Sapogenol is a Major Microbial Metabolite in Human Plasma Associated with High Protein Soy-Based Diets: The Relevance for Functional Food Formulations
Abstract
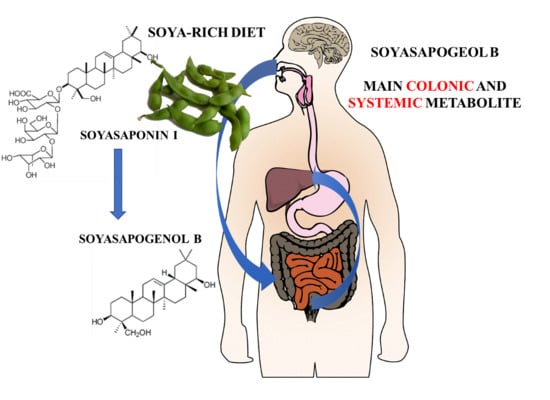
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Human Dietary Intervention Study
2.3. Extraction and Analysis of Saponins (Soyasaponin I) from Soya Food Products
2.4. Extraction and Analysis of Saponins and Sapogenols from Human Biological Samples
2.4.1. Extraction of Soyasaponin I and Soyasapogenol B from Human Faeces
2.4.2. Extraction of Soyasaponin I and Soyasapogenol B from Human Plasma
2.4.3. In vitro Microbial Transformation of Soyasaponin I by Mixed Human Faecal Microbiota.
2.4.4. LC-MS/MS Analysis of Saponins and Sapogenols from Biological Samples.
2.5. Statistical Analysis
3. Results and Discussion
3.1. Saponins (Soyasaponin I) Content of Soya-Rich Foods
3.2. Concentration of Saponins and Sapogenols (SSI and SSB) in Human Faecal Samples
3.3. In Vitro Microbial Transformation of Soyasaponin I by Human Faecal Inoculation
3.4. Concentration of Saponins and Sapogenols (SSI and SSB) in Human Plasma Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dillard, C.J.; German, J.B. Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- WHO. Diet, Nutrition and The Prevention of Chronic Diseases; Report of a Joint, WHO/FAO Expert Consultation. WHO Technical Report Series No. 916; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Rochfort, S.; Panozzo, J. Phytochemicals for health, the role of pulses. J. Agric. Food Chem. 2007, 55, 7981–7994. [Google Scholar] [CrossRef] [PubMed]
- Champ, M.M. Non-nutrient bioactive substances of pulses. Brit. J. Nutr. 2002, 88, 307–319. [Google Scholar] [CrossRef]
- Flight, I.; Clinton, P. Cereals grains and legumes in the prevention of coronary heart disease and stroke: A review of the literature. Eur. J. Clin. Nutr. 2006, 60, 1145–1159. [Google Scholar] [CrossRef] [Green Version]
- Muzquiz, M. Factores antinutricionales en fuentes proteicas. In Jornada Internacional Sobre Proteínas Alimentarías; Vioque, J., Clemente, A., Bautista, J., Millán, F., Eds.; Universidad de Sevilla: Seville, Spain, 2000. [Google Scholar]
- Oakenfull, D. Soy protein, saponins and plasma cholesterol. J. Nutr. 2001, 131, 2971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagratini, G.; Caprioli, G.; Maggi, F.; Font, G.; Giardinà, D.; Mañes, J.; Meca, G.; Ricciutelli, M.; Sirocchi, V.; Torregiani, E.; et al. Determination of soyasaponins I and βg in raw and cooked legumes by solid phase extraction (SPE) coupled to liquid chromatography (LC)—Mass Spectrometry (MS) and assessment of their bioaccessibility by an in vitro digestion model. J. Agric. Food Chem. 2013, 61, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Popovich, D.G. Chemical and biological characterization of oleanane triterpenoids from soya. Molecules 2009, 14, 2959–2975. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Badger, T.M.; Ronis, M.J.J.; Wu, X. Non-isoflavone phytochemicals in soya and their health effects. J. Agric. Food Chem. 2010, 58, 8119–8133. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kang, J. Phytochemicals in soya and their health effects. In Phytochemicals–Bioactivities and Impact on Health; Rasooli, I., Ed.; Intech: Rijeka, Croatia, 2011; pp. 43–76. [Google Scholar]
- Campos-Vega, R.; Loarca-Pina, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Guang, C.; Chen, J.; Sang, S.; Cheng, S. Biological functionality of soyasaponins and soyasapogenols. J. Agric. Food Chem. 2014, 62, 8247–8255. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, M.; Fyfe, C.; Horgan, G.; Johnstone, A.M. Appetite control and biomarkers of satiety with vegetarian (soy) and meat-based high-protein diets for weight loss in obese men: A randomized crossover trial. Am J. Clin. Nutr. 2014, 100, 548–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Reddy, M.B.; Hendrich, S.; Murphy, P. Soyasaponin I and sapogenol B have limited absorption by caco-2 intestinal cells and limited bioavailability in women. J. Nutr. 2004, 134, 1867–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamo, S.; Suzuki, S.; Sato, T. Comparison of bioavailability (I) between soyasaponins and soyasapogenols, and (II) between group A and B soyasaponins. Nutrition 2014. [CrossRef] [PubMed]
- Miyazaki, K.; Martin, J.C.; Marinsek-Logar, R.; Flint, H.J. Degradation and utilization of xylans by the rumen anaerobe Prevotella bryantii (formerly P. Ruminsicola subsp. Brevis) B(1)4. Anaerobe 1997, 3, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Kamo, S.; Suzuki, S.; Sato, T. The content of soyasaponin and soyasapogenol in soy foods and their estimated intake in the Japanese. Food Sci. Nutr. 2014, 2, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.A.; Hu, J.; Barua, K.; Hauck, C.C. Group B saponins in soy products in the U.S. department of agriculture—Iowa State University isoflavone database and their comparison with isoflavone contents. J. Agric. Food Chem. 2008, 56, 8534–8540. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zheng, Y.L.; Hyde, W.; Hendrich, S.; Murphy, P.A. Human faecal metabolism of Soyasaponin I. J. Agric Food Chem. 2004, 52, 2689–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakenfull, D.G.; Sidhu, G.S. Could saponins be a useful treatment for hypercholesterolaemia? Eur. J. Clin. Nutr. 1990, 44, 79–88. [Google Scholar] [PubMed]





| Soya Product | Soyasaponin I a |
|---|---|
| Flour | 47.01 ± 5.27 |
| Milk | 74.11 ± 4.16 |
| Meat | 38.97 ± 1.43 |
| Protein Isolate | 146.25 ± 10.55 |
| Sausage Spread | 26.47 ± 3.73 n/d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neacsu, M.; Raikos, V.; Benavides-Paz, Y.; Duncan, S.H.; Duncan, G.J.; Christie, J.S.; Johnstone, A.M.; Russell, W.R. Sapogenol is a Major Microbial Metabolite in Human Plasma Associated with High Protein Soy-Based Diets: The Relevance for Functional Food Formulations. Foods 2020, 9, 422. https://doi.org/10.3390/foods9040422
Neacsu M, Raikos V, Benavides-Paz Y, Duncan SH, Duncan GJ, Christie JS, Johnstone AM, Russell WR. Sapogenol is a Major Microbial Metabolite in Human Plasma Associated with High Protein Soy-Based Diets: The Relevance for Functional Food Formulations. Foods. 2020; 9(4):422. https://doi.org/10.3390/foods9040422
Chicago/Turabian StyleNeacsu, Madalina, Vassilios Raikos, Yara Benavides-Paz, Sylvia H. Duncan, Gary J. Duncan, James S. Christie, Alexandra M. Johnstone, and Wendy R. Russell. 2020. "Sapogenol is a Major Microbial Metabolite in Human Plasma Associated with High Protein Soy-Based Diets: The Relevance for Functional Food Formulations" Foods 9, no. 4: 422. https://doi.org/10.3390/foods9040422
APA StyleNeacsu, M., Raikos, V., Benavides-Paz, Y., Duncan, S. H., Duncan, G. J., Christie, J. S., Johnstone, A. M., & Russell, W. R. (2020). Sapogenol is a Major Microbial Metabolite in Human Plasma Associated with High Protein Soy-Based Diets: The Relevance for Functional Food Formulations. Foods, 9(4), 422. https://doi.org/10.3390/foods9040422