Comparison of Various Soybean Allergen Levels in Genetically and Non-Genetically Modified Soybeans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Soybean Sample Extraction
2.3. Immunochromatography
2.4. Electrophoresis and Immunoblotting
2.5. Antibodies Against the Major Soybean Allergens
2.6. ELISA Using Allergen-Specific Antibodies
2.7. Detection of IgE-Binding Proteins using Patient Sera
2.8. Statistical Analysis
3. Results
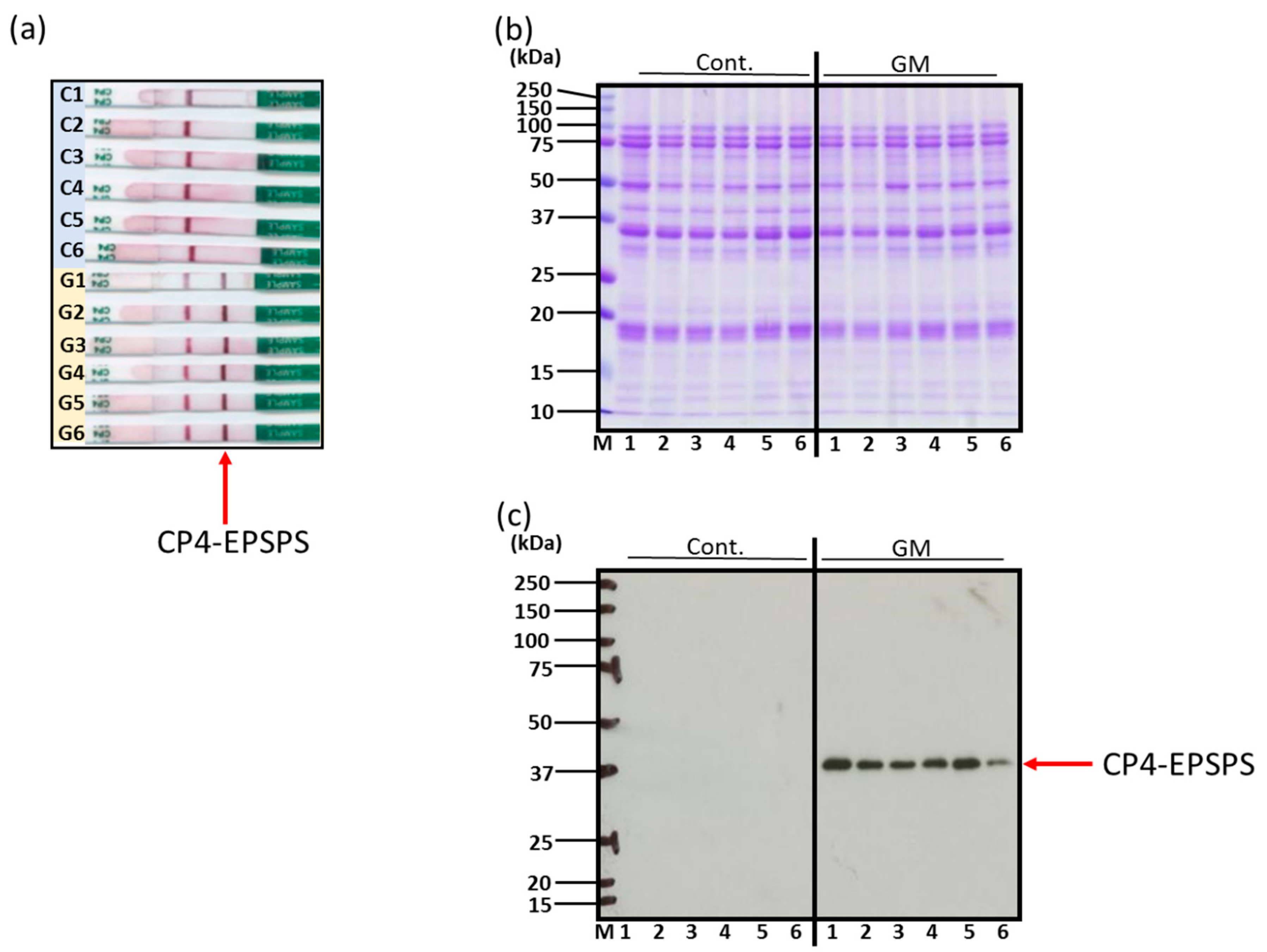
3.1. Confirmation of Genetically Modified and Non-Genetically Modified Soybeans
3.2. Comparative Levels of Pollinosis-Related Soybean Allergens (Gly m 4 and Gly m 3) in GM-Soybean and Non-GM Soybean Extracts
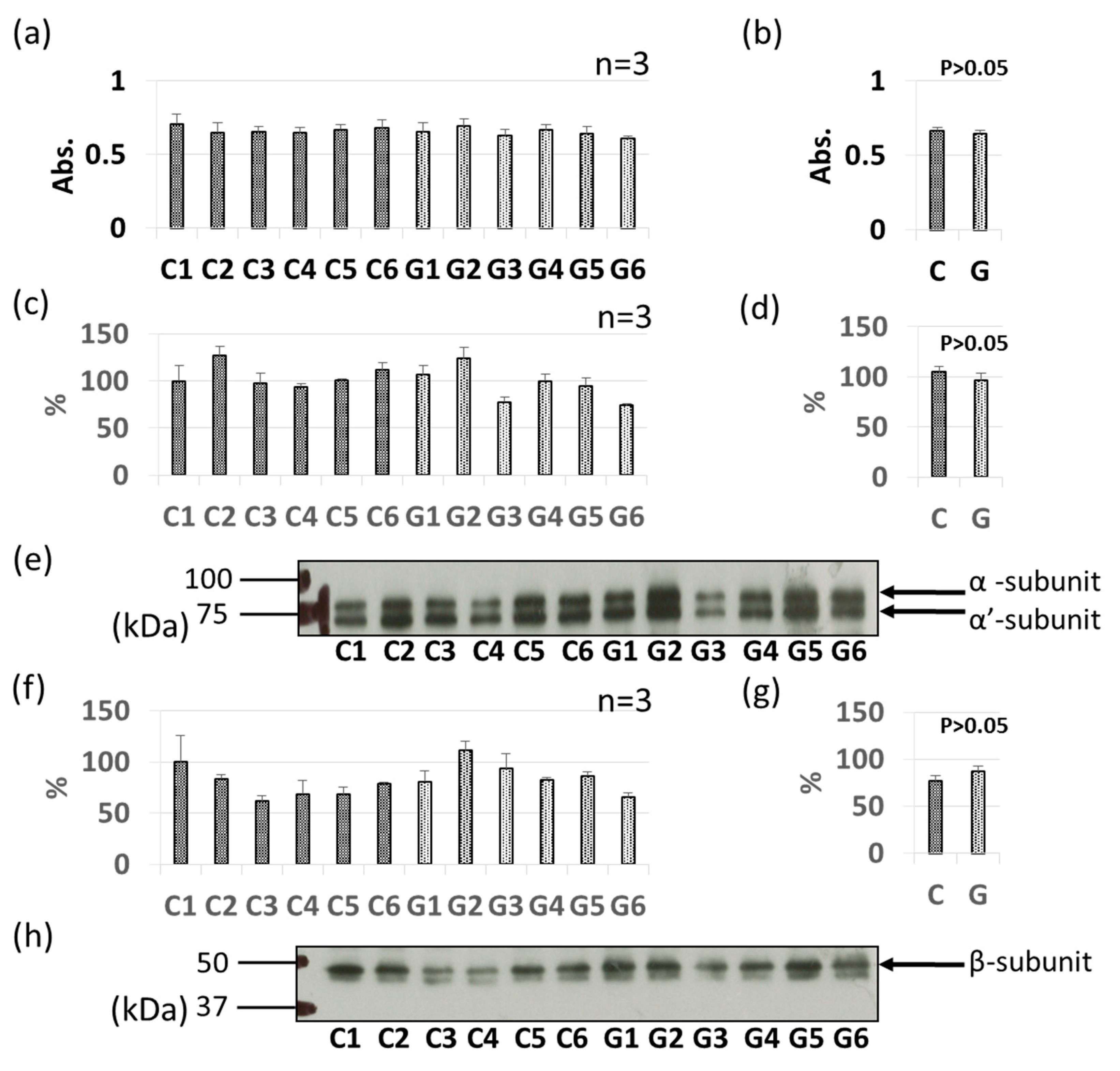
3.3. Comparative Levels of Other Soybean Allergens in GM-Soybean and non-GM Soybean
3.4. IgE-ELISA and IgE-Immunoblotting using Patient Serum
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chrispeels, M.J. Global production and consumption of genetically engineered crops. J. Huazhong Agric. Univ. 2014, 4, 120–132. [Google Scholar]
- Lua, M.; Jina, Y.; Weber, B.B.; Goodman, R.E. A comparative study of human IgE binding to proteins of a genetically modified (GM) soybean and six non-GM soybeans grown in multiple locations. Food Chem. Toxicol. 2018, 112, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ichim, M.C. The Romanian experience and perspective on the commercial cultivation of genetically modified crops in Europe. Transgenic Res. 2019, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Al-Busaidi, K.T.; Trivedi, M.; Tiwari, R.K. Status of research, regulations and challenges for genetically modified crops in India. GM Crops Food 2018, 9, 173–188. [Google Scholar] [CrossRef] [PubMed]
- ISAAA. Global Status of Commercialized Biotech/GM Crops: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years; ISAAA: Ithaca, NY, USA, 2017; pp. 100–104. ISBN 978-1-892456-67-2. [Google Scholar]
- Ogawa, T.; Samoto, M.; Takahashi, K. Soybean allergens and hypoallergenic soybean products. J. Nutr. Sci. Vitaminol. 2000, 46, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Yagami, T. Allergies to Cross-Reactive Plant Proteins. Latex-fruit syndrome is comparable with pollen-food allergy syndrome. Int. Arch. Allergy Immunol. 2002, 128, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Amlot, P.L.; Kemeny, D.M.; Zachary, C.; Parkes, P.; Lessof, M.H. Oral allergy syndrome (OAS): Symptoms of IgE-mediated hypersensitivity to foods. Clin. Allergy 1987, 17, 33–42. [Google Scholar] [CrossRef]
- Ogawa, T.; Bando, N.; Tsuji, H.; Nishikawa, K.; Kitamura, K. α-Subunit of β-Conglycinin, an allergenic protein recognized by IgE Antibodies of soybean-sensitive patients with atopic dermatitis. Biosci. Biotechnol. Biochem. 1995, 59, 831–833. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, N.; Sato, S.; Cabanos, C.; Tanaka, A.; Ito, K.; Ebisawa, M. Gly m 5/Gly m 8 fusion component as a potential novel candidate molecule for diagnosing soya bean allergy in Japanese children. Clin. Exp. Allergy 2018, 48, 1726–1734. [Google Scholar] [CrossRef]
- Holzhauser, T.; Wackermann, O.; Ballmer-Weber, B.K.; Bindslev-Jensen, C.; Scibilia, J.; Perono-Garoffo, L.; Utsumi, S.; Poulsen, L.K.; Vieths, S. Soybean (Glycine max) allergy in Europe: Gly m 5 (β-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. J. Allergy Clin. Immunol. 2009, 123, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Riascos, J.J.; Weissinger, S.M.; Weissinger, A.K.; Kulis, M.; Burks, A.W.; Pons, L. The Seed Biotinylated Protein of Soybean (Glycine max): A BoilingResistant New Allergen (Gly m 7) with the Capacity to Induce IgE Mediated Allergic Responses. J. Agric. Food Chem. 2016, 64, 3890–3900. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Tsuji, H.; Bando, N.; Kitamura, K.; Zhu, Y.L.; Hirano, H.; Nishikawa, K. Identification of the soybean allergenic protein, Gly m Bd 30K, with the soybean seed 34-kDa oil-body-associated protein. Biosci. Biotechnol. Biochem. 1993, 57, 1030–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, T.; Bando, N.; Tsuji, H.; Okajima, H.; Nishikawa, K.; Sasaoka, K. Investigation of the IgE-binding protein in soybeans by immunoblotting with the sera of the soybean-sensitive patient with atopic dermatitis. J. Nutr. Sci. Vitaminol. 1991, 37, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroz, L.A.; Yang, W.H. Kunitz Soybean Trypsin Inhibitor—A Specific Allergen in Food Anaphylaxis. N. Engl. J. Med. 1980, 302, 1126–1128. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, L.; Ying, Y.; Kong, X.; Hua, Y.; Chen, Y. The characterization of soybean oil body integral oleosin isoforms and the effects of alkaline pH on them. Food Chem. 2015, 177, 288–294. [Google Scholar] [CrossRef]
- Rihs, H.P.; Chen, Z.; Ruëff, F.; Petersen, A.; Rozynek, P.; Heimann, H.; Baur, X. IgE binding of the recombinant allergen soybean profilin (rGly m 3) is mediated by conformational epitopes. J. Allergy Clin. Immunol. 1999, 104, 1293–1301. [Google Scholar] [CrossRef]
- Yagami, A.; Inaba, Y.; Kuno, Y.; Suzuki, K.; Tanaka, A.; Sjolander, S.; Saito, H.; Matsunaga, K. Two cases of pollen-food allergy syndrome to soy milk diagnosed by skin prick test, specific serum immunoglobulin E and microarray analysis. J. Dermatol. 2009, 36, 50–55. [Google Scholar] [CrossRef]
- Kleine-Tabbe, J.; Vogel, L.; Crowell, D.N.; Haustein, U.F.; Vieths, S. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1- related PR-10 protein in soybean, SAM22. J. Allergy Clin. Immunol. 2002, 110, 797–804. [Google Scholar] [CrossRef]
- Mittag, D.; Vieths, S.; Vogel, L.; Becker, W.M.; Rihs, H.P.; Helbling, A.; Wüthrich, B.; Ballmer-Weber, B.K. Soybean allergy in patients allergic to birch pollen: Clinical investigation and molecular characterization of allergens. J. Allergy Clin. Immunol. 2004, 113, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Berkner, H.; Neudecker, P.; Mittag, D.; Ballmer-Weber, B.K.; Schweimer, K.; Vieths, S.; Rösch, P. Cross-reactivity of pollen and food allergens: Soybean Gly m 4 is a member of the Bet v 1 superfamily and closely resembles yellow lupine proteins. Biosci. Rep. 2009, 29, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Kim, H.M.; Ye, Y.M.; Kim, S.H.; Nahm, D.H.; Park, H.S.; Ryu, S.R.; Lee, B.O. Evaluating the Allergic Risk of Genetically Modified Soybean. Yonsei Med. J. 2006, 47, 505–512. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; Zhou, X.; Zhang, Y.; Huang, M.; He, J.; Shen, W. Targeting the middle region of CP4-EPSPS protein for its traceability in highly processed soy-related products. J. Food Sci. Technol. 2017, 54, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Funke, T.; Healy-Fried, M.L.; Han, H.; Alberg, D.G.; Bartlett, P.A.; Schönbrunn, E. Differential Inhibition of Class I and Class II 5-Enolpyruvylshikimate-3-phosphate Synthases by Tetrahedral Reaction Intermediate Analogues. Biochemistry 2007, 46, 13344–13351. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.J.; Chang, C.Y.; Liao, E.C. Comparison of Allergenicity at Gly m 4 and Gly m Bd 30K of Soybean after Genetic Modification. J. Agric. Food Chem. 2017, 65, 1255–1262. [Google Scholar] [CrossRef]
- Selb, R.; Wal, J.M.; Moreno, F.J.; Lovik, M.; Mills, C.; Hoffmann-Sommergruber, K.; Fernandez, A. Assessment of endogenous allergenicity of genetically modified plants exemplified by soybean—Where do we stand? Food Chem. Toxicol. 2017, 101, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Goodman, R.E.; Panda, R.; Ariyarathna, H. Evaluation of Endogenous Allergens for the Safety Evaluation of Genetically Engineered Food Crops: Review of Potential Risks, Test Methods, Examples and Relevance. J. Agric. Food Chem. 2013, 61, 8317–8332. [Google Scholar] [CrossRef]
- Graf, L.; Hayder, H.; Mueller, U. Endogenous allergens in the regulatory assessment of genetically engineered crops. Food Chem. Toxicol. 2014, 73, 17–20. [Google Scholar] [CrossRef]
- Fernandez, A.; Mills, E.N.; Lovik, M.; Spoek, A.; Germini, A.; Mikalsen, A.; Wal, J.M. Endogenous allergens and compositional analysis in the allergenicity assessment of genetically modified plants. Food Chem. Toxicol. 2013, 62, 1–6. [Google Scholar] [CrossRef]
- Panda, R.; Ariyarathna, H.; Amnuaycheewa, P.; Tetteh, A.; Pramod, S.N.; Taylor, S.L.; Ballmer-Weber, B.K.; Goodman, R.E. Challenges in testing genetically modified crops for potential increases in endogenous allergen expression for safety. Allergy 2013, 68, 142–151. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Genetically Modified Organisms (GMO). Guidance on allergenicity assessment of genetically modified plants. EFSA J. 2017, 15, e04862. [Google Scholar] [CrossRef] [Green Version]
- Kyhse-Andersen, J. Electroblotting of multiple gels: A simple apparatus without buffer tank for rapid transfer of proteins from polycrylamide to nitrocellulose. J. Biochem. Biophys. Methods 1984, 10, 203–209. [Google Scholar] [CrossRef]
- Hei, W.; Li, Z.; Ma, X.; He, P. Determination of beta-conglycinin in soybean and soybean products using a sandwich enzyme-linked immunosorbent assay. Anal. Chim. Acta 2012, 734, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Hanafusa, K.; Murakami, H.; Ueda, T.; Yano, E.; Zaima, N.; Moriyama, T. Worm wounding increases levels of pollen-related food allergens in soybean (Glycine max). Biosci. Biotechnol. Biochem. 2018, 82, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Kim, W.S.; Jang, S.; Kerley, M.S. All three subunits of soybean beta-conglycinin are potential food allergens. J. Agric. Food Chem. 2009, 57, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Horikawa, T.; Shimizu, H.; Sarayama, Y.; Ogawa, T.; Sjolander, S.; Tanaka, A.; Moriyama, T. Soybean beta-conglycinin as the main allergen in a patient with food-dependent exercise-induced anaphylaxis by tofu: Food processing alters pepsin resistance. J. Clin. Exp. Allergy 2009, 39, 167–173. [Google Scholar] [CrossRef]
- Natarajan, S.; Khan, F.; Song, Q.; Lakshman, S.; Cregan, P.; Scott, R.; Shipe, E.; Garrett, W. Characterization of Soybean Storage and Allergen Proteins Affected by Environmental and Genetic Factors. J. Agric. Food Chem. 2016, 64, 1433–1445. [Google Scholar] [CrossRef]
- Quirce, S.; Fernández-Nieto, M.; Polo, F.; Sastre, J. Soybean trypsin inhibitor is an occupational inhalant allergen. J. Allergy Clin. Immunol. 2002, 109, 178. [Google Scholar] [CrossRef]
- Amnuaycheewa, P.; Gonzalez de Mejia, E. Purification, characterisation, and quantification of the soy allergen profilin (Gly m 3) in soy products. Food Chem. 2010, 119, 1671–1680. [Google Scholar] [CrossRef]











| Allergen Name | Molecular Mass (kDa) | Features | References |
|---|---|---|---|
| Soybean food allergens (class 1 food allergens) | |||
| Gly m 5 (7S globulin) | 72, 68, 50 | Major storage protein, glycoprotein | [9,10] |
| Gly m 6 (11S globulin) | 34, 20 | Major storage protein | [11] |
| Gly m 7 | 76 | Seed-specific biotinylated protein (SBP) | [12] |
| Gly m Bd 30K (P34) | 34 (30) | Homologous to papain | [13,14] |
| Kunitz-type trypsin inhibitor | 18 | trypsin inhibitor | [15] |
| Oleosin | 22–24 | Oil body associated protein | [16] |
| Pollen-related soybean allergens (class 2 food allergens) | |||
| Gly m 3 | 14 | Profilin, Bet v 2 homolog | [17,18] |
| Gly m 4 | 17 | PR-10 family, Bet v 1 homolog | [18,19,20,21] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, A.; Matsushita, K.; Fukuzumi, A.; Tokumasu, N.; Yano, E.; Zaima, N.; Moriyama, T. Comparison of Various Soybean Allergen Levels in Genetically and Non-Genetically Modified Soybeans. Foods 2020, 9, 522. https://doi.org/10.3390/foods9040522
Matsuo A, Matsushita K, Fukuzumi A, Tokumasu N, Yano E, Zaima N, Moriyama T. Comparison of Various Soybean Allergen Levels in Genetically and Non-Genetically Modified Soybeans. Foods. 2020; 9(4):522. https://doi.org/10.3390/foods9040522
Chicago/Turabian StyleMatsuo, Ayato, Kaho Matsushita, Ayano Fukuzumi, Naoki Tokumasu, Erika Yano, Nobuhiro Zaima, and Tatsuya Moriyama. 2020. "Comparison of Various Soybean Allergen Levels in Genetically and Non-Genetically Modified Soybeans" Foods 9, no. 4: 522. https://doi.org/10.3390/foods9040522
APA StyleMatsuo, A., Matsushita, K., Fukuzumi, A., Tokumasu, N., Yano, E., Zaima, N., & Moriyama, T. (2020). Comparison of Various Soybean Allergen Levels in Genetically and Non-Genetically Modified Soybeans. Foods, 9(4), 522. https://doi.org/10.3390/foods9040522






