A Critical Appraisal of Global Testing Protocols for Zoonotic Parasites in Imported Seafood Applied to Seafood Safety in Australia
Abstract
:1. Introduction
2. Methods Used for Critical Appraisal
3. Codex Alimentarius Non-Mandatory Recommendations
3.1. Candling
3.2. Operator Constraints and Candling Accuracy
3.3. Limitations of Candling to Detect Parasites
3.3.1. Nematodes
3.3.2. Trematode Metacercariae
3.3.3. Tapeworm Plerocercoids
3.3.4. Myxozoans
3.4. Ambiguity in Codex Food Safety Guidelines
3.5. Ambiguity of Codex Salting and Brining Recommendations
4. Human Health Risks Posed by Seafood-Borne Parasites
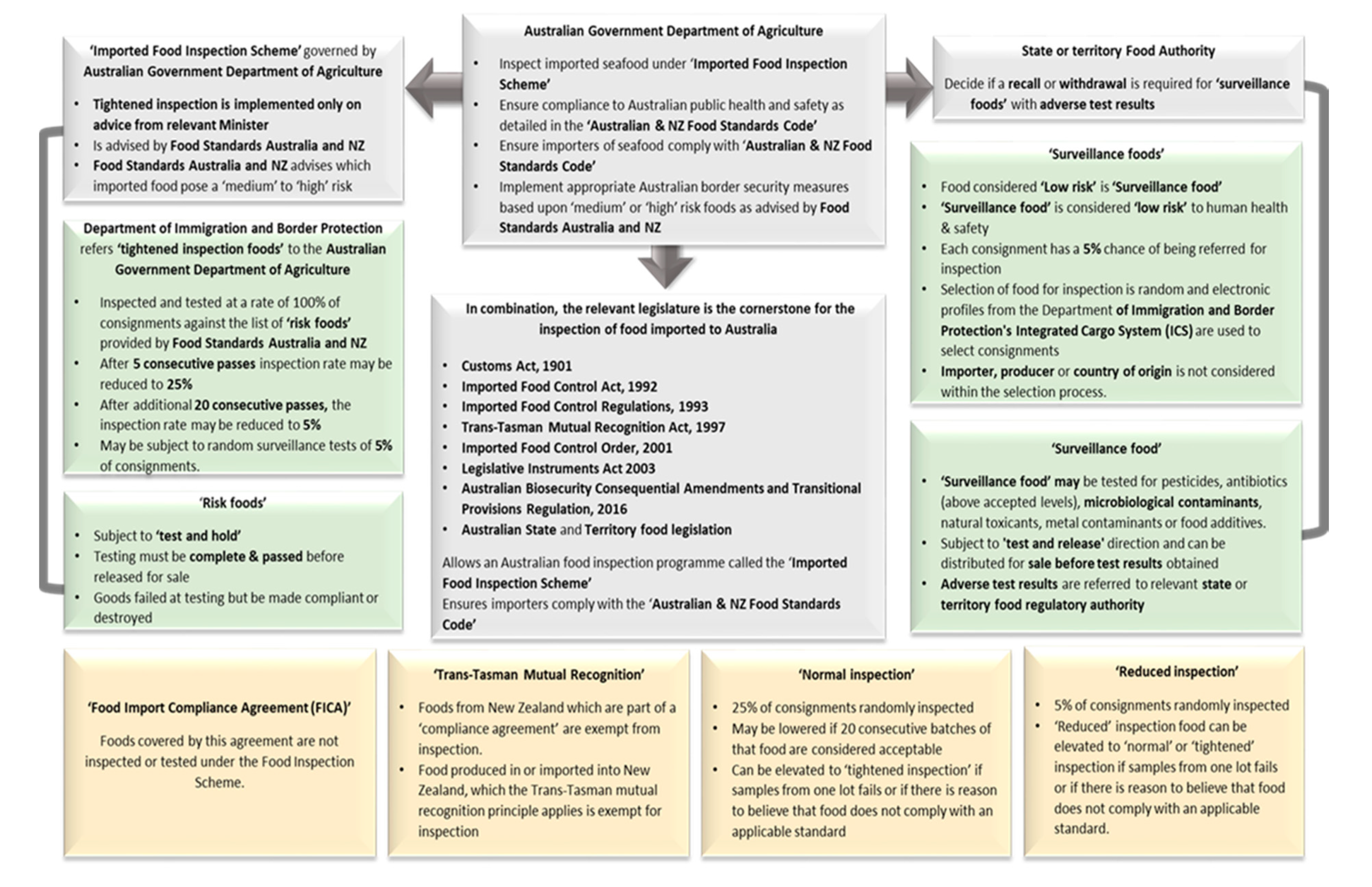
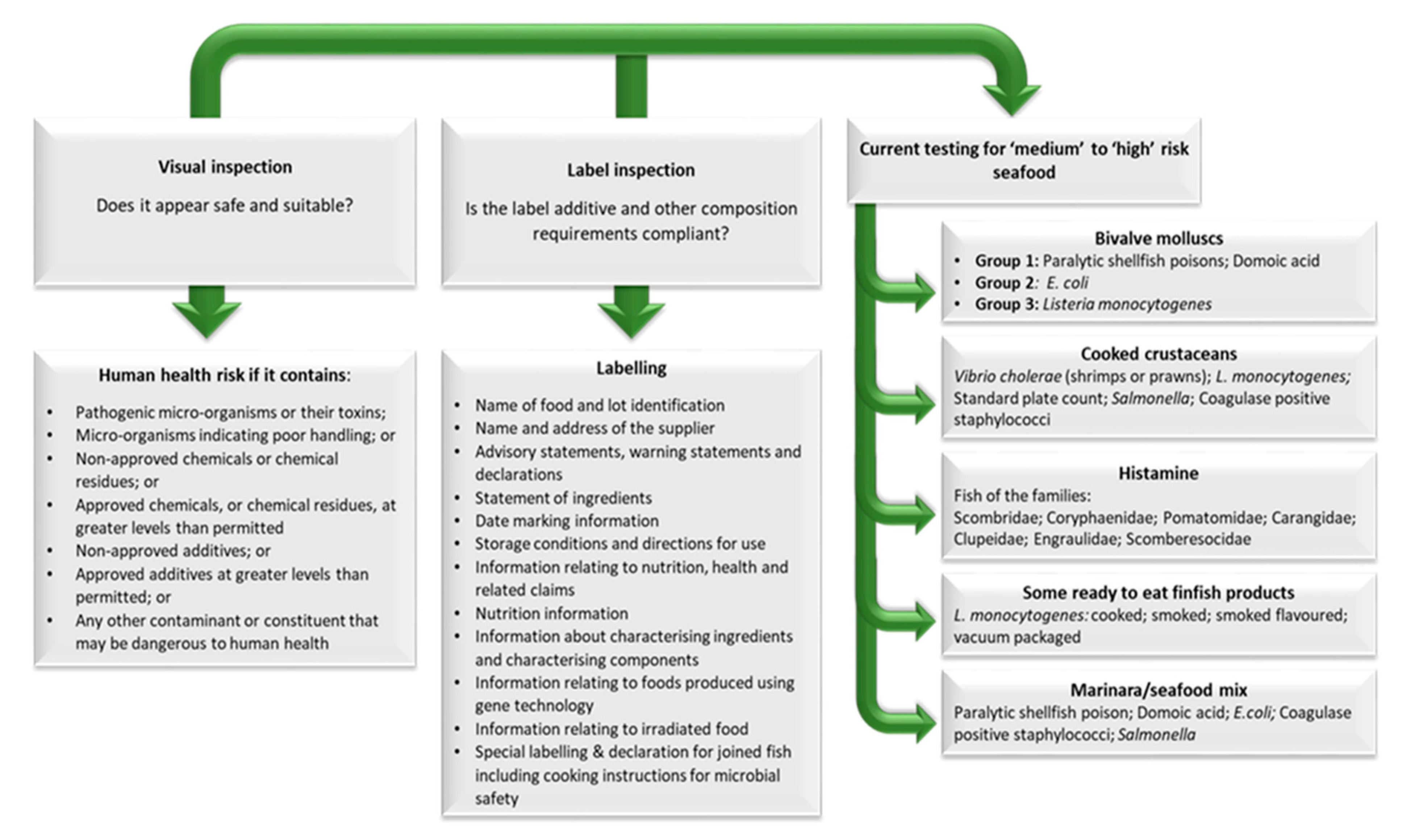
5. Imported Seafood Inspection in Australia
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Codex Codes of Practice | Codex Code of Practice which Relate to Parasite in Fish Processing |
|---|---|
| CAC/RCP 1-1969 (Last updated 2003) General principles of food hygiene | 5.3 INCOMING MATERIAL REQUIREMENTS No raw material or ingredient should be accepted by an establishment if it is known to contain parasites, undesirable micro-organisms, pesticides, veterinary drugs or toxic, decomposed or extraneous substances which would not be reduced to an acceptable level by normal sorting and/or processing. Where appropriate, specifications for raw materials should be identified and applied. |
| CAC/RCP 52-2003 (Last updated 2016) Code of practice for fish and fishery products | 2.5 Candling Passing fillets of fish over a translucent Filluminated from below to detect parasites and other defects. Hot is generally sufficient to kill parasites, to destroy non-sporulated bacterial pathogens and to injure spores of human health concern. |
| 5. Unless they can be reduced to an acceptable level by normal sorting and/or processing, no fish, shellfish or other aquatic invertebrates should be accepted if they are known to contain parasites. 5.2 Parasites of public health significance: trematodes, nematodes, cestodes | |
| 6.2 Infection with nematode parasites is absent from, or very much reduced in, farmed salmon compared with salmon caught in the wild | |
| 9.1.1. Raw, fresh or frozen fish reception Potential hazards: microbiological contamination, viable parasites | |
| Training in species identification and communication in product specification should be provided to fish handlers and appropriate personnel to ensure a safe source of incoming fish where written protocols exist. Warranting special consideration are the reception and sorting of fish species that pose a risk from parasites. 9.1.3. Frozen storage Potential hazards: microbiological contamination, toxins, viable parasites. For killing parasites harmful to human health, the freezing temperature and monitoring of duration of freezing should be combined with good inventory control to ensure sufficient cold treatment. 9.1.6 Filleting, skinning, trimming and candling Potential hazards: viable parasites, Potential defects: parasites. 9.1.6. Candling of skinless fillets by skilled personnel, in a suitable location that optimizes the illuminating effect, is an effective technique in controlling parasites (in fresh fish) and should be employed when implicated fish species are being used. | |
| 9.3.1 Freezing process Potential hazards: viable parasites. For killing parasites harmful to human health, the freezing temperature and monitoring of duration of freezing should be combined with good inventory control to ensure sufficient cold treatment. 9.4.1 Mincing fish using mechanical separation process, Potential defects: parasites. Candling is recommended for fish suspected of high infestation with parasites | |
| 10.1 General considerations of hazards and defects for frozen surimi production. Parasites will not be a hazard as the final product will be cooked or pasteurized. 10.1.2 Myxosporidia is a parasite that is common in marine groundfish such as Pacific whiting. This organism contains protease enzymes that chemically separate proteins that can ultimately affect the gel strength of surimi even at very low incidence. If species are used that are known to contain this parasite, protease inhibitors such as beef plasma protein or egg whites may be needed as additives to attain the necessary gel strength capabilities for kamaboko or crab analogue production. | |
| 12. Processing of salted and dried salted fish, 12.1 Where appropriate, fresh fish intended for processing salted fish should be checked for visible parasites. Freezing, heating or adequate combination of salt content and storage time can be used as treatment procedures for killing living parasites. 12.2 Preparing for salting. 12.2.1 Splitting, washing and rinsing, visible parasites should be removed. 12.4.1 Brining: Potential hazards: viable parasites. 12.4.2 Brine injection: Potential hazards: viable parasites. 12.4.3 Wet-salting: Potential hazards: viable parasites. 12.4.4 Dry-salting Potential hazards: viable parasites. 12.4.5 Pickling: Potential hazards: viable parasites. 12.4.6 Maturing: Potential hazards: viable parasites. | |
| 13. Smoked fish, smoke-flavoured fish and smoke-dried fish. 13.1 Processing of Smoked Fish: If raw material likely to contain viable parasites is to be used steps must be taken to eliminate this hazard during processing steps, e.g., freezing, heating or salting the product. Alternatively, the final product should be treated in a way to kill parasites. 13.1.10 Hot smoking Potential hazards: parasites. 13.1.15 Cooling or freezing Potential hazards: survival of parasites. If freezing at this process step is carried out to kill parasites, a time/temperature regime has to be chosen as laid down in Annex I of the Standard for smoked fish, smoke-flavoured fish and smoke-dried fish (CODEX STAN 311-2013). 13.3.2 Smoke-drying. Potential hazards: parasites. | |
| 17. Processing of canned fish. 17.3.5.1 Fish preparation: Potential defects: Parasites. | |
| ANNEX I. Potential hazards associated with fresh fish, shellfish and other aquatic invertebrates. 1.1 Parasites The parasites known to cause disease in humans and transmitted by fish are broadly classified as helminths or parasitic worms. These are commonly referred to as nematodes, cestodes and trematodes. Fish can be parasitized by protozoans, but there are no records of fish protozoan disease being transmitted to human beings. Parasites have complex life cycles involving one or more intermediate hosts and are generally passed to human beings through the consumption of raw, minimally processed or inadequately cooked products that contain the parasite infectious stage, causing foodborne disease. Freezing at −20 °C or below for seven days or −35 °C for about 20 h for fish intended for raw consumption will kill parasites. Processes such as brining or pickling may reduce the parasite hazard if the products are kept in the brine for a sufficient time but may not eliminate it. Candling, trimming belly flaps and physically removing the parasite cysts will also reduce the hazards but may not eliminate them. | |
| Nematodes Many species of nematode are known to occur worldwide and some species of marine fish act as secondary hosts. Among the nematodes of greatest concern are Anisakis spp., Capillaria spp., Gnathostoma spp. and Pseudoteranova spp., which can be found in the liver, belly cavity and flesh of marine fish. An example of a nematode causing disease in human beings is Anisakis simplex; the infective stage of the parasite is killed by heating (60 °C for one minute) and by freezing (−20 °C for 24 h) of the fish core | |
| Cestodes are tapeworms and the species of greatest concern associated with the consumption of fish is Dibothriocephalus latus. This parasite occurs worldwide and both fresh and marine fish are intermediate hosts. Similar to other parasitic infections, the foodborne disease occurs through the consumption of raw or under-processed fish. Similar freezing and cooking temperatures as applied to nematodes will kill the infective stages of this parasite. | |
| Trematodes Fish-borne trematode (flatworm) infections are a major public health problem endemic to approximately 20 countries around the world. The most significant species in terms of the number of people infected belong to the genera Clonorchis and Ophisthorchis (liver flukes), Paragonimus (lung flukes), and, to a lesser extent, Heterophyes and Echinochasmus (intestinal flukes). The most important definitive hosts of these trematodes are human beings or other mammals. Freshwater fish are the second intermediate host in the life cycles of Clonorchis and Ophistorchis, and freshwater crustaceans in the case of Paragonimius. Foodborne infections occur through the consumption of raw, undercooked or otherwise under-processed products containing the infective stages of these parasites. Freezing fish at −20 °C for seven days or at −35 °C for 24 h will kill the infective stages of these parasites | |
| CAC/GL 48-2004 (Last updated 2004) Model certificate for fish and fishery product | |
| CAC/GL 31-1999 (Last updated 1999) Guidelines for the Sensory Evaluation of Fish and Shellfish in Laboratories | Annexe 1. Belly cavity guts (in intact fish): intact, digested cleanliness (in gutted fish): completely gutted and cleaned, incompletely gutted, not washed belly walls: bright, clean, discoloured, digested parasites: absent, present blood: bright, red, brown |
| CAC/GL 83-2013 (Last updated 2015) Principles for the use of sampling and testing in international food trade | |
| CAC/GL 88-2016 (Last updated 2016) Guidelines on the Application of General Principles of Food Hygiene to the Control of Foodborne Parasites | |
| Codex Standard Codes | Codex Standard Guideline Which Apply to for Parasite in Seafood Product |
| CODEX STAN 3-1981 (Last updated 2011) Standard for Canned Salmon | |
| CODEX STAN 36-1981 (Last updated 2017) Standard for Quick Frozen Finfish, Uneviscerated and Eviscerated | 8.4.2 Flesh abnormalities A sample unit affected by excessive gelatinous condition of the flesh together with greater than 86% moisture found in any individual fish or sample unit with pasty texture resulting from parasitic infestation affecting more than 5% of the sample unit by weight. |
| CODEX STAN 37-1991 (Last updated 2016) Standard for Canned Shrimps or Prawns | |
| CODEX STAN 70-1981 (Last updated 2016) Standard for Canned Tuna and Bonito | |
| CODEX STAN 90-1981 (Last updated 2016) Standard for Canned Crab Meat | |
| CODEX STAN 92-1981 (Last updated 2017) Standard for Quick Frozen Shrimps or Prawns | |
| CODEX STAN 94-1981 (Last updated 2016) Standard for Canned Sardines and Sardine-Type Products | |
| CODEX STAN 95-1981 (Last updated 2017) Standard for Quick Frozen Lobsters | |
| CODEX STAN 119-1981 (Last updated 2016) Standard for Canned Finfish | |
| CODEX STAN 165-1989 (Last updated 2017) Standard for Quick Frozen Blocks of Fish Fillets, Minced Fish Flesh and Mixtures of Fillets and Minced Fish Flesh Amended (2017) | 7.4 Procedure for the Detection of Parasites for skinless blocks of fish fillets (Type I method) The entire sample unit is examined non-destructively by placing appropriate portions of the thawed sample unit on a 5-mm thick acryl sheet with 45% translucency and candled with a light source giving 1500 lux 30 cm above the sheet. |
| 8.3 Parasites The presence of two or more parasites per kg of the sample unit detected by a method described in 7.4 with a capsular diameter greater than 3 mm or a parasite not encapsulated and greater than 10 mm in length. | |
| CODEX STAN 166-1989 (Last updated 2017) Standard for Quick Frozen Fish Sticks (Fish Fingers), Fish Portions and Fish Fillets—Breaded or in Batter | |
| CODEX STAN 167-1989 (Last updated 2016) Standard for Salted Fish and Dried Salted Fish of the Gadidae Family of Fishes | |
| CODEX STAN 189-1993 (Last updated 1993) Standard for Dried Shark Fins | |
| CODEX STAN 190-1995 (Last updated 2017) Standard for Quick Frozen Fish Fillets | 7.4 Procedure for the Detection of Parasites (Type 1 Method) in skinless fillets The entire sample unit is examined non-destructively by placing appropriate portions of the thawed sample unit on a 5 mm thick acryl sheet with 45% translucency and candled with a light source giving 1500 lux 30 cm above the sheet. |
| 8.3 Parasites The presence of two or more parasites per kg of the sample unit detected by the method described in 7.4 with a capsular diameter greater than 3 mm or a parasite not encapsulated and greater than 10 mm in length. | |
| 8.6 Flesh abnormalities A sample unit affected by excessive gelatinous condition of the flesh together with greater than 86% moisture found in any individual fillet or a sample unit with pasty texture resulting from parasitic infestation affecting more than 5% of the sample unit by weight. | |
| CODEX STAN 191-1995 (Last updated 1995) Standard for Quick Frozen Raw Squid | |
| CODEX STAN 236-2003 (2003) Standard for Boiled Dried Salted Anchovies | |
| CODEX STAN 244-2004 (Last updated, 2018) Standard for Salted Atlantic Herring and Salted Sprat | 3.1 Fish Salted Atlantic herring and salted sprats shall be prepared from sound and wholesome fish which are of a quality fit to be sold fresh for human consumption after appropriate preparation. Fish flesh shall not be obviously infested by parasites. |
| 5.4 Parasites Fish flesh shall not contain living larvae of nematodes. Viability of nematodes shall be examined according to Annex I. If living nematodes are confirmed, products must not be placed on the market for human consumption before they are treated in conformity with the methods laid down in Annex II | |
| 7.1 Sampling plan for containers (Barrels) Sampling of lots for pathogenic microorganisms and parasites will be in accordance with the Principles and Guidelines for the Establishment and Application of Microbiological Criteria Related Foods (CXG 21-1997). | |
| 8.1.2 Parasites The presence of readily visible parasites in a sample of the edible portion of the sample unit detected by normal visual inspection of the fish flesh (see Annex III). ANNEX I VIABILITY TEST FOR NEMATODES Principle Nematodes are isolated from fish fillets by digestion, transferred into 0.5% Pepsin digestion solution and inspected visually for viability. Digestion conditions correspond to conditions found in the digestive tracts of mammals and guarantee the survival of nematodes. Equipment—Stacked sieves (diameter: 14 cm or larger, mesh size: 0.5 mm)—Magnetic stirrer with thermostated heating plate—Normal laboratory equipment Chemicals—Pepsin 2000 FIP-U/g—Hydrochloric acid Solution A: 0.5% (w/v) Pepsin in 0.063 M HCl Procedure Fillets of approximately 200 g are manually shredded and placed in a 2 l beaker containing 1 l Pepsin solution A. The mixture is heated on a magnet stirrer to 37 °C for 1–2 h under continuous slow stirring. If the flesh is not dissolved, the solution is poured through a sieve, washed with water and the remaining flesh is quantitatively replaced in the beaker. 700 mL digestion solution A is added and the mixture stirred again under gentle heating (max. 37 °C) until there are no large pieces of flesh left. The digestion solution is decanted through a sieve and the content of the sieve rinsed with water. Nematodes are carefully transferred by means of small forceps into Petri dishes containing fresh Pepsin solution A. The dishes are placed on a candling dish, and care has to be taken not to exceed 37 °C. Viable nematodes show visible movements or spontaneous reactions when gently probed with dissecting needles. A single relaxation of coiled nematodes, which sometimes occurs, is not a clear sign of viability. Nematodes must show spontaneous movement. Attention When checking for viable nematodes in salted or sugar salted products, reanimation time of nematodes can last up to two hours and more. Remarks Several other methods exist for the determination of viability of nematodes (e.g., ref. 2, 3). The described method has been chosen because it is easy to perform and combines isolation of nematodes and viability test within one step. ANNEX II Treatment procedures sufficient to kill living nematodes—e.g., freezing to −20 °C for not less than 24 h in all parts of the product—the adequate combination of salt content and storage time (To be elaborated)—or by other processes with the equivalent effect (To be elaborated) ANNEX III Determination of the presence of visible parasites 1. The presence of readily visible parasites in a sample unit that is broken into normal bite-size pieces 20–30 mm of flesh by the thickness of the fillet. Only the normal edible portion is considered even if other material is included with the fillet. Examination should be done in an adequately lighted room (where a newspaper may be read easily), without magnification, for evidence of parasites. 2. Notwithstanding paragraph 1, the verification of the presence of parasites in intermediate entire fishery products in bulk intended for further processing could be carried out at a later stage. | |
| CODEX STAN 291-2010 (Last updated 2018) Standard for Sturgeon Caviar | |
| CODEX STAN 292-2008 (2015) Standard for Live and Raw Bivalve Molluscs | |
| CODEX STAN 302-2011 (Last updated 2018) Standard for Fish Sauce | |
| CODEX STAN 311-2013 (Last updated 2018) Standard for Smoked Fish, Smoke-Flavoured Fish and Smoke-Dried Fish | 2.1.2 “Hot smoking” is a process in which fish is smoked at an appropriate combination of temperature and time sufficient to cause the complete coagulation of the proteins in the fish flesh. Hot smoking is generally sufficient to kill parasites, to destroy non-sporulated bacterial pathogens and to injure spores of human health concern. |
| 6.3 Parasites Products covered by this Standard shall not contain living parasites and particular attention needs to be paid to cold smoked or smoke-flavoured products, which should be frozen before or after smoking if a parasite hazard is present (see Annex 1). Viability of nematodes, cestodes and trematodes shall be examined according to Section 8.10 and/or 8.11. | |
| 8.10 Determination of the viability of parasites Methods used for extracting and testing the viability of parasites could include the method set out in Annex I for nematodes in the Standard for Salted Atlantic Herring and Salted Sprat (CXS 244-2004) or other validated methods for parasites acceptable to the competent authority having jurisdiction. | |
| 8.11 Determination of visible parasites The entire sample unit is examined for the presence of parasites non-destructively by placing appropriate portions of the thawed (if necessary) sample unit on a 5 mm thick acryl sheet with 45% translucency and candled with a light source giving 1500 lux 30 cm above the sheet. | |
| 9.2 Parasites The presence of two or more visible parasites per kg of the sample unit detected by the method described in 8.11 with a capsular diameter greater than 3 mm or a parasite not encapsulated and greater than 10 mm in length. | |
| ANNEX I Procedures sufficient to kill parasites A method that is acceptable to the competent authority having jurisdiction shall be used to kill parasites. Where freezing is required to kill parasites (i.e., cold smoked fish and smoke-flavoured fish) the fish must be frozen either before or after processing to a temperature time combination sufficient to kill the living parasites. Examples of freezing processes that may be sufficient to kill some or all parasites are:
| |
| CODEX STAN 312-2013 (Last updated 2016) Standard for Live Abalone and for Raw Fresh Chilled or Frozen Abalone for Direct Consumption or for further Processing | |
| CODEX STAN 315-2014 (Last updated 2017) Standard for Fresh and Quick Frozen Raw Scallop Products (2014) | 8.6 Examination for Parasites The presence of readily visible parasites in a sample unit detected by normal visual inspection of the scallops. |
| 9.4 Parasites The presence of parasites should not be at an objectionable level. | |
| CODEX STAN 329-2017 (Last updated 2017) Standard for Fish Oils |
References
- Jeggo, M. The Australian perspective, the biosecurity continuum from preborder, to border and postborder. In Improving Food Safety Through a One Health Approach: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2012; p. 198. [Google Scholar]
- Australian Government. Biosecurity in Australia. 2019. Available online: http://www.agriculture.gov.au/biosecurity/australia (accessed on 15 September 2019).
- Craik, W.; Palmer, D.; Sheldrake, R. Priorities for Australia’s Biosecurity System. An Independent Review of the Capacity of the National Biosecurity System and Its Underpinning Intergovernmental Agreement; Department of Agriculture and Water Resources: Canberra, Australia, 2017.
- FRDC. Summary Overview: Economic Impact of 2016 White Spot Disease Outbreak; FRDC: Canberra, Australia, 2016.
- Lightner, D.V.; Redman, R.; Poulos, B.; Nunan, L.; Mari, J.; Hasson, K. Risk of spread of penaeid shrimp viruses in the Americas by the international movement of live and frozen shrimp. Sci. Tech. Rev. -Off. Int. Épizooties 1997, 16, 146–160. [Google Scholar] [CrossRef]
- Durand, S.; Tang, K.; Lightner, D.V. Frozen commodity shrimp: Potential avenue for introduction of white spot syndrome virus and yellow head virus. J. Aquat. Anim. Health 2000, 12, 128–135. [Google Scholar] [CrossRef]
- Nunan, L.; Poulos, B.; Lightner, D. The detection of white spot syndrome virus (WSSV) and yellow head virus (YHV) in imported commodity shrimp. Aquaculture 1998, 160, 19–30. [Google Scholar] [CrossRef]
- Sheeshka, J.; Murkin, E. Nutritional aspects of fish compared with other protein sources. Comments Toxicol. 2002, 8, 375–397. [Google Scholar] [CrossRef]
- McManus, A.; Howieson, J.; Nicholson, C. Review of Literature and Resources Relating to the Health Benefit of Regular Consumption of Seafood as Part of a Healthy Diet; CoESSH: Perth, Australia, 2009; pp. 1–113. [Google Scholar]
- Aadland, E.K.; Lavigne, C.; Graff, I.E.; Eng, Ø.; Paquette, M.; Holthe, A.; Mellgren, G.; Jacques, H.; Liaset, B. Lean-seafood intake reduces cardiovascular lipid risk factors in healthy subjects: Results from a randomized controlled trial with a crossover design1,2. Am. J. Clin. Nutr. 2015, 102, 582–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senior, N.; Stewardson, C. Promoting sustainable Australian fish and seafood. Food Aust. 2019, 71, 34. [Google Scholar]
- Australian Bureau of Statistics. Selected Living Cost Indexes, CAT Number 6467.0. Available online: http://www.abs.gov.au/AUSSTATS/[email protected]/97adb482c0aba769ca2570460017d0e7/0e1fe191c5e59d18ca2570d7001ad238!OpenDocument#Timeseries (accessed on 24 October 2018).
- Manetta. Manetta Seafood Market Retail Price. Available online: https://www.manettas.com.au/product/flathead-fillets/ (accessed on 24 October 2008).
- FRDC. Australian Fish Names Database. Available online: www.fishnames.com.au (accessed on 10 October 2018).
- Greenpeace. Label My Fish: Why Accurate Seafood Labelling Matters. Available online: https://www.marineconservation.org.au/data/14-115_OCN_LabelMyFish_Report_lr.pdf (accessed on 1 October 2018).
- United Nations. International Covenant on Economic, Social and Cultural Rights; Opened for Signature 16 December 1966 UNTS 2200A (XXI) (Entered into Force 3 January 1976) Art 27; United Nations: Geneva, Switzerland, 1976. [Google Scholar]
- FAO. Voluntary Guidelines to Support the Progressive Realization of the Right to Adequate Food in the Context of National Food Security. Available online: http://www.fao.org/docrep/009/y7937e/y7937e00.htm (accessed on 4 June 2018).
- WTO. Sanitary and phytosanitary measures In The WTO Agreement Series; World Trade Organisation: Geneva, Switzerland, 1994. [Google Scholar]
- Codex Alimentarius CAC/RCP 52-2003. Code of Practice for Fish and Fishery Products. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCAC%2BRCP%2B52-2003%252FCXP_052e.pdf (accessed on 1 July 2019).
- Karl, H.; Leinemann, M. A fast and quantitative detection method for nematodes in fish fillets and fishery products. J. Food Saf. Food Qual. 1993, 44, 124–125. [Google Scholar]
- Andreoletti, O.; Budka, H.; Buncic, S.; Collins, J.D.; Griffin, J.; Hald, T.; Havelaar, A.H.; Hope, J.; Klein, G.; Koutsumanis, K.; et al. Scientific opinion on risk assessment of parasites in fishery products. EFSA J. 2010, 8, 1–91. [Google Scholar]
- Codex Alimentarius CODEX STAN 165-1989. Standard for Quick Frozen Blocks of Fish Fillets, Minced Fish Flesh and Mixtures of Fillets and Minced Fish Flesh. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B165-1989%252FCXS_165e.pdf (accessed on 1 July 2019).
- Codex Alimentarius CODEX STAN 190-1995. Standard for Quick Frozen Fish Fillets. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B190-1995%252FCXS_190e.pdf (accessed on 1 October 2018).
- Codex Alimentarius CODEX STAN 311-2013. Standard for Smoked Fish, Smoke-Flavoured Fish and Smoke-Dried Fish. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B311-2013%252FCXS_311e.pdf (accessed on 1 October 2019).
- Petrie, A.; Wootten, R.; Bruno, D.; MacKenzie, K.; Bron, J. A Survey of Anisakis and Pseudoterranova in Scottish Fisheries and the Efficacy of Current Detection Methods; FSAS Project S14008; Food Standards Scotland: Aberdeen, UK, 2007.
- Krupinski, E.A.; Berbaum, K.S.; Caldwell, R. Impact of Visual Fatigue on Observer Performance in Medical Imaging 2009: Image Perception, Observer Performance, and Technology Assessment; SPIE: Bellingham, WA, USA, 2009; Volume 7263. [Google Scholar]
- Wootten, R.; Cann, D. Round Worms in Fish; Marine Laboratory of the Department of Agriculture and Fisheries for Scotland and the Torry Research Station of the Ministry of Agriculture: Aberdeen, Scotland, 2001.
- Hafsteinsson, H.; Rizvi, S.S. A review of the sealworm problem: Biology, implications and solutions. J. Food Prot. 1987, 50, 70–84. [Google Scholar] [CrossRef]
- McGladdery, S.E. Anisakis simplex (Nematoda: Anisakidae) Infection of the Musculature and Body Cavity of Atlantic Herring (Clupea harengus harengus). Can. J. Fish. Aquat. Sci. 1986, 43, 1312–1317. [Google Scholar] [CrossRef]
- Codex Alimentarius CAC/RCP 1-1969. General Principles of Food Hygiene. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCAC%2BRCP%2B1-1969%252FCXP_001e.pdf (accessed on 1 July 2019).
- Levsen, A.; Lunestad, B.T.; Berland, B. Low detection efficiency of candling as a commonly recommended inspection method for nematode larvae in the flesh of pelagic fish. J. Food Prot. 2005, 68, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.M.; Murrell, K.D.; Cross, J.H. Parasites of fish and risks to public health. Rev. Sci. Tech. 1997, 16, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Murrell, C.; Sohn. Fishborne Zoonotic Paraistes in South East Asian Freshwater Aquaculture: Detection and Risk Mitigation throughout the Commercial Production Chain [powerpoint]. Available online: http://old.iss.it/binary/crlp/cont/Murrell_EU_Rome.pdf (accessed on 1 June 2018).
- Dos Santos, C.L. The possible use of HACCP in the prevention and control of food-borne trematode infections in aquacultured fish. In Seafood Safety, Processing, and Biotechnology; Technomic Publishing: Lancaster, PA, USA, 1997; pp. 53–64. [Google Scholar]
- Rim, H.-J.; Sohn, W.-M.; Yong, T.-S.; Eom, K.S.; Chai, J.-Y.; Min, D.-Y.; Lee, S.-H.; Hoang, E.-H.; Phommasack, B.; Insisengmay, S. Fishborne trematode metacercariae detected in freshwater fish from Vientiane Municipality and Savannakhet Province, Lao PDR. Korean J. Parasitol. 2008, 46, 253. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.-M.; Eom, K.S.; Min, D.-Y.; Rim, H.-J.; Hoang, E.-H.; Yang, Y.; Li, X. Fishborne trematode metacercariae in freshwater fish from Guangxi Zhuang Autonomous Region, China. Korean J. Parasitol. 2009, 47, 249–257. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation. Codex Alimentarius: International Food Standards. Available online: http://www.fao.org/fao-who-codexalimentarius/about-codex/jp/ (accessed on 24 June 2019).
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I. Aquaculture: Global status and trends. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef]
- Rozas, M.; Bohle, H.; Sandoval, A.; Ildefonso, R.; Navarrete, A.; Bustos, P. First molecular identification of Diphyllobothrium dendriticum Plerocercoids from feral rainbow trout (Oncorhynchus mykiss) in Chile. J. Parasitol. 2012, 98, 1220–1226. [Google Scholar] [CrossRef]
- Torres, P.; Puga, S. Comparative efficacy of candling and glass plate compression for detection of diphyllobothriosis in rainbow trout (Oncorhynchus mykiss) musculature. Revue Scientifique et Technique-OIE 2011, 30, 831. [Google Scholar] [CrossRef]
- Henricson, J. The abundance and distribution of Diphyllobothrium dendriticum (Nitzsch) and D. ditremum (Creplin) in the char Salvelinus alpinus (L.) in Sweden. J. Fish Biol. 1977, 11, 231–248. [Google Scholar] [CrossRef]
- Wicht, B.; Gustinelli, A.; Fioravanti, M.; Invernizzi, S.; Peduzzi, R. Prevalence of the broad tapeworm Diphyllobothrium latum in perch (Perca fluviatilis) and analysis of abiotic factors influencing its occurrence in Lake Lario (Como, Italy). Bull. Eur. Assoc. Fish Pathol. 2009, 29, 58–65. [Google Scholar]
- Codex Alimentarius. Code of Practice for Fish and Fishery Products; CAC/RCP 52-2003; FAO/WHO Food Standards Programme: Rome, Italy, 2016. [Google Scholar]
- Kasai, A.; Li, Y.-C.; Mafie, E.; Sato, H. New host records of monacanthid fish for three Kudoa spp.(K. septempunctata, K. thyrsites, and K. shiomitsui) prevalent in the olive flounder (Paralichthys olivaceus), with the description of K. parathyrsites n. sp. from a black scraper (Thamnaconus modestus). Parasitol. Res. 2016, 115, 2741–2755. [Google Scholar]
- Matsukane, Y.; Sato, H.; Tanaka, S.; Kamata, Y.; Sugita-Konishi, Y. Kudoa septempunctata n. sp.(Myxosporea: Multivalvulida) from an aquacultured olive flounder (Paralichthys olivaceus) imported from Korea. Parasitol. Res. 2010, 107, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Suzuki, J.; Shirakashi, S. Kudoa hexapunctata n. sp.(Myxozoa: Multivalvulida) from the somatic muscle of Pacific bluefin tuna Thunnus orientalis and re-description of K. neothunni in yellowfin tuna T. albacares. Parasitol. Int. 2014, 63, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, N.; Maehara, T. Molecular characterization of kudoid parasites (Myxozoa: Multivalvulida) from somatic muscles of Pacific bluefin (Thunnus orientalis) and Yellowfin (T. albacores) tuna. Acta Parasitol. 2013, 58, 226–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, J.; Murata, R.; Yokoyama, H.; Sadamasu, K.; Kai, A. Detection rate of diarrhoea-causing Kudoa hexapunctata in Pacific bluefin tuna Thunnus orientalis from Japanese waters. Int. J. Food Microbiol. 2015, 194, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius CAC/GL 88-2016. Guidelines on the Application of General Principles of Food Hygiene to the Control of Foodborne Parasites. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/tr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCAC%2BGL%2B88-2016%252FCXG_088e.pdf (accessed on 1 July 2019).
- Codex Alimentarius CODEX STAN 244-2004. Standard for Salted Atlantic Herring and Salted Sprat. Codex Standard 244-2004. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 2 October 2018).
- Horbowy, J.; Podolska, M. Modelling infection of Baltic herring (Clupea harengus membras) by larval Anisakis simplex. ICES J. Mar. Sci. 2001, 58, 321–330. [Google Scholar] [CrossRef]
- Lubieniecki, B. Salted and spiced herring tested for the presence and viability of parasitic nematode larvae. Biul. MIR Rocz. I 1970, 4, 12–16. [Google Scholar]
- Grabda, J.; Bier, J.W. Cultivation as an estimate for infectivity of larval Anisakis simplex from processed herring. J. Food Prot. 1988, 51, 734–736. [Google Scholar] [CrossRef]
- Oh, S.-R.; Zhang, C.-Y.; Kim, T.-I.; Hong, S.-J.; Ju, I.-S.; Lee, S.-H.; Kim, S.-H.; Cho, J.-I.; Ha, S.-D. Inactivation of Anisakis larvae in salt-fermented squid and pollock tripe by freezing, salting, and combined treatment with chlorine and ultrasound. Food Control 2014, 40, 46–49. [Google Scholar] [CrossRef]
- Ching, H.L. Fish tapeworm infections (diphyllobothriasis) in Canada, particularly British Columbia. Can. Med. Assoc. J. 1984, 130, 1125. [Google Scholar]
- Fan, P. Viability of metacercariae of Clonorchis sinensis in frozen or salted freshwater fish. Int. J. Parasitol. 1998, 28, 603–605. [Google Scholar] [CrossRef]
- Shamsi, S.; Sheorey, H. Seafood-borne parasitic diseases in Australia: Are they rare or underdiagnosed? Int. Med. J. 2018, 48, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gómez, A.; Moreno-Ancillo, A.; López-Serrano, M.; Suarez-de-Parga, J.; Daschner, A.; Caballero, M.; Barranco, P.; Cabañas, R. Anisakis simplex only provokes allergic symptoms when the worm parasitises the gastrointestinal tract. Parasitol. Res. 2004, 93, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Audicana, M.T.; Fernández de Corres, L.; Muñoz, D.; Fernández, E.; Navarro, J.A.; del Pozo, M.D. Recurrent anaphylaxis caused by Anisakis simplex parasitizing fish. J. Allergy Clin. Immunol. 1995, 96, 558–560. [Google Scholar] [CrossRef]
- FAO/WHO. Multicriteria-based ranking for risk management of food-borne parasites. In Report of a Joint FAO/WHO Expert Meeting, 3–7 September 2012, FAO Headquarters, Rome, Italy; FAO/WHO: Rome, Italy, 2014; Volume 23. [Google Scholar]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. Available online: https://www.paho.org/hq/dmdocuments/2015/2015-cha-who-estimates-global-foodborne-diseases.pdf (accessed on 1 June 2018).
- Sumner, J.; Ross, T. A semi-quantitative seafood safety risk assessment. Int. J. Food Microbiol. 2002, 77, 55–59. [Google Scholar] [CrossRef]
- Anantanawat, S.; Kiermeier, A.; McLeod, C.; Sumner, J. A Semi-Quantitative Risk Assessment of Harmful Parasites in Australian Finfish; South Australian Research & Development Institute: Urrbrae, Australia, 2012.
- Sumner, J.; Antananawat, S.; Kiermeier, A.; McLeod, C.; Shamsi, S. Raw fish consumption in Australia: How safe is it? Food Aust. 2015, 67, 24. [Google Scholar]
- NSW, D. Risk Assessment of the Seafood Safety Scheme; Department of Primary Industry Food Authority: Newington, Australia, 2017.
- Warner, K.; Timme, W.; Lowell, B.; Stiles, M. Persistent seafood fraud found in South Florida; Oceana: Washington, DC, USA, 2012; pp. 1–20. [Google Scholar]
- Sirisinha, S.; Chawengkirttikul, R.; Sermswan, R. Immunodiagnosis of opisthorchiasis. Southeast Asian J. Trop. Med. Public Health 1991, 22, 179–183. [Google Scholar]
- Yossepowitch, O.; Gotesman, T.; Assous, M.; Marva, E.; Zimlichman, R.; Dan, M. Opisthorchiasis from imported raw fish.(Research). Emerg. Infect. Dis. 2004, 10, 2122. [Google Scholar] [CrossRef]
- Rohela, M.; Surin, J.; Jamaiah, I.; Init, I.; Lee, S. Acute cholecystitis caused by Clonorchis sinensis. SE Asian J. Trop. Med. 2006, 37, 648–651. [Google Scholar]
- Park, G.-M. Genetic comparison of liver flukes, Clonorchis sinensis and Opisthorchis viverrini, based on rDNA and mtDNA gene sequences. Parasitol. Res. 2006, 100, 351. [Google Scholar] [CrossRef]
- Mas, C.S.; Bargues, M.D. Human liver flukes: A review. Res. Rev. Parasitol. 1997, 57, 145–218. [Google Scholar]
- Prasongwatana, J.; Laummaunwai, P.; Boonmars, T.; Pinlaor, S. Viable metacercariae of Opisthorchis viverrini in northeastern Thai cyprinid fish dishes—As part of a rational program for control of O. viverrini-associated cholangiocarcinoma. Parasitol. Res. 2013, 112, 1323–1327. [Google Scholar] [CrossRef]
- Murrell, D.; Chai, J.; Sohn, W. FIBOZOPA laboratory manual on: Identification of zoonotic metacercariae from fish. FIBOZOPA 2005, 1–33. [Google Scholar]
- Pastor-Valle, J.; González, L.M.; Martín-Clemente, J.P.; Merino, F.J.; Gottstein, B.; Gárate, T. Molecular diagnosis of diphyllobothriasis in Spain, most presumably acquired via imported fish, or sojourn abroad. New Microbes New Infect. 2014, 2, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban, J.; Muñoz-Antoli, C.; Borras, M.; Colomina, J.; Toledo, R. Human infection by a “fish tapeworm”, Diphyllobothrium latum, in a non-endemic country. Infection 2014, 42, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, R.; Esteban, J.-G.; Brabec, J.; Scholz, T. Misidentification of Diphyllobothrium species related to global fish trade, Europe. Emerg. Infect. Dis. 2014, 20, 1955–1957. [Google Scholar] [CrossRef]
- Paugam, A.; Yera, H.; Poirier, P.; Lebuisson, A.; Dupouy-Camet, J. Diphyllobothrium nihonkaiense Bothricephalosis: A new risk linked to the consumption of salmon. La Presse Médicale 2009, 38, 675–677. [Google Scholar] [CrossRef]
- Yera, H.; Estran, C.; Delaunay, P.; Gari-Toussaint, M.; Dupouy-Camet, J.; Marty, P. Putative Diphyllobothrium nihonkaiense acquired from a Pacific salmon (Oncorhynchus keta) eaten in France; genomic identification and case report. Parasitol. Int. 2006, 55, 45–49. [Google Scholar] [CrossRef]
- Shimizu, H.; Kawakatsu, H.; Shimizu, T.; Yamada, M.; Tegoshi, T.; Uchikawa, R.; Arizono, N. Diphyllobothriasis nihonkaiense: Possibly acquired in Switzerland from imported Pacific Salmon. Intern. Med. J. 2008, 47, 1359–1362. [Google Scholar] [CrossRef] [Green Version]
- Wicht, B.; de Marval, F.; Peduzzi, R. Diphyllobothrium nihonkaiense (Yamane et al., 1986) in Switzerland: First molecular evidence and case reports. Parasitol. Int. 2007, 56, 195–199. [Google Scholar] [CrossRef]
- Wicht, B.; Marval, F.; Gottstein, B.; Peduzzi, R. Imported diphyllobothriasis in Switzerland: Molecular evidence of Diphyllobothrium dendriticum (Nitsch, 1824). Parasitol. Res. 2008, 102, 201–204. [Google Scholar] [CrossRef]
- Ko, K.K.K.; Cao, D.Y.H.; Wee, L.W.Y.; Hsu, L.Y.; Yamasaki, H. First two confirmed cases of Dibothriocephalus nihonkaiensis in Southeast Asia (Singapore). Pathology 2019, 51, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Nawa, Y.; Akahane, H.; Diaz Camacho, S.P.; Lamothe-Argumedo, R.; Cruz-Reyes, A. Short report: Gnathostomiasis in Mexico. Am. J. Trop. Med. Hyg. 1998, 58, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, R.A.; Choudhury, A.; Nico, L.G.; Griffin, K.M. Gnathostoma spp. in live Asian swamp eels (Monopterus spp.) from food markets and wild populations, United States. Emerg. Infect. Dis. 2014, 20, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nico, L.; Sharp, P.; Collins, T. Imported Asian swamp eels (Synbranchidae: Monopterus) in North American live food markets: Potential vectors of non-native parasites. Aquat. Invasions J. 2011, 6, 69–76. [Google Scholar] [CrossRef]
- Attia, R.; Tolba, M.; Yones, D.; Bakir, H.; Eldeek, H.; Kamel, S. Capillaria philippinensis in Upper Egypt: Has it become endemic? Am. J. Trop. Med. Hyg. 2012, 86, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Mossali, C.; Palermo, S.; Capra, E.; Piccolo, G.; Botti, S.; Bandi, C.; D’Amelio, S.; Giuffra, E. Sensitive detection and quantification of anisakid parasite residues in food products. Foodborne Pathog. Dis. 2010, 7, 391–397. [Google Scholar] [CrossRef]
- Caballero, M.L.; Moneo, I. Several allergens from Anisakis simplex are highly resistant to heat and pepsin treatments. Parasitol. Res. 2004, 93, 248–251. [Google Scholar] [CrossRef]
- Herrero, B.; Vieites, J.M.; Espiñeira, M. Detection of anisakids in fish and seafood products by real-time PCR. Food Control 2011, 22, 933–939. [Google Scholar] [CrossRef]
- FRDC. Seafood Import and Export by Commodity. Available online: https://app.powerbi.com/view?r=eyJrIjoiZTg2NTlhYmQtM2ZhMy00ZGUyLTkwMTktMDJhODE5MmUxNjQ2IiwidCI6Ijk3M2YzMGJjLTNlMWYtNGQwMC05NmEyLTVjZGM2NTE5YjQxOCIsImMiOjEwfQ%3D%3D (accessed on 22 July 2018).
- Mattiucci, S.; Fazii, P.; De Rosa, A.; Paoletti, M.; Megna, A.S.; Glielmo, A.; De Angelis, M.; Costa, A.; Meucci, C.; Calvaruso, V.; et al. Anisakiasis and gastroallergic reactions associated with Anisakis pegreffii infection, Italy. Emerg. Infect. Dis. 2013, 19, 496. [Google Scholar] [CrossRef]
- Mattiucci, S.; Paoletti, M.; Colantoni, A.; Carbone, A.; Gaeta, R.; Proietti, A.; Frattaroli, S.; Fazii, P.; Bruschi, F.; Nascetti, G. Invasive anisakiasis by the parasite Anisakis pegreffii (Nematoda: Anisakidae): Diagnosis by real-time PCR hydrolysis probe system and immunoblotting assay.(Report). BMC Infect. Dis. 2017, 17, 530. [Google Scholar] [CrossRef]
- Moschella, C.; Mattiucci, S.; Mingazzini, P.; De Angelis, G.; Assenza, M.; Lombardo, F.; Monaco, S.; Paggi, L.; Modini, C. Intestinal anisakiasis in Italy: Case report. J. Helminthol. 2004, 78, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Daschner, A.; Moreno-Ancillo, A. Anaphylaxis with Anisakis simplex in the gastric mucosa. N. Engl. J. Med. 1997, 337, 350. [Google Scholar] [CrossRef] [PubMed]
- Australian Government. Imported Food Control Act 1992; Federal Register of Legislation, Ed.; Compilation No. 25; Australian Government: Canberra, Australia, 2018; pp. 1–86.
- Australian Government. Imported Food Control Regulations 1993; Federal Register of Legislation, Ed.; Latest Version F2017C00345. Amended 19 April 2017; Statutory Rules No. 100, 1993; Australian Government: Canberra, Australia, 2017.
- FSANZ. Australia and New Zealand Food Standards Code. In The FSANZ; Information Australia: Melbourne, Australia, 1997. [Google Scholar]
- FSANZ. The Australian and New Zealand Food Standards Code. In Imported Foods: Imported Foods Inspection Scheme; Food Standards Australia and New Zealand: Canberra, Australia, 2018; Volume 2018. [Google Scholar]
- Australian Government. Imported Food Control Order 2019. In In Force 13 February 2020; Federal Register of Legislation, Ed.; Australian Government: Canberra, Australia, 2019. [Google Scholar]
- Shamsi, S.; Suthar, J. Occurrence of Terranova larval types (Nematoda: Anisakidae) in Australian marine fish with comments on their specific identities. PeerJ 2016, 4, e1722. [Google Scholar] [CrossRef] [Green Version]
- Llarena-Reino, M.; Piñeiro, C.; Antonio, J.; Outeriño, L.; Vello, C.; González, Á.F.; Pascual, S. Optimization of the pepsin digestion method for anisakids inspection in the fishing industry. Vet. Parasitol. 2013, 191, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Agriculture. Failing Food Reports. Available online: http://www.agriculture.gov.au/import/goods/food/inspection-compliance/failing-food-reports (accessed on 12 December 2018).
- Australian Government. Imported Food Control Amendment Bill; Federal Register Legislation, Ed.; No. 108; Australian Government: Canberra, Australia, 2018; pp. 1–49.
- Parliament of Australian Government. Progress of the Imported Food Control Amendment Bill 2017; Agriculture and Water Resources, Ed.; Parliament of Australian Government: Canberra, Australia, 2018.
- Pyburne, P. Imported Food Control Amendment Bill 2017; Parliamentary Library Information and Advice; BILLS DIGEST NO. 12, 2017–2018; Parliament of Australia: Canberra, Australia, 2017.
- Department of Agriculture and Water Resources. Imported Food Reforms-Decision Regulation Impact Statement; Department of Agriculture and Water Resources: Canberra, Australia, 2016.
- Standards-Australia. Food safety management systems—Requirements for any organization in the food chain. In AS ISO 22000—2005; Council of Standards Australia, Ed.; Standards Australia: Sydney, Australia, 2005. [Google Scholar]
- ANZFSC. Standard 1.6.1—Microbiological limits in food. In Food Standards as Amended, Taking into Account Amendments up to Food Standards (Proposal P1048—Code Revision (2018)) Variation; F2018C00939; Federal Register of Legislation: Canberra, Australia, 2018. [Google Scholar]
- Australian Government. Export Control (Fish and Fish Products) Orders 2005; Federal Register of Legislation, Ed.; Australian Government: Canberra, Australia, 2014.
- Food Safety Information Council. Seafood. Available online: https://foodsafety.asn.au/seafood/ (accessed on 1 June 2018).
- FSANZ. Chapter 4 of the Australia New Zealand Food Standards Code (Australia only). In A guide to the Australian Primary Production and Processing Standard for Seafood Guide to Standard 4.2.1; Food Standards Australia New Zealand: Canberra, Australia, 2005. [Google Scholar]
- FSANZ. The Australian and New Zealand Food Standards Code. In The Compendium of Microbiological Criteria for Food; Food Standards Australia and New Zealand: Canberra, Australia, 2018. [Google Scholar]
- FRDC. Seafood Safety Fact Sheets. Available online: https://www.safefish.com.au/Reports/Food-Safety-Fact-Sheets (accessed on 1 October 2018).
- Kirk, M.; Ford, L.; Glass, K.; Hall, G. Foodborne illness, Australia, circa 2000 and circa 2010. Emerg. Infect. Dis. 2014, 20, 1857. [Google Scholar] [CrossRef]
- Hussain, M.; Saputra, T.; Szabo, E.; Nelan, B. An Overview of Seafood Supply, Food Safety and Regulation in New South Wales, Australia. Foods 2017, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, S. Seafood-borne parasitic diseases in Australia: How much do we know about them? Microbiol. Aust. 2016, 37, 27–29. [Google Scholar] [CrossRef] [Green Version]
- WTO. Marrakesh Agreement Establishing the World Trade Organisation; Opened for Signature 15 April 1994, 1867 UNTS 3 (Entered into Force 1 January 1995) Annex 1A (‘Agreement on Technical Barriers to Trade’); WTO: Geneva, Switzerland, 1995. [Google Scholar]
- World Organisation for Animal Health. The Aquatic Animal Health Code. Available online: http://www.oie.int/standard-setting/aquatic-code/access-online/ (accessed on 1 June 2018).


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, M.; Hernandez-Jover, M.; Shamsi, S. A Critical Appraisal of Global Testing Protocols for Zoonotic Parasites in Imported Seafood Applied to Seafood Safety in Australia. Foods 2020, 9, 448. https://doi.org/10.3390/foods9040448
Williams M, Hernandez-Jover M, Shamsi S. A Critical Appraisal of Global Testing Protocols for Zoonotic Parasites in Imported Seafood Applied to Seafood Safety in Australia. Foods. 2020; 9(4):448. https://doi.org/10.3390/foods9040448
Chicago/Turabian StyleWilliams, Michelle, Marta Hernandez-Jover, and Shokoofeh Shamsi. 2020. "A Critical Appraisal of Global Testing Protocols for Zoonotic Parasites in Imported Seafood Applied to Seafood Safety in Australia" Foods 9, no. 4: 448. https://doi.org/10.3390/foods9040448






