Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study
Abstract
:1. Introduction
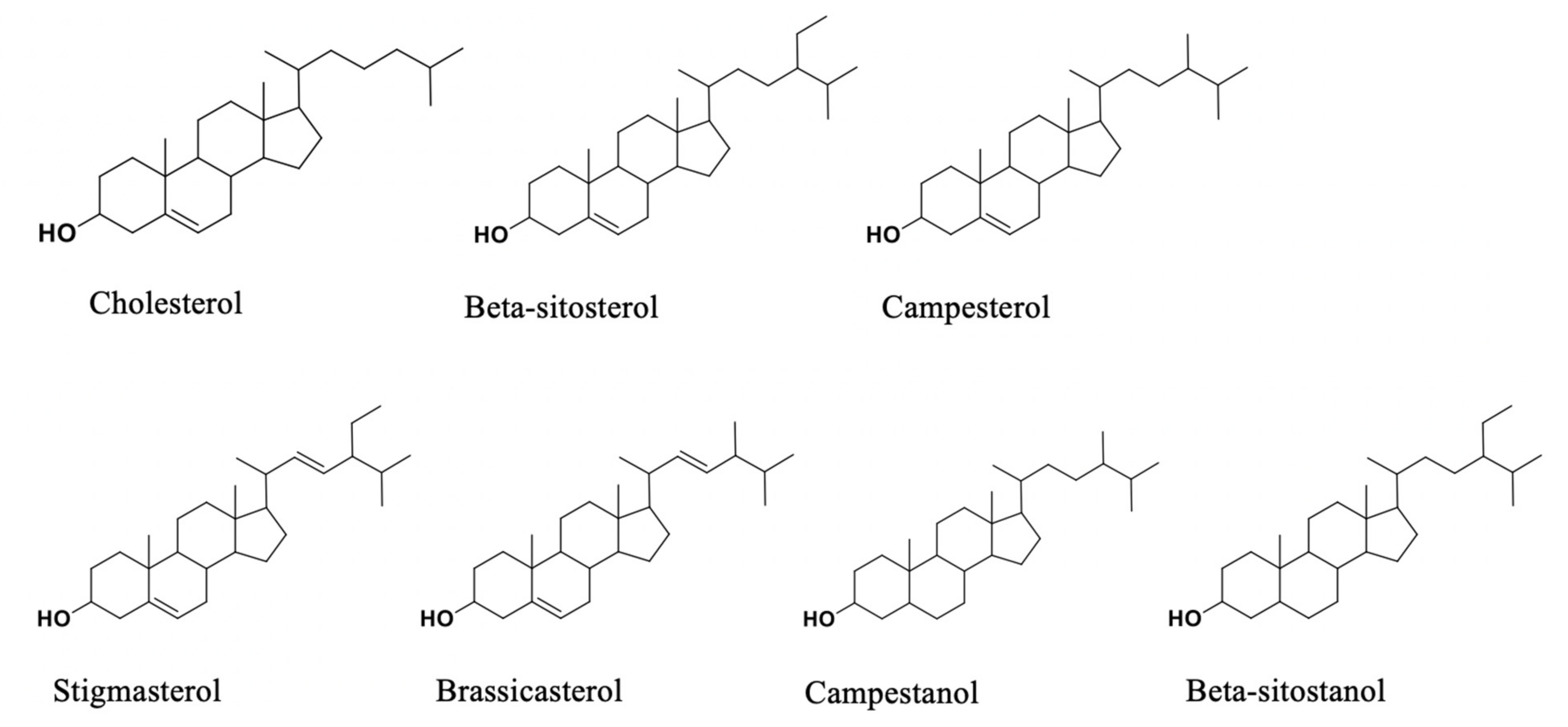
2. Effects and Action Mechanisms of Phytosterols
3. Phytosterols as Natural Source or Added in Foods
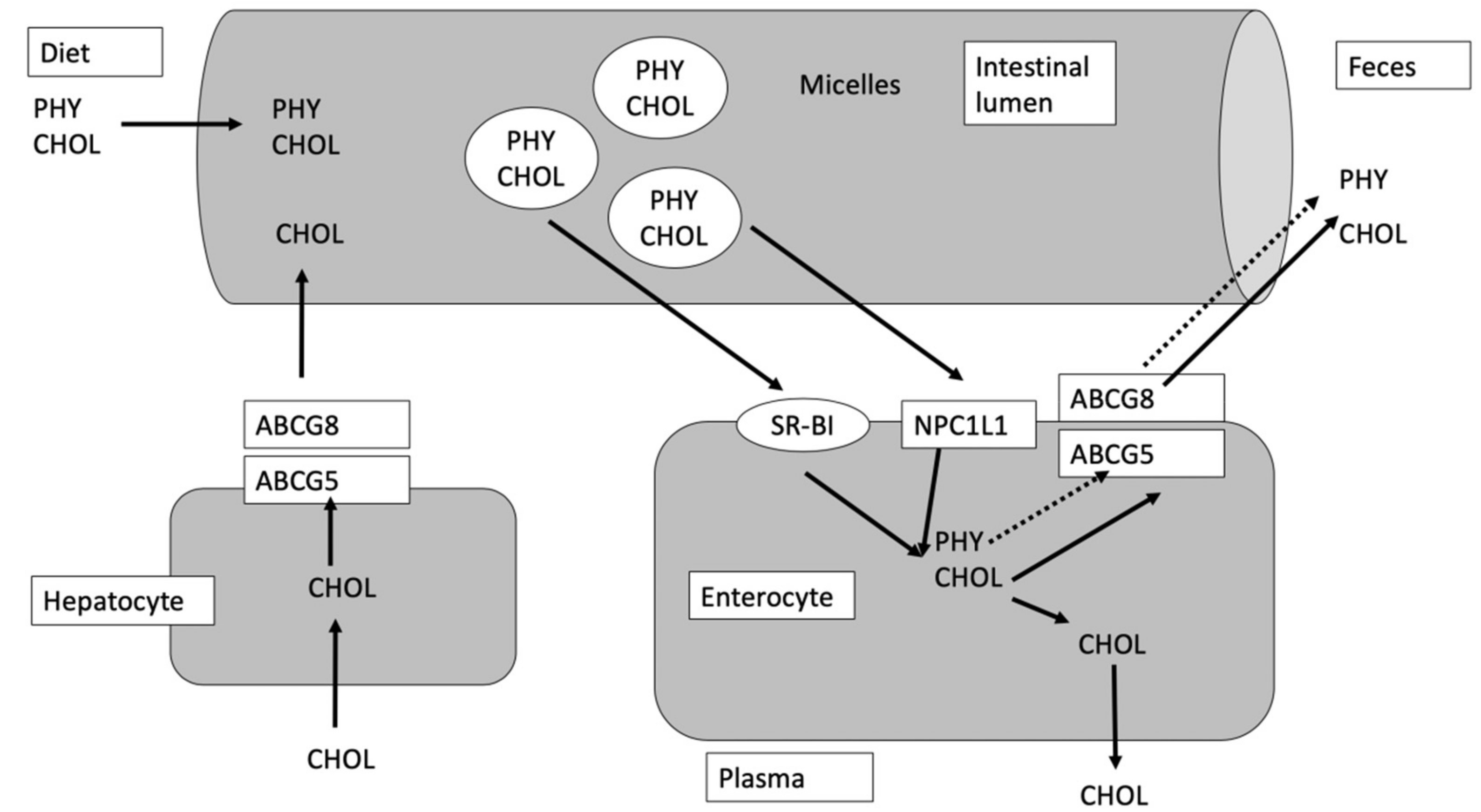
4. Stability of Phytosterols in Foods
5. Micro/Nanoencapsulation of Phytosterols
6. Use of Microencapsulated Phytosterols for Functional Foods’ Production
7. Legislation
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Merah, O.; Mouloungui, Z. Tetraploid wheats: Valuable source of phytosterols and phytostanols. Agronomy 2019, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, R.C.G.; Peralta, R.M.; Bracht, A.; Ferreira, I.C.F.R. The emerging use of mycosterols in food industry along with the current trend of extended use of bioactive phytosterols. Trends Food Sci. Technol. 2017, 67, 19–35. [Google Scholar] [CrossRef]
- Racette, S.B.; Lin, X.; Lefevre, M.; Spearie, C.A.; Most, M.M.; Ma, L.; Ostlund, R.E., Jr. Dose effects of dietary phytosterols on cholesterol metabolism: A controlled feeding study. Am. J. Clin. Nutr. 2009, 91, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolve, R.; Condelli, N.; Can, A.; Tchuenbou-Magaia, F.L. Development and characterization of phytosterol-enriched oil microcapsules for foodstuff application. Food Bioproc. Tech. 2018, 11, 152–163. [Google Scholar] [CrossRef]
- Jesch, E.D.; Carr, T.P. Food ingredients that inhibit cholesterol absorption. Prev. Nutr. Food Sci. 2017, 22, 67–80. [Google Scholar] [PubMed]
- Hasler, C.M. Functional foods: Their role in disease prevention and health promotion. Food Technol.-Champaign Chicago 1998, 52, 63–147. [Google Scholar]
- Dias, M.I.; Ferreira, I.C.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef] [Green Version]
- da Silva, B.V.; Barreira, J.C.; Oliveira, M.B.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Abbas, S.; Da Wei, C.; Hayat, K.; Xiaoming, Z. Ascorbic acid: Microencapsulation techniques and trends—A review. Food Rev. Int. 2012, 28, 343–374. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Timilsena, Y.P.; Wang, B.; Adhikari, R.; Adhikari, B. Advances in microencapsulation of polyunsaturated fatty acids (PUFAs)-rich plant oils using complex coacervation: A review. Food Hydrocoll. 2017, 69, 369–381. [Google Scholar] [CrossRef]
- Berger, A.; Jones, P.J.H.; Abumweis, S.S. Plant sterols: Factors affecting their efficacy and safety as functional food ingredients. Lipid Health Dis. 2004, 3, 5–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostlund, R.E., Jr. Phytosterols and cholesterol metabolism. Curr. Opin. Lipidol. 2004, 15, 37–41. [Google Scholar] [CrossRef] [PubMed]
- MacKay, D.S.; Jones, P.J.H. Phytosterols in human nutrition: Type, formulation, delivery, and physiological function. Eur. J. Lipid Sci. Tech. 2011, 113, 1427–1432. [Google Scholar] [CrossRef]
- de Jong, A.; Plat, J.; Mensink, R.P. Metabolic effects of plant sterols and stanols. J. Nutr. Biochem. 2003, 14, 362–369. [Google Scholar] [CrossRef]
- Izar, M.C.; Tegani, D.M.; Kasmas, S.H.; Fonseca, F.A. Phytosterols and phytosterolemia: Gene–diet interactions. Genes Nutr. 2011, 6, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Bain, B.J.; Chakravorty, S. Phytosterolemia. Am. J. Hematol. 2016, 91, 643. [Google Scholar] [CrossRef] [Green Version]
- Tzavella, E.; Hatzimichael, E.; Kostara, C.; Bairaktari, E.; Elisaf, M.; Tsimihodimos, V. Sitosterolemia: A multifaceted metabolic disorder with important clinical consequences. J. Clin. Lipidol. 2017, 11, 1095–1100. [Google Scholar] [CrossRef]
- Vergès, B.; Fumeron, F. Potential risks associated with increased plasma plant-sterol levels. Diabetes Metab. 2015, 41, 76–81. [Google Scholar] [CrossRef]
- Fardet, A.; Morise, A.; Kalonji, E.; Margaritis, I.; Mariotti, F. Influence of phytosterol and phytostanol food supplementation on plasma liposoluble vitamins and provitamin A carotenoid levels in humans: An updated review of the evidence. Crit. Rev. Food Sci. 2017, 57, 1906–1921. [Google Scholar] [CrossRef]
- Penchala Raju, M.; Aravind Babu, D.G.; Rakesh Kumar, B.; Rajashekar, C.H. The role of phytosterols enriched foods-A review. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 7, 40–47. [Google Scholar] [CrossRef]
- Sanclemente, T.; Marques-Lopes, I.; Fajó-Pascual, M.; Cofán, M.; Jarauta, E.; Ros, E.; Puzo, J.; García-Otín, A.L. Naturally-occurring phytosterols in the usual diet influence cholesterol metabolism in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A. Composition of plant sterols and stanols in supplemented food products. J. AOAC Int. 2015, 98, 685–690. [Google Scholar] [CrossRef]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.; Duchateau, G.S.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. Continuous dose-response relationship of the LDL-cholesterol–lowering effect of phytosterol intake. J. Nutr. 2009, 139, 271–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, P.C. Occurrence and levels of phytosterols in food. In Phytosterols as Functional Food Components and Nutraceuticals; Dutta, P.C., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 1–27. [Google Scholar]
- Vaghini, A.S.; Cilla, A.; Garcia-Llatas, G.; Lagarda, M.J. Bioaccessibility study of plant sterol-enriched fermented milks. Food Funct. 2016, 7, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Sala, A.; Garcia-Llatas, G.; Cilla, A.; Barberá, R.; Sánchez-Siles, L.M.; Lagarda, M.J. Impact of lipid components and emulsifiers on plant sterols bioaccessibility from milk-based fruit beverages. J. Agric. Food Chem. 2016, 64, 5686–5691. [Google Scholar] [CrossRef] [PubMed]
- Izadi, Z.; Nasirpour, A.; Garoosi, G.A.; Tamjidi, F. Rheological and physical properties of yogurt enriched with phytosterol during storage. J. Food Sci. Technol. 2014, 52, 5341–5346. [Google Scholar] [CrossRef] [Green Version]
- Duong, S.; Strobel, N.; Buddhadasa, S.; Stockham, K.; Auldist, M.; Wales, B.; Orbell, J.; Cran, M. Rapid measurement of phytosterols in fortified food using gas chromatography with flame ionization detection. Food Chem. 2016, 211, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Grasso, S.; Brunton, N.P.; Monahan, F.J.; Harrison, S.M. Development of a method for the analysis of sterols in sterol-enriched deli-style turkey with GC-FID. Food Anal. Methods 2016, 9, 724–728. [Google Scholar] [CrossRef]
- Menéndez-Carreño, M.; Knol, D.; Janssen, H.G. Development and validation of methodologies for the quantification of phytosterols and phytosterol oxidation products in cooked and baked food products. J. Chromatogr. A 2016, 1428, 316–325. [Google Scholar] [CrossRef]
- Lagarda, M.J.; García-Llatas, G.; Farré, R. Analysis of phytosterols in foods. J. Pharmaceut. Biomed. 2006, 41, 1486–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Larena, M.; Garcia-Llatas, G.; Clemente, G.; Barberá, R.; Lagarda, M.J. Plant sterol oxides in functional beverages: Influence of matrix and storage. Food Chem. 2015, 173, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Guth, S.; Engel, K.H.; Steinberg, P. Phytosterol oxidation products in enriched foods: Occurrence, exposure, and biological effects. Mol. Nutr. Food Res. 2015, 59, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Vanmierlo, T.; Husche, C.; Schött, H.F.; Pettersson, H.; Lütjohann, D. Plant sterol oxidation products—Analogs to cholesterol oxidation products from plant origin? Biochimie 2013, 95, 464–472. [Google Scholar] [CrossRef]
- Lin, Y.; Knol, D.; Trautwein, E.A. Phytosterol oxidation products (POP) in foods with added phytosterols and estimation of their daily intake: A literature review. Eur. J. Lipid Sci. Tech. 2016, 118, 1423–1438. [Google Scholar] [CrossRef] [Green Version]
- González-Larena, M.; Cilla, A.; García-Llatas, G.; Barberá, R.; Lagarda, M.J. Plant sterols and antioxidant parameters in enriched beverages: Storage stability. J. Agric. Food Chem. 2012, 60, 4725–4734. [Google Scholar] [CrossRef]
- Botelho, P.B.; Galasso, M.; Dias, V.; Mandrioli, M.; Lobato, L.P.; Rodriguez-Estrada, M.T.; Castro, I.A. Oxidative stability of functional phytosterol-enriched dark chocolate. LWT- Food Sci. Technol. 2014, 55, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Rudzińska, M.; Przybylski, R.; Wąsowicz, E. Degradation of phytosterols during storage of enriched margarines. Food Chem. 2014, 142, 294–298. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Cardenia, V.; Mandrioli, M.; Muste, S.; Borsari, A.; Rodriguez-Estrada, M.T. Stability of flavoured phytosterol-enriched drinking yogurts during storage as affected by different packaging materials. J. Sci. Food Agric. 2016, 96, 2782–2787. [Google Scholar] [CrossRef]
- Tolve, R.; Galgano, F.; Caruso, M.C.; Tchuenbou-Magaia, F.L.; Condelli, N.; Favati, F.; Zhang, Z. Encapsulation of health-promoting ingredients: Applications in foodstuffs. Int. J. Food Sci. Nutr. 2016, 67, 888–918. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Chen, Q.; McGillivray, D.; Wen, J.; Zhong, F.; Quek, S.Y. Co-encapsulation of fish oil with phytosterol esters and limonene by milk proteins. J. Food Eng. 2013, 117, 505–512. [Google Scholar] [CrossRef]
- Chen, Q.; Zhong, F.; Wen, J.; McGillivray, D.; Quek, S.Y. Properties and stability of spray-dried and freeze-dried microcapsules co-encapsulated with fish oil, phytosterol esters, and limonene. Dry. Technol. 2013, 31, 707–716. [Google Scholar] [CrossRef]
- Chan, Y.H.; Chen, B.H.; Chiu, C.P.; Lu, Y.F. The influence of phytosterols on the encapsulation efficiency of cholesterol liposomes. Int. J. Food Sci. Tech. 2004, 39, 985–995. [Google Scholar] [CrossRef]
- Alexander, M.; Lopez, A.A.; Fang, Y.; Corredig, M. Incorporation of phytosterols in soy phospholipids nanoliposomes: Encapsulation efficiency and stability. LWT- Food Sci. Technol. 2012, 47, 427–436. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Leong, W.F.; Lai, O.M.; Long, K.; Che Man, Y.B.; Misran, M.; Tan, C.P. Preparation and characterisation of water-soluble phytosterol nanodispersions. Food Chem. 2011, 129, 77–83. [Google Scholar] [CrossRef]
- Auweter, H.; Bohn, H.; Hasselwander, O.; Runge, F. Process for Producing Pulvurulent Phytosterols Formulations. U.S. Patent 2009/0047355 A1, 19 February 2009. [Google Scholar]
- Choi, M.J.; Briançon, S.; Bazile, D.; Royere, A.; Min, S.G.; Fessi, H. Effect of cryoprotectant and freeze-drying process on the stability of W/O/W emulsions. Dry. Technol. 2007, 25, 809–819. [Google Scholar] [CrossRef]
- Alvim, I.D.; Souza, F.D.S.D.; Koury, I.P.; Jurt, T.; Dantas, F.B.H. Use of the spray chilling method to deliver hydrophobic components: Physical characterization of microparticles. LWT- Food Sci. Technol. 2013, 33, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Ling, H.W.; Lin, N.K. In Vitro release study of freeze-dried and oven-dried microencapsulated kenaf seed oil. Malays. J. Nutr. 2017, 23, 139–149. [Google Scholar]
- Lim, W.T.; Nyam, K.L. Characteristics and controlled release behaviour of microencapsulated kenaf seed oil during in-vitro digestion. J. Food Eng. 2016, 182, 26–32. [Google Scholar] [CrossRef]
- Khalid, N.; Kobayashi, I.; Neves, M.A.; Uemura, K.; Nakajima, M.; Nabetani, H. Encapsulation of β-sitosterol plus γ-oryzanol in O/W emulsions: Formulation characteristics and stability evaluation with microchannel emulsification. Food Bioprod. Process. 2017, 102, 222–232. [Google Scholar] [CrossRef]
- Comunian, T.A.; Nogueira, M.; Scolaro, B.; Thomazini, M.; Ferro-Furtado, R.; de Castro, I.A.; Favaro-Trindade, C.S. Enhancing stability of echium seed oil and beta-sitosterol by their coencapsulation by complex coacervation using different combinations of wall materials and crosslinkers. Food Chem. 2018, 252, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.K.; Jessie, L.Y.L.; Tan, C.P.; Long, K.; Nyam, K.L. Effect of accelerated storage on microencapsulated kenaf seed oil. J. Am. Oil Chem. Soc. 2013, 90, 1023–1029. [Google Scholar] [CrossRef]
- Di Battista, C.A.; Constenla, D.; Ramiréz-Rigo, M.V.; Piña, J. The use of arabic gum, maltodextrin and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technol. 2015, 286, 193–201. [Google Scholar] [CrossRef]
- Winkler, J.K.; Warner, K. The effect of phytosterol concentration on oxidative stability and thermal polymerization of heated oils. Eur. J. Lipid Sci. Technol. 2008, 110, 455–464. [Google Scholar] [CrossRef]
- Tolve, R.; Condelli, N.; Caruso, M.C.; Genovese, F.; Di Renzo, G.C.; Mauriello, G.; Galgano, F. Preparation and characterization of microencapsulated phytosterols for the formulation of functional foods: Scale up from laboratory to semi-technical production. Food Res. Int. 2019, 116, 1274–1281. [Google Scholar] [CrossRef]
- Bagherpour, S.; Alizadeh, A.; Ghanbarzadeh, S.; Mohammadi, M.; Hamishehkar, H. Preparation and characterization of betasitosterol-loaded nanostructured lipid carriers for butter enrichment. Food Biosci. 2017, 20, 51–55. [Google Scholar] [CrossRef]
- Comunian, T.A.; Chaves, I.E.; Thomazini, M.; Moraes, I.C.F.; Ferro-Furtado, R.; de Castro, I.A.; Favaro-Trindade, C.S. Development of functional yogurt containing free and encapsulated echium oil, phytosterol and sinapic acid. Food Chem. 2017, 237, 948–956. [Google Scholar] [CrossRef]
- Tolve, R.; Condelli, N.; Caruso, M.C.; Barletta, D.; Favati, F.; Galgano, F. Fortification of dark chocolate with microencapsulated phytosterols: Chemical and sensory evaluation. Food Funct. 2018, 9, 1265–1273. [Google Scholar] [CrossRef]
- Regulation No 258/97 of the European Parliament and of the Council of 27 January 1997. O J E U 1997, L43, 14.2.
- Commission Regulation No 608/2004/EC of 31 March 2004. O J E U 2004, L15, 288.
- Commission Regulation No 2013/718/EU of 25 July 2013. O J E U 2013, L201, 49.
- Commission Decision No 2004/336/EC of 31 March 2004. O J E U 2004, L105, 49.
- FSANZ, Food Standards Australia New Zealand. Food Standards Code. Commonwealth of Australia Gazette No. FSC 31; Food Standards Australia New Zealand: Canberra, Australia, 2006. [Google Scholar]
- Health Canada. Notice of Assessment of Certain Categories of Foods Containing Added Phytosterols; Bureau of Nutritional Sciences Food Directorate, Health Products and Food Branch Health Canada: Montreal, QC, Canada, 2010. [Google Scholar]
- FOSHU (Food for Specified Health Uses) Japanese Ministry of Health, Labour and Welfare. 2006. Available online: http://www.mhlw.go.jp/english/topics/foodsafety/fhc/02.html (accessed on 24 February 2018).
- Raisio, PLC. Approval by the Chinese Ministry of Health Administration for Benecol Ingredient. 2008. Available online: https://www.globenewswire.com/news-release/2008/12/04/24962/0/en/APPROVAL-BY-THE-CHINESE-MINISTRY-OF-HEALTH-FOR-BENECOL-INGREDIENT.html (accessed on 7 December 2014).
- Kim, J.Y.; Dai, B.K.; Hyong, J.L. Regulations on Health/functional Foods in Korea; Nutrition and Functional Food Headquarters, Korea Food and Drug Administration: Seoul, Korea, 2005. [Google Scholar]
- Zawistowski, J.; Jones, P. Regulatory aspects related to plant sterol and stanol supplemented foods. J. AOAC Int. 2015, 98, 750–756. [Google Scholar] [CrossRef] [PubMed]
| Phytosterol Food Sources | Phytosterols Content (mg/kg of Fresh Weight) | Reference |
|---|---|---|
| Oils | ||
| Corn | 7150–9520 | [23] |
| Olive | 1140–1150 | [25] |
| Palm | 490–610 | [25] |
| Peanut | 1670–2290 | [25] |
| Rice bran | 10,550 | [23] |
| Soybean | 2210–3280 | [23] |
| Sunflower | 2030–3280 | [25] |
| Vegetables | ||
| Broccoli | 367–390 | [25] |
| Carrot | 153–160 | [25] |
| Cauliflower | 310–400 | [25] |
| Onion | 84–93 | [25] |
| Potato | 38–73 | [25] |
| Tomato | 47–148 | [25] |
| Fruits | ||
| Apple | 130–183 | [25] |
| Banana | 116–161 | [25] |
| Grapes | 40–200 | [25] |
| Orange | 228–240 | [25] |
| Nuts | ||
| Almond | 1380–1430 | [25] |
| Peanuts | 600–1608 | [25] |
| Cereals | ||
| Barley | 720–801 | [25] |
| Buckwheat | 963–1980 | [25] |
| Corn | 662–1205 | [25] |
| Oats | 350–491 | [25] |
| Rye | 707–1134 | [25] |
| Wheat | 447–830 | [25] |
| Core Material | Shell Material | Encapsulation Technique | Food Inclusion | Principal Outcomes | Reference |
|---|---|---|---|---|---|
| Fish oil, phytosterol esters and limonene | Whey protein isolate and sodium caseinate | Spray drying | N.R. | Higher protection from oxidation than non-encapsulated phytosterols | [43] |
| Phytosterols mixture | Lipid mixture of low trans hydrogenated vegetable fats and stearic acid | Spray chilling | N.R. | Good quality microcapsules with a mean diameter varied between 13.8 and 32.2 μm | [51] |
| Kenaf seed oil containing phytosterols | Alginate with high methoxy pectin and chitosan | Oven-dried | N.R. | Increase in phytosterols bioavailability evaluated through in vitro release | [52] |
| Kenaf seed oil containing phytosterols | Carboxymethyl-cellulose, maltodextrin and soy lecithin | Spray drying | N.R. | Increase in phytosterols bioavailability evaluated through in vitro release | [53] |
| Beta-sitosterol and γ-oryzanol | Medium chain triglycerice oil | O/W microchannel emulsification | N.R. | Phytosterols retention ranged from 50 to 80%, according to the use of tween 20 or decaglycerol monolaurate as surfactant agent, when stored for 30 days at 4 and 25 °C | [54] |
| Beta-sitosterol and echium oil | Arabic gum, cashew gum | Complex coacervation | N.R. | Phytosterols retention ranged from 70.74 to 73.78% depending upon the absence or the use of sinapic acid as crosslinking when stored for 30 days at 37 °C | [55] |
| Kenaf seed oil containing phytosterols | Sodium caseinate and of maltodextrin | Spray drying | N.R. | Phytosterols concentration was stable when microcapsules were stored at 65 °C for 24 days | [56] |
| Phytosterol mixture | Arabic gum, maltodextrin | Spray drying | N.R. | The microcapsules particle size was lower than 25 μm, which is required to ensure the phytosterols inclusion in the intestinal micellar phase | [57] |
| Phytosterol mixture | Whey protein isolate, inulin and chitosan | O/W emulsion + spray drying | N.R. | Unexpected, the peroxide values of the obtained microcapsules were relatively high even just after the production | [4] |
| Phytosterol mixture | Whey protein isolate, inulin and chitosan | Spray drying | N.R. | Possibility to scale up the production of microcapsules without affect their features using a laboratory dryer or a spray dryer for semi- technical production | [59] |
| Beta-sitosterol | Lipid mixture of Precirol and Miglyol | Hot melt homogenization method | Butter | Beta-sitosterol loaded lipid nanocarriers, showed good stability during three months’ storage period Moreover, the use of this technique does not alter the texture and the organoleptic characteristics of the product | [60] |
| Echium oil and beta-sitosterol | Arabic gum and gelatin | Complex coacervation | Yogurt | Yogurt containing microcapsules did not show a significant difference in terms of physicochemical, rheological and sensorial properties with respect to control | [61] |
| Phytosterols mixture | Whey protein isolate | Spray drying | Dark chocolates | No matter the microencapsulated phytosterols concentration, fortified dark chocolate was widely accepted by consumers | [62] |
| Country | Current Legislation | Health Claim |
|---|---|---|
| European Union (EU) | Novel Food Regulations (EC 258/97) | “Plant sterols (stanols) have been shown to lower/reduce blood cholesterols. High cholesterol is a risk factor in the development of coronary disease” “Plant sterols/stanols contribute to the maintenance of normal blood cholesterol levels” |
| United States of America | GRAS notification and self-GRAS regulation; Dietary Supplement Health and Education ACT (DSHEA) | “Helps maintain normal cholesterol levels”; “May reduce the risk of heart disease” |
| Australia and New Zealand | Novel Food Standard; Food Standards Australia New Zealand (FSANZ) | “Reduces blood cholesterol” |
| Canada | Part B, Division 28 (Novel Foods) of the Food and Drug Regulations | “Plant sterols help reduce/lower cholesterol. High cholesterol is a risk factor for heart disease” |
| Japan | Food for Specified Health Uses (FOSHU) | “Good for those concerned about serum cholesterol” “Good for those having relatively high serum cholesterol and triglycerides with mild obesity” |
| China | State Food and Drug Administration (SFDA) | “This product is not a substitute for medicine” |
| Taiwan | Health Food Control Act | “Regulating blood lipids”; “An animal study shows that consumption of this product may help lower blood total cholesterol” |
| South Korea | Korea Health Functional Food Act (HFFA) by Korean Food and Drug Agency (KFDA) | “Phytosterols may reduce the risk of coronary heart disease” |
| Malaysia | Food Safety and Quality Division under Malaysian Regulations of the Food Act | “Helps lower or reduce cholesterol” |
| Indonesia | Indonesian National Agency for Drug and Food Control (NADFC) | “May reduce the risk of coronary heart disease” |
| Thailand | Thai Food and Drug Administration | “May help lower cholesterol” |
| Philippines | Philippine Food Fortification Act | “This product contains plant sterols that help lower cholesterol” |
| Singapore | Implemented by Agri-Food and Veterinary Authority with the Health Promotion Board | “Plant sterols/stanols have been shown to lower/reduce blood cholesterol. High blood cholesterol is a risk factor in the development of coronary heart disease”; “Intended exclusively for people who want to lower their blood cholesterol level” |
| Brazil | National Health Surveillance Agency | “Helps to maintain healthy level of cholesterol when associated with a healthy diet and life style” |
| Mexico | Mexican General Health Law | “Proven to reduce cholesterol” |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolve, R.; Cela, N.; Condelli, N.; Di Cairano, M.; Caruso, M.C.; Galgano, F. Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study. Foods 2020, 9, 470. https://doi.org/10.3390/foods9040470
Tolve R, Cela N, Condelli N, Di Cairano M, Caruso MC, Galgano F. Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study. Foods. 2020; 9(4):470. https://doi.org/10.3390/foods9040470
Chicago/Turabian StyleTolve, Roberta, Nazarena Cela, Nicola Condelli, Maria Di Cairano, Marisa C. Caruso, and Fernanda Galgano. 2020. "Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study" Foods 9, no. 4: 470. https://doi.org/10.3390/foods9040470
APA StyleTolve, R., Cela, N., Condelli, N., Di Cairano, M., Caruso, M. C., & Galgano, F. (2020). Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study. Foods, 9(4), 470. https://doi.org/10.3390/foods9040470








