.000 | 72.5 | 18.7 | |
| A. actinomycetemcomitans | 30 | 7.2 × 106 ± 6.4 × 106 | 6.8 × 106 ± 4.7 × 106 | 5.6 × 106 ± 3.0 × 106 | 1.000 | 1.000 | 5.6 | 22.2 |
| 60 | 5.2 × 106 ± 3.5 × 106 | 4.6 × 106 ± 4.4 × 106 | 5.2 × 106 ± 4.9 × 106 | 1.000 | 1.000 | 11.5 | 0.0 | |
| P. gingivalis | 30 | 1.7 × 106 ± 7.0 × 105 | 1.1 × 106 ± 5.2 × 105 | 1.6 × 106 ± 1.8 × 106 | 0.434 | 1.000 | 35.3 | 5.9 |
| 60 | 8.9 × 105 ± 6.8 × 105 | 5.4 × 105 ± 1.8 × 105 | 1.0 × 106 ± 7.0 × 105 | 1.000 | 1.000 | 39.3 | 12.3 | |
| F. nucleatum | 30 | 3.8 × 105 ± 3.1 × 105 | 2.3 × 105 ± 1.5 × 105 † | 3.5 × 105 ± 1.3 × 105 | 0.164 | 1.000 | 39.5 | 7.9 |
| 60 | 1.5 × 105 ± 1.0 × 105 | 3.7 × 104 ± 3.0 × 104 † | 1.8 × 105 ± 1.5 × 105 | 0.448 | 1.000 | 75.3 | 0.0 | |
| Treatment | Mean Difference (I–J) | Standard Error | Sig.a | 95% Confidence Interval for Difference | |||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Antimicrobial effect | |||||||
| 30 s | PBS | Cranberry | 0.763 | 0.071 | 0.000 | 0.567 | 0.960 |
| DMSO | 0.663 | 0.071 | 0.000 | 0.467 | 0.860 | ||
| Cranberry | DMSO | −0.100 | 0.071 | 0.550 | −0.297 | 0.097 | |
| 60 s | PBS | Cranberry | 0.687 | 0.071 | 0.000 | 0.490 | 0.883 |
| DMSO | 0.467 | 0.071 | 0.000 | 0.270 | 0.663 | ||
| Cranberry | DMSO | −0.220 | 0.071 | 0.027 | −0.417 | −0.023 | |
| Anti-biofilm effect | |||||||
| 6 h | PBS | Cranberry | 0.35000 | 0.14575 | 0.160 | −0.1292 | 0.8292 |
| DMSO | 0.40000 | 0.14575 | 0.101 | −0.0792 | 0.8792 | ||
| Cranberry | DMSO | 0.05000 | 0.14575 | 1.000 | −0.4292 | 0.5292 | |
| Viable CFUs mL−1 [mean (SD)] | p-Value When Compared to Negative Control | % of Reduction of Viable CFUs mL−1 Respect to Negative Control | |||||
|---|---|---|---|---|---|---|---|
| Negative Control (PBS) | Cranberry Extract | DMSO | Cranberry Extract | DMSO | Cranberry Extract | DMSO | |
| S. oralis | 1.2 × 105 ± 2.5 × 104 | 1.3 × 103 ± 5.3 × 102 | 5.5 × 102 ± 2.6 × 102 | 0.000 | 0.000 | 98.9 | 99.5 |
| A. naeslundii | 4.8 × 104 ± 3.1 × 104 | 7.8 × 104 ± 7.6 × 104 | 6.4 × 104 ± 1.9 × 104 | 0.608 | 1.000 | - | - |
| V. parvula | 2.3 × 104 ± 1.5 × 104 | 2.1 × 103 ± 2.2 × 103 | 2.0 × 104 ± 7.3 × 103 | 0.000 | 1.000 | 90.9 | 13.0 |
| A. actinomycetemcomitans | 7.5 × 105 ± 2.8 × 105 | 1.2 × 105 ± 9.5 × 104 | 3.8 × 105 ± 1.4 × 105 | 0.000 | 0.001 | 84.0 | 50.7 |
| P. gingivalis | 4.0 × 104 ± 2.9 × 104 | 1.1 × 103 ± 1.1 × 103 | 1.0 × 104 ± 9.9 × 103 | 0.000 | 0.0047 | 97.2 | 75.0 |
| F. nucleatum | 1.1 × 105 ± 3.8 × 104 | 2.7 × 104 ± 2.0 × 104 | 5.9 × 104 ± 2.0 × 104 | 0.000 | 0.005 | 75.4 | 46.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, M.C.; Ribeiro-Vidal, H.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.V.; Sanz, M.; Herrera, D. New Evidences of Antibacterial Effects of Cranberry Against Periodontal Pathogens. Foods 2020, 9, 246. https://doi.org/10.3390/foods9020246
Sánchez MC, Ribeiro-Vidal H, Bartolomé B, Figuero E, Moreno-Arribas MV, Sanz M, Herrera D. New Evidences of Antibacterial Effects of Cranberry Against Periodontal Pathogens. Foods. 2020; 9(2):246. https://doi.org/10.3390/foods9020246
Chicago/Turabian StyleSánchez, María C., Honorato Ribeiro-Vidal, Begoña Bartolomé, Elena Figuero, M. Victoria Moreno-Arribas, Mariano Sanz, and David Herrera. 2020. "New Evidences of Antibacterial Effects of Cranberry Against Periodontal Pathogens" Foods 9, no. 2: 246. https://doi.org/10.3390/foods9020246
APA StyleSánchez, M. C., Ribeiro-Vidal, H., Bartolomé, B., Figuero, E., Moreno-Arribas, M. V., Sanz, M., & Herrera, D. (2020). New Evidences of Antibacterial Effects of Cranberry Against Periodontal Pathogens. Foods, 9(2), 246. https://doi.org/10.3390/foods9020246






