Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles
Abstract
:1. Introduction
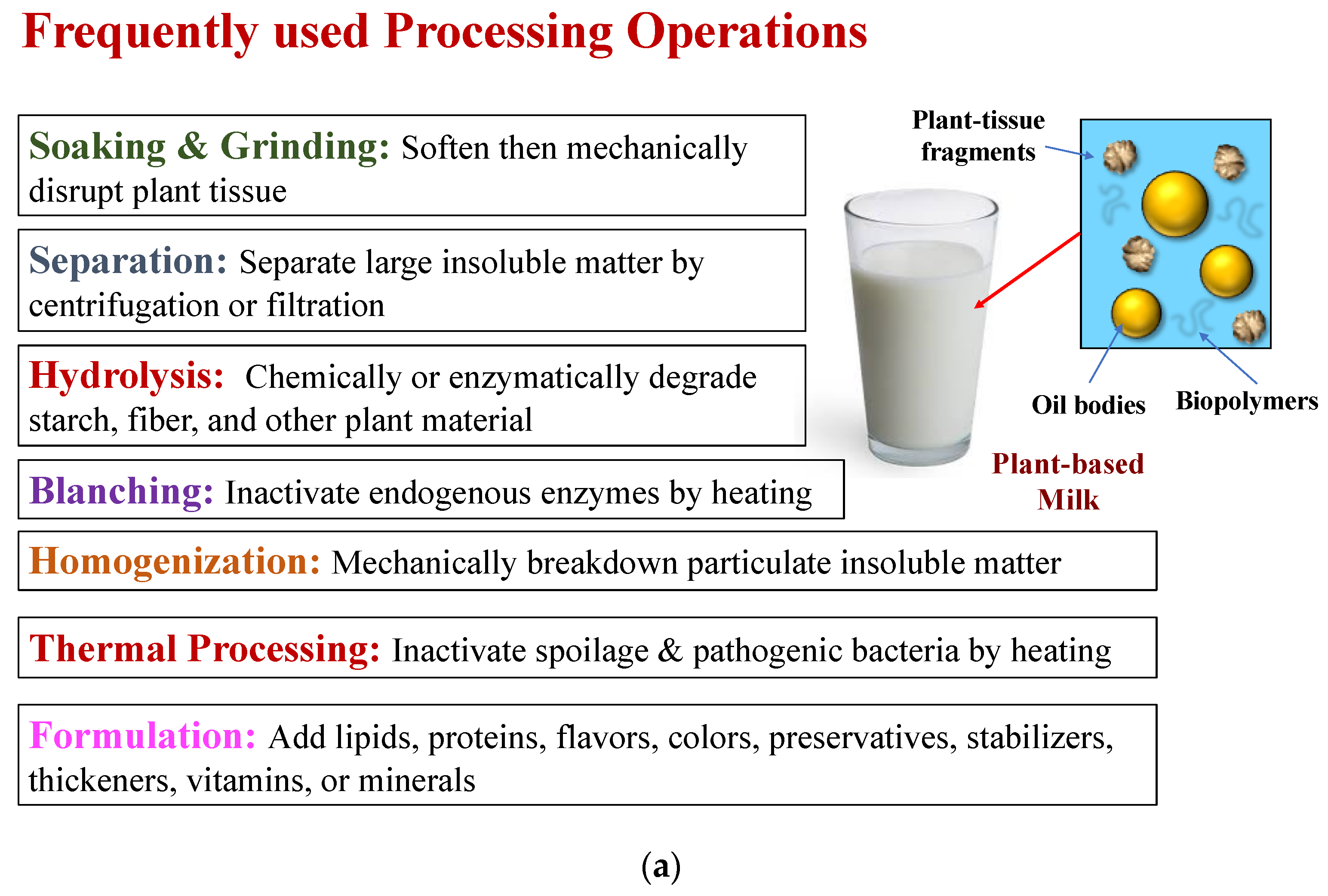
2. Plant-Based Milk Production
2.1. Extracted Oil Bodies
2.2. Simulated Fat Globules
2.2.1. Ingredients
2.2.2. Manufacturing Operations
3. Structural Basis of Physicochemical Properties
3.1. Visual Attributes
3.1.1. Physical Basis of Visual Attributes
3.1.2. Examples of Plant-Based Milk Appearance
3.2. Textural Attributes
3.2.1. Physical Basis of Textural Attributes
3.2.2. Examples of Plant-Based Milk Texture
3.3. Stability
3.3.1. Physical Instability
3.3.2. Chemical Instability
3.3.3. Examples of Instability in Plant-Based Milk Substitutes
3.4. Sensory Properties
4. Nutritional Fortification
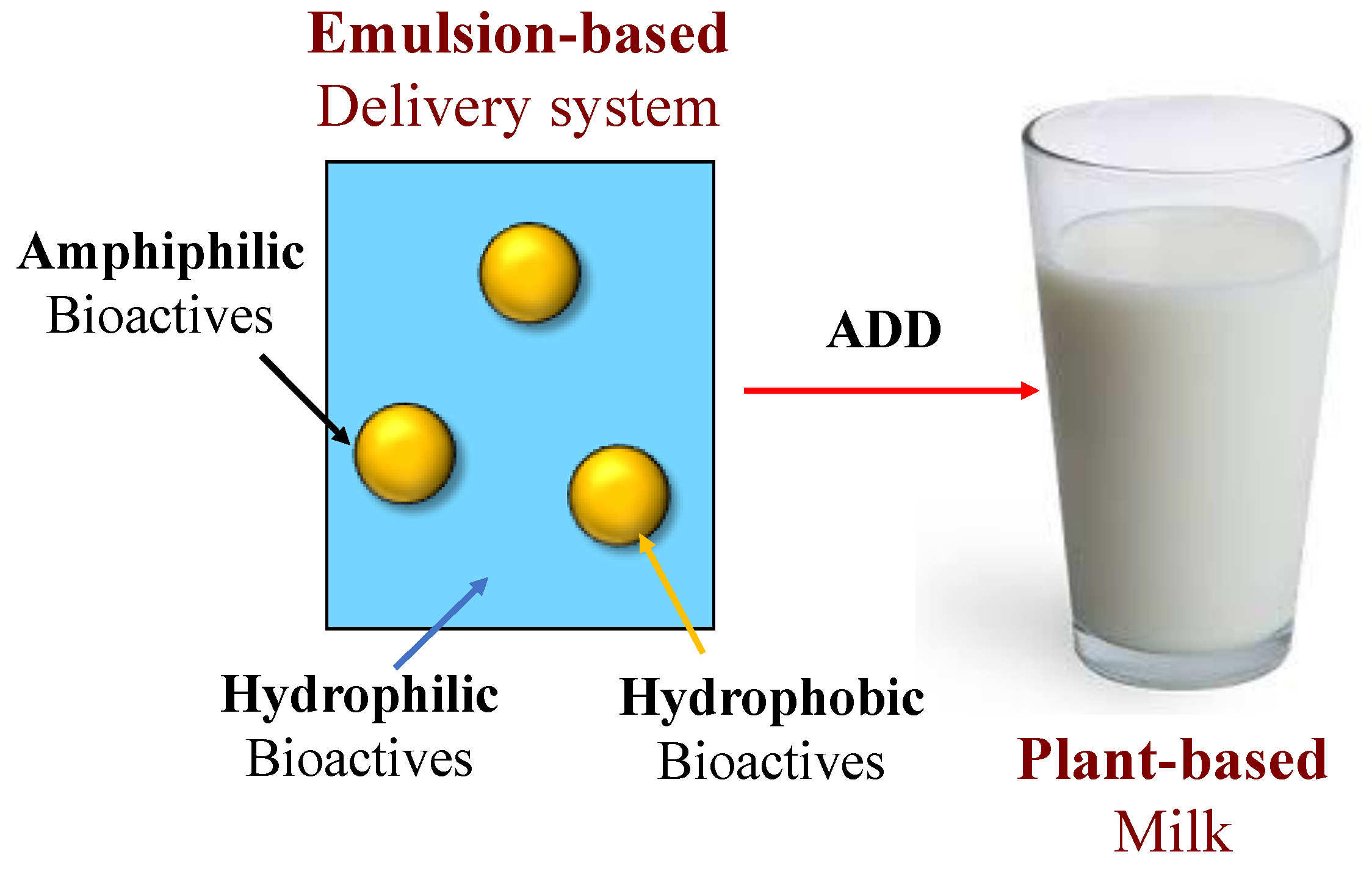
4.1. Incorporation of Bioactive Agents into Plant-Based Milk Substitutes
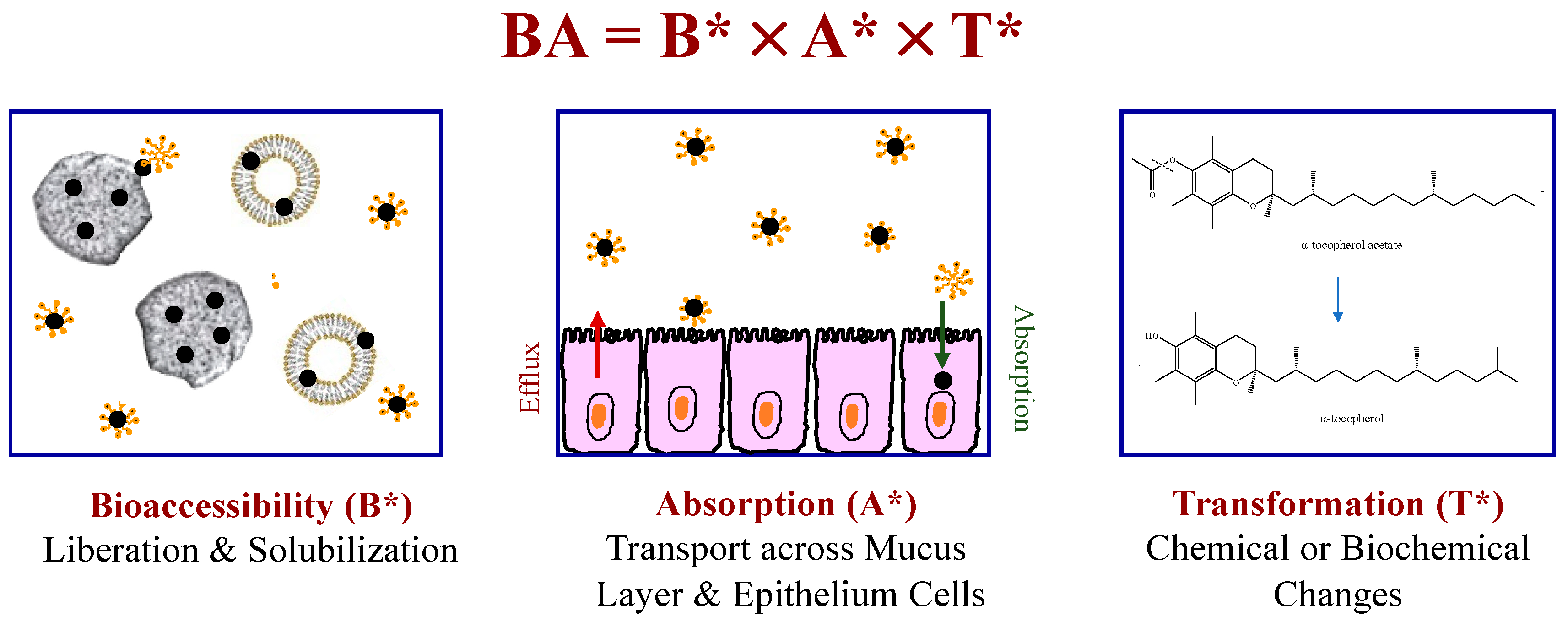
4.2. Bioavailability
4.3. Factors Impacting Overall Bioavailability
4.4. Optimizing Delivery Systems for Fortifying Plant-Based Milk Substitutes
4.4.1. Oil Phase Composition
4.4.2. Oil Phase Concentration
4.4.3. Droplet Size
4.4.4. Emulsifier Type
4.4.5. Food Matrix Effects
5. Conclusions
Funding
Conflicts of Interest
References
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; de Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Future Foods: How Modern Science is Transforming the Way We Eat; Springer Scientific: New York, NY, USA, 2019. [Google Scholar]
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. Mysore 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant based alternatives to conventional milk, production, potential and health concerns. Crit. Rev. Food Sci. Nutr. 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [Green Version]
- Jukkola, A.; Rojas, J.O. Milk fat globules and associated membranes: Colloidal properties and processing effects. Adv. Colloid Interface Sci. 2017, 245, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Do, D.T.; Singh, J.; Oey, I.; Singh, H. Biomimetic plant foods: Structural design and functionality. Trends Food Sci. Technol. 2018, 82, 46–59. [Google Scholar] [CrossRef]
- Pyc, M.; Cai, Y.Q.; Greer, M.S.; Yurchenko, O.; Chapman, K.D.; Dyer, J.M.; Mullen, R.T. Turning over a New Leaf in Lipid Droplet Biology. Trends Plant Sci. 2017, 22, 596–609. [Google Scholar] [CrossRef]
- Nikiforidis, C.V. Structure and functions of oleosomes (oil bodies). Adv. Colloid Interface Sci. 2019, 274, 102039. [Google Scholar] [CrossRef] [PubMed]
- Michalski, M.C. Specific molecular and colloidal structures of milk fat affecting lipolysis, absorption and postprandial lipemia. Eur. J. Lipid Sci. Technol. 2009, 111, 413–431. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Matsakidou, A.; Kiosseoglou, V. Composition, properties and potential food applications of natural emulsions and cream materials based on oil bodies. RSC Adv. 2014, 4, 25067–25078. [Google Scholar] [CrossRef] [Green Version]
- Campbell, K.A.; Glatz, C.E.; Johnson, L.A.; Jung, S.; de Moura, J.M.N.; Kapchie, V.; Murphy, P. Advances in Aqueous Extraction Processing of Soybeans. J. Am. Oil Chem. Soc. 2011, 88, 449–465. [Google Scholar] [CrossRef]
- Iwanaga, D.; Gray, D.A.; Fisk, I.D.; Decker, E.A.; Weiss, J.; McClements, D.J. Extraction and characterization of oil bodies from soy beans: A natural source of pre-emulsified soybean oil. J. Agric. Food Chem. 2007, 55, 8711–8716. [Google Scholar] [CrossRef]
- McHugh, T. How Plant-Based Milks Are Processed. Food Technol. 2018, 72, 63–64. [Google Scholar]
- Drake, M.A. Invited review: Sensory analysis of dairy foods. J. Dairy Sci. 2007, 90, 4925–4937. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Food Emulsions: Principles, Practice, and Techniques, 2nd ed.; CRC Series in Contemporary Food Science; CRC PRESS: Boca Raton, FL, USA, 2015. [Google Scholar]
- McClements, D.J.; Bai, L.; Chung, C. Recent Advances in the Utilization of Natural Emulsifiers to Form and Stabilize Emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasala, B.A.; Mayfield, S.P. Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth. Res. 2015, 123, 227–239. [Google Scholar] [CrossRef]
- Celik, E.; Calik, P. Production of recombinant proteins by yeast cells. Biotechnol. Adv. 2012, 30, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A. Emulsion Formation by Homogenization: Current Understanding and Future Perspectives. Annu. Rev. Food Sci. Technol. 2019, 10, 239–258. [Google Scholar] [CrossRef]
- Stocker, S.; Foschum, F.; Krauter, P.; Bergmann, F.; Hohmann, A.; Happ, C.S.; Kienle, A. Broadband Optical Properties of Milk. Appl. Spectrosc. 2017, 71, 951–962. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Theoretical prediction of emulsion color. Adv. Colloid Interface Sci. 2002, 97, 63–89. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of Physicochemical and Glycaemic Properties of Commercial Plant-Based Milk Substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.S.; Chang, S.K.C. Nutritional profile and physicochemical properties of commercial soymilk. J. Food Process. Preserv. 2013, 37, 651–661. [Google Scholar] [CrossRef]
- Jeske, S.; Bez, J.; Arendt, E.K.; Zannini, E. Formation, stability, and sensory characteristics of a lentil-based milk substitute as affected by homogenisation and pasteurisation. Eur. Food Res. Technol. 2019, 245, 1519–1531. [Google Scholar] [CrossRef]
- Park, S.H.; Min, S.G.; Jo, Y.J.; Chun, J.Y. Effect of High Pressure Homogenization on the Physicochemical Properties of Natural Plant-based Model Emulsion Applicable for Dairy Products. Korean J. Food Sci. Anim. Resour. 2015, 35, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.D.; Dai, Y.Q.; Wu, H.; Liu, X.L.; Wang, Y.; Cao, J.P.; Zhou, J.Z. Effects of pressure and multiple passes on the physicochemical and microbial characteristics of lupin-based beverage treated with high-pressure homogenization. J. Food Process. Preserv. 2019, 43, e13912. [Google Scholar] [CrossRef]
- Chung, C.; Sher, A.; Rousset, P.; McClements, D.J. Influence of homogenization on physical properties of model coffee creamers stabilized by quillaja saponin. Food Res. Int. 2017, 99, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Sher, A.; Rousset, P.; McClements, D.J. Use of natural emulsifiers in model coffee creamers: Physical properties of quillaja saponin-stabilized emulsions. Food Hydrocoll. 2017, 67, 111–119. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, X.; Lin, H.; McClements, D.J. Loading natural emulsions with nutraceuticals using the pH-driven method: Formation & stability of curcumin-loaded soybean oil bodies. Food Funct. 2019, 10, 5473–5484. [Google Scholar] [PubMed]
- Zheng, B.; Zhang, X.; Peng, S.; McClements, D.J. Impact of curcumin delivery system format on bioaccessibility: Nanocrystals, nanoemulsion droplets, and natural oil bodies. Food Funct. 2019, 10, 4339–4349. [Google Scholar] [CrossRef] [PubMed]
- Morison, K.R.; Phelan, J.P.; Bloore, C.G. Viscosity and Non-Newtonian Behaviour of Concentrated Milk and Cream. Int. J. Food Prop. 2013, 16, 882–894. [Google Scholar] [CrossRef]
- Aditya, N.P.; Espinosa, Y.G.; Norton, I.T. Encapsulation systems for the delivery of hydrophilic nutraceuticals: Food application. Biotechnol. Adv. 2017, 35, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Bernat, N.; Chafer, M.; Rodriguez-Garcia, J.; Chiralt, A.; Gonzalez-Martinez, C. Effect of high pressure homogenisation and heat treatment on physical properties and stability of almond and hazelnut milks. LWT-Food Sci. Technol. 2015, 62, 488–496. [Google Scholar] [CrossRef]
- Deswal, A.; Deora, N.S.; Mishra, H.N. Effect of Concentration and Temperature on the Rheological Properties of Oat Milk. Food Bioprocess Technol. 2014, 7, 2451–2459. [Google Scholar] [CrossRef]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Formation and Stability of omega-3 Oil Emulsion-Based Delivery Systems Using Plant Proteins as Emulsifiers: Lentil, Pea, and Faba Bean Proteins. Food Biophys. 2017, 12, 186–197. [Google Scholar] [CrossRef]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Impact of legume protein type and location on lipid oxidation in fish oil-in-water emulsions: Lentil, pea, and faba bean proteins. Food Res. Int. 2017, 100, 175–185. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Role of Iron and Hydroperoxides in the Degradation of Lycopene in Oil-in-Water Emulsions. J. Agric. Food Chem. 2009, 57, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and chemical stability of beta-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Du, Z.Y.; Zhang, G.D.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Mezzena, M. Incorporation of quercetin in lipid microparticles: Effect on photo- and chemical-stability. J. Pharm. Biomed. Anal. 2009, 49, 90–94. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Nutraceutical delivery systems: Resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem. 2015, 167, 205–212. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Sapino, S.; Trotta, M.; Battaglia, L.; Vione, D.; Pelizzetti, E. Photostability and stability over time of retinyl palmitate in an O/W emulsion and in SLN introduced in the emulsion. J. Dispers. Sci. Technol. 2005, 26, 125–138. [Google Scholar] [CrossRef]
- Koo, C.K.W.; Chung, C.; Fu, J.T.R.; Sher, A.; Rousset, P.; McClements, D.J. Impact of sodium caseinate, soy lecithin and carrageenan on functionality of oil-in-water emulsions. Food Res. Int. 2019, 123, 779–789. [Google Scholar] [CrossRef]
- Chung, C.; Sher, A.; Rousset, P.; McClements, D.J. Impact of oil droplet concentration on the optical, rheological, and stability characteristics of O/W emulsions stabilized with plant-based surfactant: Potential application as non-dairy creamers. Food Res. Int. 2018, 105, 913–919. [Google Scholar] [CrossRef]
- Patil, U.; Benjakul, S. Coconut Milk and Coconut Oil: Their Manufacture Associated with Protein Functionality. J. Food Sci. 2018, 83, 2019–2027. [Google Scholar] [CrossRef]
- Euston, S.R.; Al-Bakkush, A.A.; Campbell, L. Comparing the heat stability of soya protein and milk whey protein emulsions. Food Hydrocoll. 2009, 23, 2485–2492. [Google Scholar] [CrossRef]
- Ryan, M.; McEvoy, E.; McSweeney, S.L.; O’Callaghan, D.M.; FitzGerald, R.J. Thermal behavior of emulsions manufactured with soy protein ingredients. Food Res. Int. 2008, 41, 813–818. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Makinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.S.; Lopetcharat, K.; Drake, M.A. Milk fat threshold determination and the effect of milk fat content on consumer preference for fluid milk. J. Dairy Sci. 2017, 100, 1702–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiano, A.N.; Harwood, W.S.; Drake, M.A. A 100-Year Review: Sensory analysis of milk. J. Dairy Sci. 2017, 100, 9966–9986. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Dhankhar, J.; Sharma, A. Development of Non Dairy Milk Alternative Using Soymilk and Almond Milk. Curr. Res. Nutr. Food Sci. 2018, 6, 203–210. [Google Scholar] [CrossRef]
- Vanga, S.K.; Raghavan, V. How well do plant based alternatives fare nutritionally compared to cow’s milk? J. Food Sci. Technol. Mysore 2018, 55, 10–20. [Google Scholar] [CrossRef]
- Sebastiani, G.; Herranz Barbero, A.; Borras-Novell, C.; Casanova, M.A.; Aldecoa-Bilbao, V.; Andreu-Fernandez, V.; Tutusaus, M.P.; Martinez, S.F.; Roig, M.D.G.; Garcia-Algar, O. The Effects of Vegetarian and Vegan Diet during Pregnancy on the Health of Mothers and Offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [Green Version]
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.; de Groot, L.; Eussen, S. Vitamin B12 Intake From Animal Foods, Biomarkers, and Health Aspects. Front. Nutr. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Hunt, M.W. Veganism and Children: Physical and Social Well-Being. J. Agric. Environ. Ethics 2019, 32, 269–291. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. Mysore 2015, 52, 2522–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J. Nanoscale Nutrient Delivery Systems for Food Applications: Improving Bioactive Dispersibility, Stability, and Bioavailability. J. Food Sci. 2015, 80, N1602–N1611. [Google Scholar] [CrossRef]
- McClements, D.J. Enhanced delivery of lipophilic bioactives using emulsions: A review of major factors affecting vitamin, nutraceutical, and lipid bioaccessibility. Food Funct. 2018, 9, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Walia, N.; Chen, L.Y. Pea protein based vitamin D nanoemulsions: Fabrication, stability and in vitro study using Caco-2 cells. Food Chem. 2020, 305, 125475. [Google Scholar] [CrossRef]
- Schoener, A.L.; Zhang, R.J.; Lv, S.S.; Weiss, J.; McClements, D.J. Fabrication of plant-based vitamin D-3-fortified nanoemulsions: Influence of carrier oil type on vitamin bioaccessibility. Food Funct. 2019, 10, 1826–1835. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Chen, S.; Wang, Y.Y.; Wang, X.B.; Ku, N.; Jiang, L.Z. Stability and in vitro digestion simulation of soy protein isolate-vitamin D-3 nanocomposites. LWT-Food Sci. Technol. 2020, 117, 108647. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, A.; Ahmed, Z.; Ahmad, M.S. Optimization of soya lecithin and Tween 80 based novel vitamin D nanoemulsions prepared by ultrasonication using response surface methodology. Food Chem. 2019, 289, 664–670. [Google Scholar] [CrossRef]
- Maurya, V.K.; Bashir, K.; Aggarwal, M. Vitamin D microencapsulation and fortification: Trends and technologies. J. Steroid Biochem. Mol. Biol. 2020, 196, 105489. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, F.; Xiao, H. The Nutraceutical Bioavailability Classification Scheme: Classifying Nutraceuticals According to Factors Limiting their Oral Bioavailability. Annu. Rev. Food Sci. Technol. 2015, 6, 299–327. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Van de Kamp, E.; Rompelberg, C.J.M. Development and Applicability of an In Vitro Digestion Model in Assessing the Bioaccessibility of Contaminants from Food; Inspectorate of Health Inspection: Utrecht, The Netherlands, 2004. [Google Scholar]
- Bauer, E.; Jakob, S.; Mosenthin, R. Principles of physiology of lipid digestion. Asian Australas. J. Anim. Sci. 2005, 18, 282–295. [Google Scholar] [CrossRef]
- Fave, G.; Coste, T.C.; Armand, M. Physicochemical properties of lipids: New strategies to manage fatty acid bioavailability. Cell. Mol. Biol. 2004, 50, 815–831. [Google Scholar] [PubMed]
- Bermudez, B.; Pacheco, Y.M.; Lopez, S.; Abia, R.; Muriana, F.J.G. Digestion and absorption of olive oil. Grasas Y Aceites 2004, 55, 1–10. [Google Scholar]
- Mohanty, C.; Das, M.; Sahoo, S.K. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Exp. Opin. Drug Deliv. 2012, 9, 1347–1364. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Horne, D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog. Lipid Res. 2008, 48, 92–100. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res. Int. 2016, 81, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Horter, D.; Dressman, J.B. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv. Drug Deliv. Rev. 2001, 46, 75–87. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Enhancing nutraceutical bioavailability using excipient emulsions: Influence of lipid droplet size on solubility and bioaccessibility of powdered curcumin. J. Funct. Foods 2015, 15, 72–83. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Food-grade nanoparticles for encapsulation, protection and delivery of curcumin: Comparison of lipid, protein, and phospholipid nanoparticles under simulated gastrointestinal conditions. RSC Adv. 2016, 6, 3126–3136. [Google Scholar] [CrossRef]
- Xu, D.X.; Hou, Z.Q.; Liu, G.R.; Cao, Y.P.; Panya, A.; Xiao, H.; Dixon, W. Influence of Rosemary Extract Addition in Different Phases on the Oxidation of Lutein and WPI in WPI-Stabilized Lutein Emulsions. J. Food Qual. 2020, 2020, 5217–5226. [Google Scholar] [CrossRef] [Green Version]
- Sharif, H.R.; Goff, H.D.; Majeed, H.; Liu, F.; Nsor-Atindana, J.; Haider, J.; Liang, R.; Zhong, F. Physicochemical stability of beta-carotene and alpha-tocopherol enriched nanoemulsions: Influence of carrier oil, emulsifier and antioxidant. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 550–559. [Google Scholar] [CrossRef]
- McClements, D.J.; Henson, L.; Popplewell, L.M.; Decker, E.A.; Choi, S.J. Inhibition of Ostwald Ripening in Model Beverage Emulsions by Addition of Poorly Water Soluble Triglyceride Oils. J. Food Sci. 2012, 77, C33–C38. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Zou, L.; Zhang, R.; Salvia-Trujillo, L.; Kumosani, T.; Xiao, H. Enhancing Nutraceutical Performance Using Excipient Foods: Designing Food Structures and Compositions to Increase Bioavailability. Compr. Rev. Food Sci. Food Saf. 2015, 14, 824–847. [Google Scholar] [CrossRef]
- Waraho, T.; McClements, D.J.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Nuchi, C.D.; McClements, D.J.; Decker, E.A. Impact of tween 20 hydroperoxides and iron on the oxidation of methyl linoleate and salmon oil dispersions. J. Agric. Food Chem. 2001, 49, 4912–4916. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Nanoemulsion delivery systems for oil-soluble vitamins: Influence of carrier oil type on lipid digestion and vitamin D-3 bioaccessibility. Food Chem. 2015, 187, 499–506. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Vitamin E bioaccessibility: Influence of carrier oil type on digestion and release of emulsified alpha-tocopherol acetate. Food Chem. 2013, 141, 473–481. [Google Scholar] [CrossRef]
- Zhang, R.J.; Zhang, Z.P.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of lipid type on gastrointestinal fate of oil-in-water emulsions: In vitro digestion study. Food Res. Int. 2015, 75, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.F.; Xiao, H.; McClements, D.J. Delivery of Lipophilic Bioactives: Assembly, Disassembly, and Reassembly of Lipid Nanoparticles. Annu. Rev. Food Sci. Technol. 2014, 5, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.J.; Zhang, Z.P.; Zou, L.Q.; Xiao, H.; Zhang, G.D.; Decker, E.A.; McClements, D.J. Enhancing Nutraceutical Bioavailability from Raw and Cooked Vegetables Using Excipient Emulsions: Influence of Lipid Type on Carotenoid Bioaccessibility from Carrots. J. Agric. Food Chem. 2015, 63, 10508–10517. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on beta-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Qian, C.; Martin-Belloso, O.; McClements, D.J. Modulating beta-carotene bioaccessibility by controlling oil composition and concentration in edible nanoemulsions. Food Chem. 2013, 139, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.J.; Decker, E.A.; Xiao, H.; McClements, D.J. Nutraceutical nanoemulsions: Influence of carrier oil composition (digestible versus indigestible oil) on -carotene bioavailability. J. Sci. Food Agric. 2013, 93, 3175–3183. [Google Scholar] [CrossRef]
- Tan, Y.B.; Liu, J.N.; Zhou, H.L.; Mundo, J.M.; McClements, D.J. Impact of an indigestible oil phase (mineral oil) on the bioaccessibility of vitamin D-3 encapsulated in whey protein-stabilized nanoemulsions. Food Res. Int. 2019, 120, 264–274. [Google Scholar] [CrossRef]
- Xia, Z.Y.; McClements, D.J.; Xiao, H. Influence of Lipid Content in a Corn Oil Preparation on the Bioaccessibility of -Carotene: A Comparison of Low-Fat and High-Fat Samples. J. Food Sci. 2017, 82, 373–379. [Google Scholar] [CrossRef]
- Cho, H.T.; Salvia-Trujillo, L.; Kim, J.; Park, Y.; Xiao, H.; McClements, D. Droplet size and composition of nutraceutical nanoemulsions influences bioavailability of long chain fatty acids and Coenzyme Q10. Food Chem. 2014, 156, 117–122. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martin-Belloso, O.; McClements, D.J. Influence of particle size on lipid digestion and beta-carotene bioaccessibility in emulsions and nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef]
- Moelants, K.R.N.; Lemmens, L.; Vandebroeck, M.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Relation between Particle Size and Carotenoid Bioaccessibility in Carrot- and Tomato-Derived Suspensions. J. Agric. Food Chem. 2012, 60, 11995–12003. [Google Scholar] [CrossRef]
- Zhang, R.J.; Zhang, Z.P.; Zou, L.Q.; Xiao, H.; Zhang, G.D.; Decker, E.A.; McClements, D.J. Enhancement of carotenoid bioaccessibility from carrots using excipient emulsions: Influence of particle size of digestible lipid droplets. Food Funct. 2016, 7, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Verkempinck, S.H.E.; Sun, L.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Lipid digestion, micelle formation and carotenoid bioaccessibility kinetics: Influence of emulsion droplet size. Food Chem. 2017, 229, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Fumiaki, B.; Park, Y.; McClements, D.J. The influence of lipid droplet size on the oral bioavailability of vitamin D-2 encapsulated in emulsions: An in vitro and in vivo study. Food Funct. 2017, 8, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.S.; Zhang, Y.H.; Tan, H.Y.; Zhang, R.J.; McClements, D.J. Vitamin E Encapsulation within Oil-in-Water Emulsions: Impact of Emulsifier Type on Physicochemical Stability and Bioaccessibility. J. Agric. Food Chem. 2019, 67, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Tan, Y.B.; Dai, T.T.; Zhang, R.J.; Fu, G.M.; Wan, Y.; Liu, C.M.; McClements, D.J. Bioaccessibility and stability of beta-carotene encapsulated in plant-based emulsions: Impact of emulsifier type and tannic acid. Food Funct. 2019, 10, 7239–7252. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.G.; McClements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.Q.; Liu, W.; Liu, C.M.; Xiao, H.; McClements, D.J. Designing excipient emulsions to increase nutraceutical bioavailability: Emulsifier type influences curcumin stability and bioaccessibility by altering gastrointestinal fate. Food Funct. 2015, 6, 2475–2486. [Google Scholar] [CrossRef]
- Li, Y.; McClements, D.J. Inhibition of lipase-catalyzed hydrolysis of emulsified triglyceride oils by low-molecular weight surfactants under simulated gastrointestinal conditions. Eur. J. Pharm. Biopharm. 2011, 79, 423–431. [Google Scholar] [CrossRef]
- Fernandez-Garcia, E.; Carvajal-Lerida, I.; Jaren-Galan, M.; Garrido-Fernandez, J.; Perez-Galvez, A.; Hornero-Mendez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef]
- Tan, Y.B.; Li, R.Y.; Zhou, H.L.; Liu, J.N.; Mundo, J.M.; Zhang, R.J.; McClements, D.J. Impact of calcium levels on lipid digestion and nutraceutical bioaccessibility in nanoemulsion delivery systems studied using standardized INFOGEST digestion protocol. Food Funct. 2020, 11, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Zeeb, B.; Weiss, J.; McClements, D.J. Electrostatic modulation and enzymatic cross-linking of interfacial layers impacts gastrointestinal fate of multilayer emulsions. Food Chem. 2015, 180, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.B.; Li, R.Y.; Liu, C.Z.; Mundo, J.M.; Zhou, H.L.; Liu, J.N.; McClements, D.J. Chitosan reduces vitamin D bioaccessibility in food emulsions by binding to mixed micelles. Food Funct. 2020, 11, 187–199. [Google Scholar] [CrossRef] [PubMed]






| Milk | Viscosity [mPa∙s] | Flow Index | D3,2 | D43 | Separation Rate (%h) | Whiteness Index |
|---|---|---|---|---|---|---|
| [μm] | [μm] | |||||
| Almond | 4.6 | 0.82 | 2.4 | 0.9 | 52.4 | 68.4 |
| Almond | 19.1 | 0.70 | 1.1 | 1.8 | 1.4 | 72.6 |
| Almond | 26.3 | 0.56 | 2.1 | 6.0 | 30.2 | 76.0 |
| Almond | 3.9 | 0.98 | 1.5 | 2.6 | 51.7 | 51.6 |
| Cashew | 5.6 | 0.97 | 2.3 | 29.2 | 27.5 | 65.6 |
| Coconut | 47.8 | 0.40 | 1.3 | 1.7 | 37.4 | 67.8 |
| Hazelnut | 24.8 | 0.67 | 1.5 | 2.2 | 1.3 | 56.3 |
| Hemp | 25.0 | 0.73 | 1.1 | 1.5 | 4.4 | 68.5 |
| Macadamia | 2.2 | 1.00 | 1.8 | 3.4 | 54.4 | 51.7 |
| Oat | 6.8 | 0.89 | 1.7 | 3.8 | 40.1 | 60.2 |
| Quinoa | 13.2 | 0.76 | 1.1 | 81.5 | 32.0 | 71.4 |
| Rice | 2.8 | 0.97 | 0.88 | 10.5 | 42.8 | 66.5 |
| Brown Rice | 2.2 | 1.00 | 0.63 | 0.72 | 50.9 | 63.5 |
| Soy | 7.6 | 0.90 | 0.94 | 1.3 | 11.3 | 70.3 |
| Soy | 3.5 | 1.00 | 0.80 | 1.0 | 8.6 | 74.5 |
| Soy | 2.6 | 1.00 | 0.85 | 1.0 | 13.3 | 69.3 |
| Soy | 6.0 | 0.92 | 0.94 | 1.2 | 22.6 | 74.6 |
| Cow’s | 3.2 | 1.00 | 0.36 | 0.60 | 3.9 | 81.9 |
| Interaction Type | Strategy to Improve Stability |
|---|---|
| Steric repulsion | The steric repulsion between the particles can be increased by increasing the thickness of any adsorbed polymer layers. |
| Electrostatic repulsion | The electrostatic repulsion can be increased by increasing the magnitude of the charge or by reducing the ionic strength. This can often be achieved by controlling the pH and salt levels. For instance, the pH should be a long way from the isoelectric point. |
| Hydrophobic attraction | The hydrophobic attraction can be reduced by ensuring there are few non-polar patches on the surfaces of the colloidal particles. This might be achieved by controlling the degree of thermal denaturation of any proteins or by adding surfactants to cover non-polar patches. |
| Bridging attraction | The bridging attraction can be reduced by ensuring that there are no polymers or other substances that can simultaneously bind to the surfaces of multiple particles. Most importantly, charged polymers may link together oppositely charged particles. |
| Depletion attraction | The depletion attraction can be reduced by ensuring that the concentration of non-adsorbed polymers is below a critical level (which depends on their molecular weight and conformation). |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McClements, D.J. Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods 2020, 9, 421. https://doi.org/10.3390/foods9040421
McClements DJ. Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods. 2020; 9(4):421. https://doi.org/10.3390/foods9040421
Chicago/Turabian StyleMcClements, David Julian. 2020. "Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles" Foods 9, no. 4: 421. https://doi.org/10.3390/foods9040421
APA StyleMcClements, D. J. (2020). Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods, 9(4), 421. https://doi.org/10.3390/foods9040421






