Review of Research into the Determination of Acrylamide in Foods
Abstract
1. Introduction
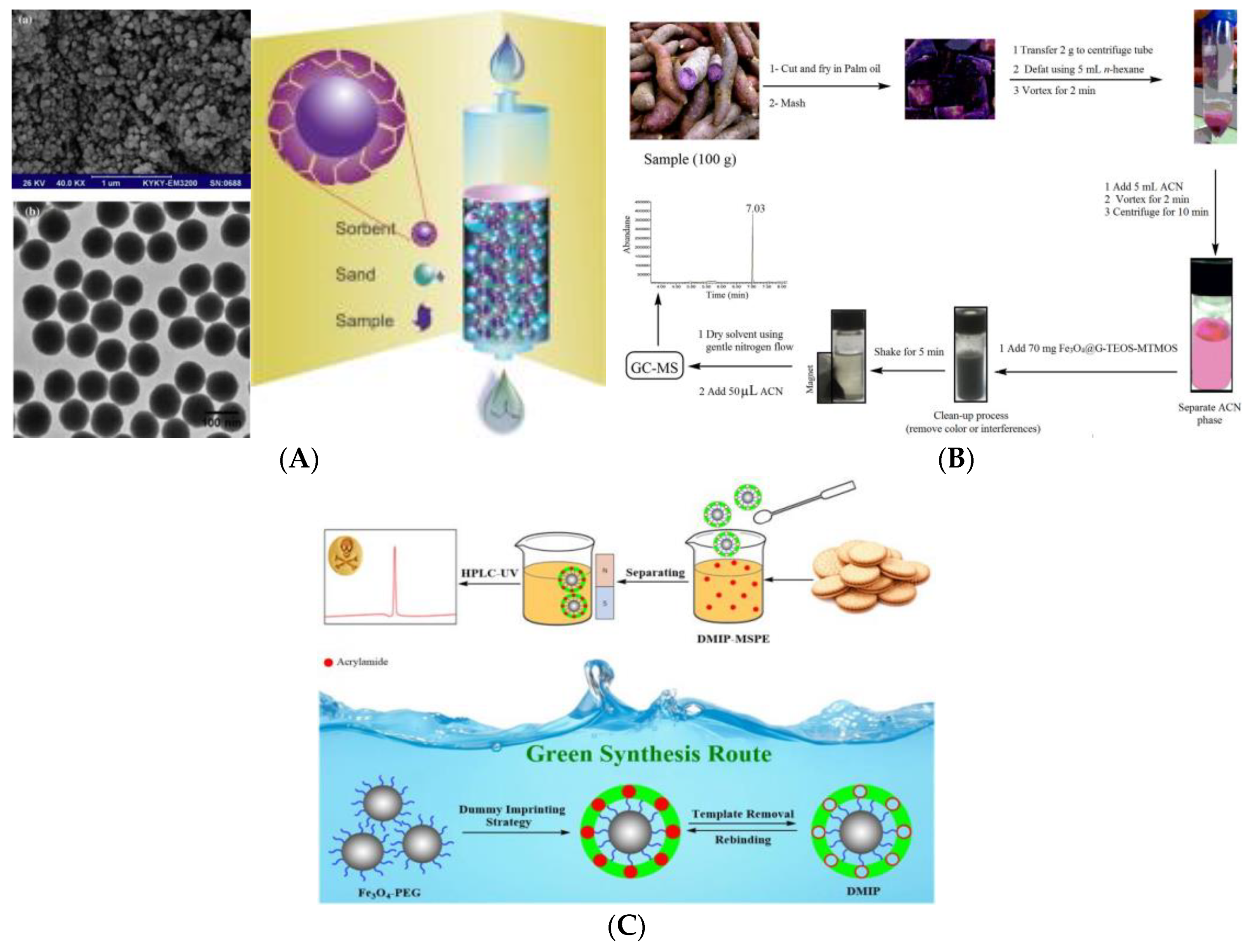
2. Instrumental Analysis Strategies for AA Content in Foods
3. New Strategies for AA Analysis
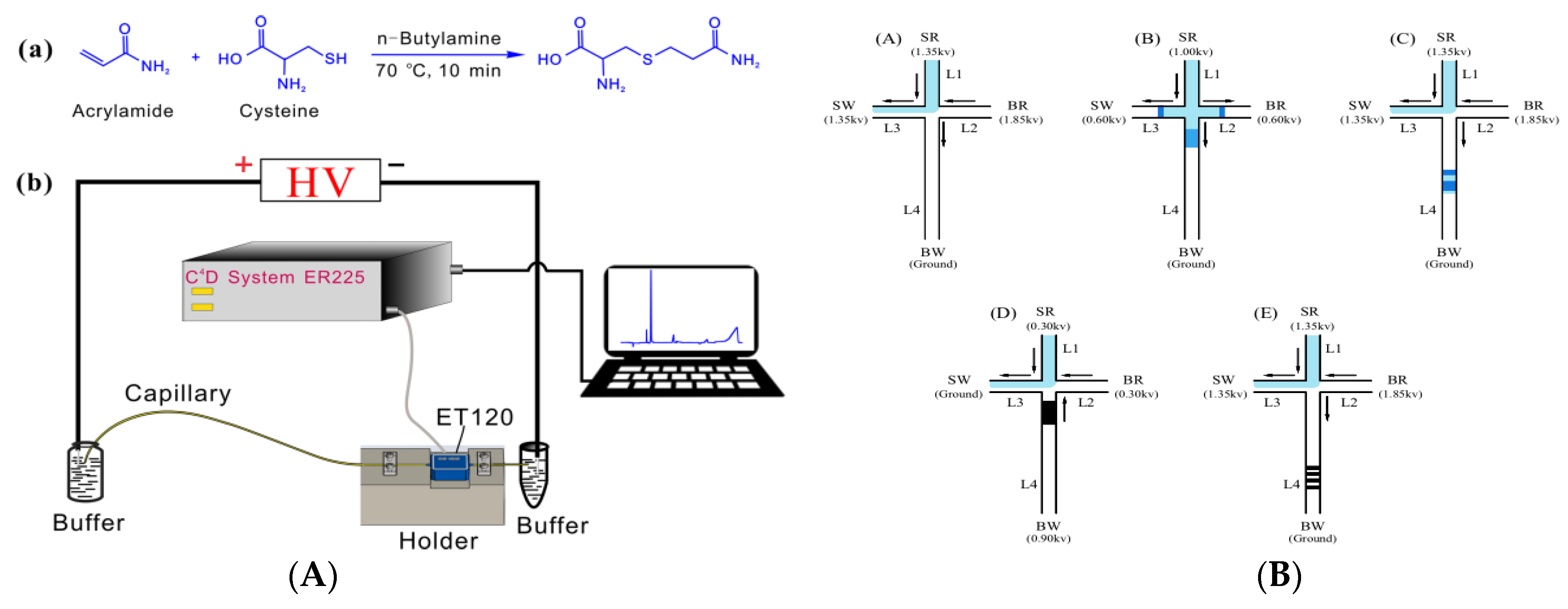
3.1. Capillary Electrophoresis
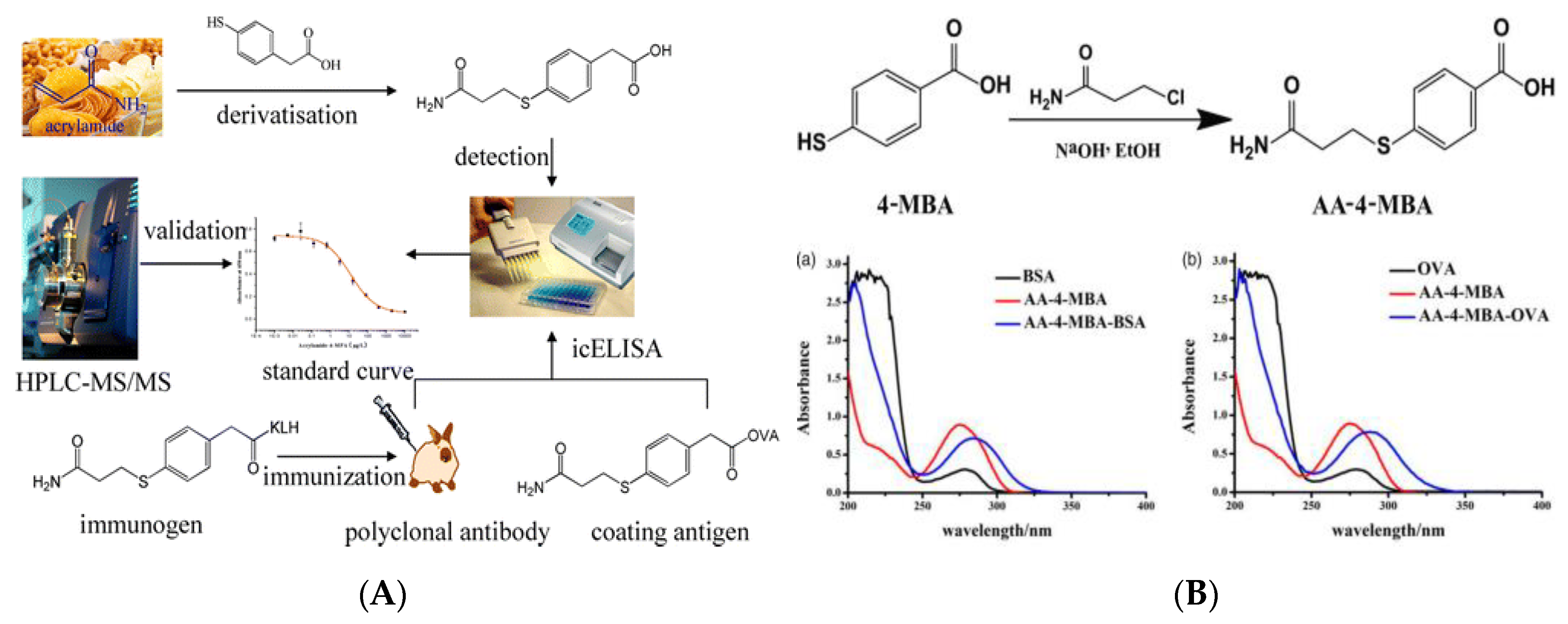
3.2. Immunoassay Method
3.3. Sensor Analysis Technique
3.3.1. Electrochemical Sensing Analysis Based on Biomolecules
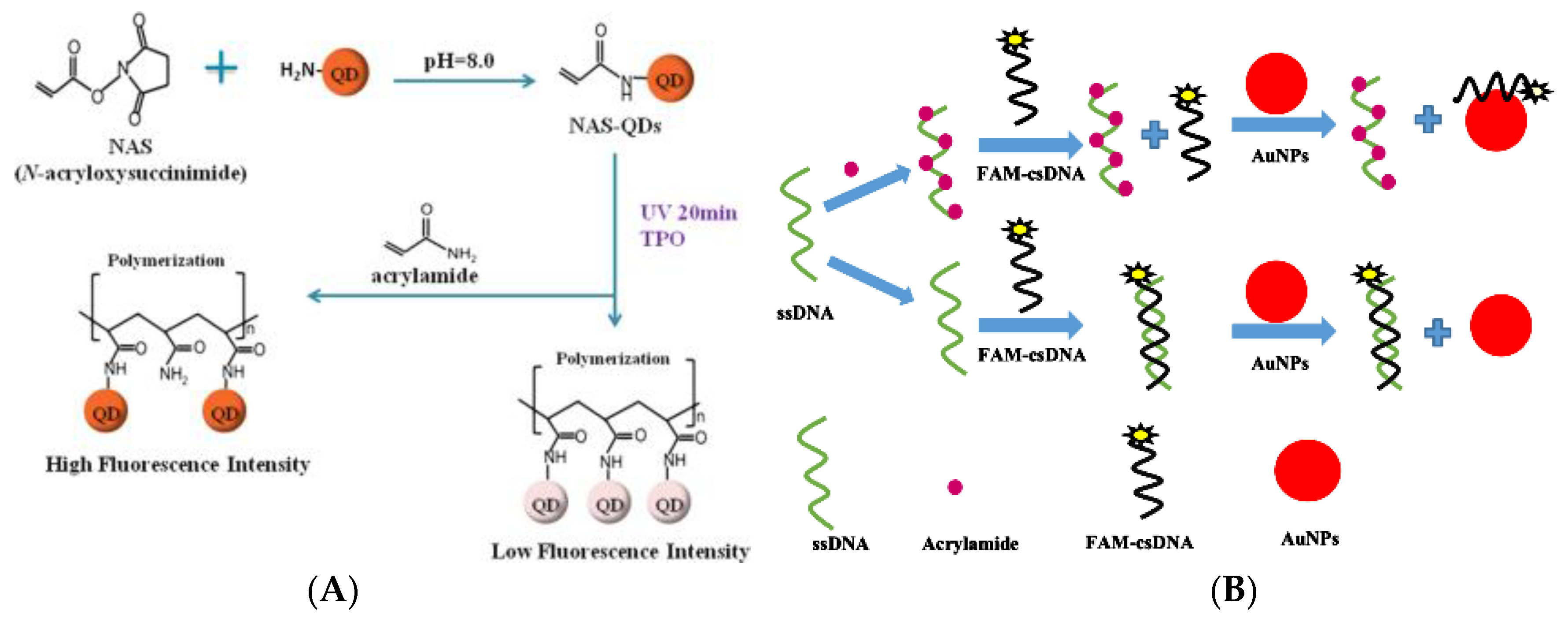
3.3.2. Fluorescence Sensing Analysis Method
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Friedman, M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- Altunay, N.; Gürkan, R.; Orhan, U. A preconcentration method for indirect determination of acrylamide from chips, crackers and cereal-based baby foods using flame atomic absorption spectrometry. Talanta 2016, 161, 143–150. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1994; Volume 60, pp. 389–433. [Google Scholar]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Claeys, W.L.; De Vleeschouwer, K.; Hendrickx, M.E. Kinetics of acrylamide formation and elimination during heating of an asparagine-sugar model system. J. Agric. Food Chem. 2005, 53, 9999–10005. [Google Scholar] [CrossRef]
- Ehling, S.; Hengel, M.; Shibamoto, T. Formation of acrylamide from lipids. In Chemistry and Safety of Acrylamide in Food; Friedman, M., Mottram, D., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2005; Volume 561, pp. 223–233. [Google Scholar]
- Perez-Nevado, F.; Cabrera-Banegil, M.; Repilado, E.; Martillanes, S.; Martin-Vertedor, D. Effect of different baking treatments on the acrylamide formation and phenolic compounds in Californian-style black olives. Food Control 2018, 94, 22–29. [Google Scholar] [CrossRef]
- Ehling, S.; Shibamoto, T. Correlation of acrylamide generation in thermally processed model systems of asparagine and glucose with color formation, amounts of pyrazines formed, and antioxidative properties of extracts. J. Agric. Food Chem. 2005, 53, 4813–4819. [Google Scholar] [CrossRef]
- Casado, F.J.; Montano, A. Influence of processing conditions on acrylamide content in black ripe olives. J. Agric. Food Chem. 2008, 56, 2021–2027. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Influence of California-style black ripe olive processing on the formation of acrylamide. J. Agric. Food Chem. 2014, 62, 8716–8721. [Google Scholar] [CrossRef]
- Esposito, F.; Fasano, E.; De Vivo, A.; Velotto, S.; Sarghini, F.; Cirillo, T. Processing effects on acrylamide content in roasted coffee production. Food Chem. 2020, 319, 126550. [Google Scholar] [CrossRef]
- Commission Recommendation (EU) 2017/2158. Commission Regulation (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017, L304, 24–44. [Google Scholar]
- Oracz, J.; Nebesny, E.; Zyzelewicz, D. New trends in quantification of acrylamide in food products. Talanta 2011, 86, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.Q.; Xu, X.H.; Fu, Y.C.; Li, Y.B. Rapid methods for detecting acrylamide in thermally processed foods: A review. Food Control 2015, 56, 135–146. [Google Scholar] [CrossRef]
- Commission Recommendation (EU) 2019/1888. Commision Recommendation (EU) 2019/1888 of 7 November 2019 on the Monitoring of the Presence of Acrylamide in Certain Foods; European Union: Brussels, Belgium, 2019; Volume L291, pp. 31–33, (document 32019H1888). [Google Scholar]
- Gokmen, V.; Palazoglu, T.K. Acrylamide Formation in Foods during Thermal Processing with a Focus on Frying. Food Bioprocess Technol. 2008, 1, 35–42. [Google Scholar] [CrossRef]
- El-Assouli, S.M. Acrylamide in selected foods and genotoxicity of their Extracts. J. Egypt Public Health Assoc. 2009, 84, 371–392. [Google Scholar] [PubMed]
- Erkekoglu, P.; Baydar, T. Toxicity of acrylamide and evaluation of its exposure in baby foods. Nutr. Res. Rev. 2010, 23, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Das, S.; Teoh, S.L. Dietary acrylamide and the risks of developing cancer: Facts to ponder. Front. Nutr. 2018, 5, 14. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit. Rev. Food Sci. 2019. [Google Scholar] [CrossRef]
- Tardiff, R.G.; Gargas, M.L.; Kirman, C.R.; Carson, M.L.; Sweeney, L.M. Estimation of safe dietary intake levels of acrylamide for humans. Food Chem. Toxicol. 2010, 48, 658–667. [Google Scholar] [CrossRef]
- Singh, P.; Singh, P.; Raja, R.B. Determination of acrylamide concentration in processed food products using normal phase high-performance liquid chromatography (HPLC). Afr. J. Biotechnol. 2010, 9, 8085–8091. [Google Scholar]
- Geng, Z.M.; Wang, P.; Liu, A.M. Determination of acrylamide in starch-based foods by HPLC with pre-column ultraviolet derivatization. J. Chromatogr. Sci. 2011, 49, 818–824. [Google Scholar] [CrossRef]
- Xu, L.H.; Zhang, L.M.; Qiao, X.G.; Xu, Z.X.; Song, J.M. Determination of trace acrylamide in potato chip and bread crust based on SPE and HPLC. Chromatographia 2012, 75, 269–274. [Google Scholar]
- Sun, S.Y.; Fang, Y.; Xia, Y.M. A facile detection of acrylamide in starchy food by using a solid extraction-GC strategy. Food Control 2012, 26, 220–222. [Google Scholar] [CrossRef]
- Yao, W.J. Direct determination of acrylamide in food by gas chromatography with nitrogen chemiluminescence detection. J. Sep. Sci. 2015, 38, 2272–2277. [Google Scholar]
- Saraji, M.; Javadian, S. Single-drop microextraction combined with gas chromatography-electron capture detection for the determination of acrylamide in food samples. Food Chem. 2019, 274, 55–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Jiao, J.; Li, D.; Zhang, Y. Ultra high-performance liquid chromatography-tandem mass spectrometry for the simultaneous analysis of asparagine, sugars, and acrylamide in Maillard reactions. Anal. Chem. 2011, 83, 3297–3304. [Google Scholar] [CrossRef]
- De Paola, E.L.; Montevecchi, G.; Masino, F.; Garbini, D.; Barbanera, M.; Antonelli, A. Determination of acrylamide in dried fruits and edible seeds using QuEChERS extraction and LC separation with MS detection. Food Chem. 2017, 217, 191–195. [Google Scholar] [CrossRef]
- Fernández, A.; Talaverano, M.I.; Pérez-Nevado, F.; Boselli, E.; Cordeiro, A.M.; Martillanes, S.; Foligni, R.; Martín-Vertedor, D. Evaluation of phenolics and acrylamide and their bioavailability in high hydrostatic pressure treated and fried table olives. J. Food Process Pres. 2020. [Google Scholar] [CrossRef]
- Cagliero, C.; Ho, T.D.; Zhang, C.; Bicchi, C.; Anderson, J.L. Determination of acrylamide in brewed coffee and coffee powder using polymeric ionic liquid-based sorbent coatings in solid-phase microextraction coupled to gas chromatography-mass spectrometry. J. Chromatogr. A 2016, 1449, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ren, Y.; Liu, J.; Wen, X.D. Investigation of a rapid and sensitive non-aqueous reaction system for the determination of acrylamide in processed foods by gas chromatography-mass spectrometry. Anal. Methods 2016, 8, 5970–5977. [Google Scholar] [CrossRef]
- Jozinovic, A.; Sarkanj, B.; Ackar, D.; Balentic, J.P.; Subaric, D.; Cvetkovic, T.; Ranilovic, J.; Guberac, S.; Babic, J. Simultaneous determination of acrylamide and hydroxymethylfurfural in extruded products by LC-MS/MS method. Molecules 2019, 24, 1971. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.L.; Carvalho, D.O.; Guido, L.F. Determination of acrylamide in biscuits by high-resolution orbitrap mass spectrometry: A novel application. Foods 2019, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Calbiani, F.; Careri, M.; Elviri, L.; Mangia, A.; Zagnoni, I. Development and single-laboratory validation of a reversed-phase liquid chromatography-electrospray-tandem mass spectrometry method for identification and determination of acrylamide in foods. J. AOAC Int. 2004, 87, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Galuch, M.B.; Magon, T.F.S.; Silveira, R.; Nicacio, A.E.; Pizzo, J.S.; Bonafe, E.G.; Maldaner, L.; Santos, O.O.; Visentainer, J.V. Determination of acrylamide in brewed coffee by dispersive liquid-liquid microextraction (DLLME) and ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). Food Chem. 2019, 282, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Tolgyesi, A.; Sharma, V.K. Determination of acrylamide in gingerbread and other food samples by HILIC-MS/MS: A dilute-and-shoot method. J. Chromatogr. B 2020, 1136, 121933. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Qian, S.; Xie, Y.H.; Xie, Z.W.; Li, H.X. Simultaneous determination of acrylamide, aniline and benzidine in water sample by high performance liquid chromatography-tandem mass spectrometry. Chin. J. Anal. Chem. 2014, 42, 93–98. [Google Scholar]
- Lee, K.J.; Lee, G.H.; Kim, H.S.; Oh, M.S.; Chu, S.; Hwang, I.J.; Lee, J.Y.; Choi, A.; Kim, C.I.; Park, H.M. Determination of heterocyclic amines and acrylamide in agricultural products with liquid chromatography-tandem mass spectrometry. Toxicol. Res. 2015, 31, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Wang, L.; Guo, X.B.; Li, H.; Yu, S.J. Simultaneous detection of 4(5)-methylimidazole and acrylamide in biscuit products by isotope-dilution UPLC-MS/MS. Food Control 2019, 105, 64–70. [Google Scholar] [CrossRef]
- Pugajeva, I.; Jaunbergs, J.; Bartkevics, V. Development of a sensitive method for the determination of acrylamide in coffee using high-performance liquid chromatography coupled to a hybrid quadrupole orbitrap mass spectrometer. Food Addit. Contam. A 2015, 32, 170–179. [Google Scholar] [CrossRef]
- Omar, M.M.A.; Elbashir, A.A.; Schmitz, O.J. Determination of acrylamide in Sudanese food by high performance liquid chromatography coupled with LTQ Orbitrap mass spectrometry. Food Chem. 2015, 176, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Ghaedi, M.; Ostovan, A. Development of dummy molecularly imprinted based on functionalized silica nanoparticles for determination of acrylamide in processed food by matrix solid phase dispersion. Food Chem. 2016, 210, 78–84. [Google Scholar] [CrossRef]
- Chang, T.T.; Yan, X.Y.; Liu, S.M.; Liu, Y.X. Magnetic dummy template silica sol-gel molecularly imprinted polymer nanospheres as magnetic solid-phase extraction material for the selective and sensitive determination of bisphenol A in plastic bottled beverages. Food Anal. Method 2017, 10, 3980–3990. [Google Scholar] [CrossRef]
- Nasir, A.N.M.; Yahaya, N.; Zain, N.N.M.; Lim, V.; Kamaruzaman, S.; Saad, B.; Nishiyama, N.; Yoshida, N.; Hirota, Y. Thiol-functionalized magnetic carbon nanotubes for magnetic micro-solid phase extraction of sulfonamide antibiotics from milks and commercial chicken meat products. Food Chem. 2019, 276, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Lv, S.W.; Yuan, X.Y.; Liu, H.L.; Wang, S. Facile construction of magnetic core-shell covalent organic frameworks as efficient solid-phase extraction adsorbents for highly sensitive determination of sulfonamide residues against complex food sample matrices. RSC Adv. 2019, 9, 14247–14253. [Google Scholar] [CrossRef]
- Nodeh, H.R.; Ibrahim, W.A.W.; Kamboh, M.A.; Sanagi, M.M. Magnetic graphene sol-gel hybrid as clean-up adsorbent for acrylamide analysis in food samples prior to GC-MS. Food Chem. 2018, 239, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Arabi, M.; Ghaedi, M.; Ostovan, A.; Wang, X.Y.; Li, J.H.; Chen, L.X. Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta 2019, 195, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, E.; Ghaemi, E.; Alizadeh, N. Determination of acrylamide in potato-based foods using headspace solid-phase microextraction based on nanostructured polypyrrole fiber coupled with ion mobility spectrometry: A heat treatment study. Anal. Method 2017, 9, 5127–5134. [Google Scholar] [CrossRef]
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Ghasemi, J.; Hosseini-Esfahani, F.; Mohammadi, A. Investigation and determination of acrylamide in the main group of cereal products using advanced microextraction method coupled with gas chromatography-mass spectrometry. J. Cereal. Sci. 2019, 87, 157–164. [Google Scholar] [CrossRef]
- Wawrzyniak, R.; Jasiewicz, B. Straightforward and rapid determination of acrylamide in coffee beans by means of HS-SPME/GC-MS. Food Chem. 2019, 301, 125264. [Google Scholar] [CrossRef]
- Commission Recommendation (EU). 2013/647 of 8 November 2013 on investigations into the levels of acrylamide in food. Off. J. Eur. Union 2013, L301, 15–17. [Google Scholar]
- Zokaei, M.; Abedi, A.S.; Kamankesh, M.; Shojaee-Aliababadi, S.; Mohammadi, A. Ultrasonic-assisted extraction and dispersive liquid-liquid microextraction combined with gas chromatography-mass spectrometry as an efficient and sensitive method for determining of acrylamide in potato chips samples. Food Chem. 2017, 234, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Hamdamali, M.; Aryanasab, F.; Shabanian, M. 2-Naphthalenthiol derivatization followed by dispersive liquid-liquid microextraction as an efficient and sensitive method for determination of acrylamide in bread and biscuit samples using high-performance liquid chromatography. J. Chromatogr. A 2018, 1558, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.; Kamankesh, M.; Mohammadi, A.; Jazaeri, S. Acrylamide in cookie samples: Analysis using an efficient co-derivatization coupled with sensitive microextraction method followed by gas chromatography-mass spectrometry. Food Anal. Method 2019, 12, 1439–1447. [Google Scholar] [CrossRef]
- Lim, H.H.; Shin, H.S. Ultra trace level determinations of acrylamide in surface and drinking water by GC-MS after derivatization with xanthydrol. J. Sep. Sci. 2013, 36, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Backe, W.J.; Yingling, V.; Johnson, T. The determination of acrylamide in environmental and drinking waters by large-volume injection-hydrophilic-interaction liquid chromatography and tandem mass spectrometry. J. Chromatogr. A 2014, 1334, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lee, A.W.; Shuang, S.; Choi, M.M. SPE/HPLC/UV studies on acrylamide in deep-fried flour-based indigenous Chinese foods. Microchem. J. 2008, 89, 90–97. [Google Scholar] [CrossRef]
- GB/T 5009. 204–2014. Determination of Acrylamide in Food; Standard Press: Beijing, China, 2015. [Google Scholar]
- Lim, H.H.; Shin, H.S. A new derivatization approach with d-cysteine for the sensitive and simple analysis of acrylamide in foods by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1361, 117–124. [Google Scholar] [CrossRef]
- Ferrer-Aguirre, A.; Romero-Gonzalez, R.; Vidal, J.L.M.; Frenich, A.G. Simple and fast determination of acrylamide and metabolites in potato chips and grilled asparagus by liquid chromatography coupled to mass spectrometry. Food Anal. Method 2016, 9, 1237–1245. [Google Scholar] [CrossRef]
- Yoshioka, T.; Izumi, Y.; Nagatomi, Y.; Miyamoto, Y.; Suzuki, K.; Bamba, T. A highly sensitive determination method for acrylamide in beverages, grains, and confectioneries by supercritical fluid chromatography tandem mass spectrometry. Food Chem. 2019, 294, 486–492. [Google Scholar] [CrossRef]
- Başkan, S.; Erim, F.B. NACE for the analysis of acrylamide in food. Electrophoresis 2007, 28, 4108–4113. [Google Scholar] [CrossRef] [PubMed]
- Voeten, R.L.C.; Ventouri, I.K.; Haselberg, R.; Somsen, G.W. Capillary electrophoresis: Trends and recent advances. Anal. Chem. 2018, 90, 1464–1481. [Google Scholar] [CrossRef]
- Abd El-Hady, D.; Albishri, H.M. Simultaneous determination of acrylamide, asparagine and glucose in food using short chain methyl imidazolium ionic liquid based ultrasonic assisted extraction coupled with analyte focusing by ionic liquid micelle collapse capillary electrophoresis. Food Chem. 2015, 188, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.P.; Li, Y.T.; Li, F.; Yang, Z.Y.; Quan, F.F.; Zhou, L.; Pu, Q.S. Thiol-ene click derivatization for the determination of acrylamide in potato products by capillary electrophoresis with capacitively coupled contactless conductivity detection. J. Agric. Food Chem. 2019, 67, 8053–8060. [Google Scholar] [CrossRef]
- Wu, M.L.; Chen, W.J.; Wang, G.; He, P.G.; Wang, Q.J. Analysis of acrylamide in food products by microchip electrophoresis with on-line multiple-preconcentration techniques. Food Chem. 2016, 209, 154–161. [Google Scholar] [CrossRef]
- Kitagawa, F.; Kawai, T.; Sueyoshi, K.; Otsuka, K. Recent progress of on-line sample preconcentration techniques in microchip electrophoresis. Anal. Sci. 2012, 28, 85–93. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Dionisopoulou, N. Acrylamide: Formation, occurrence in food products, detection methods, and legislation. Crit. Rev. Food Sci. 2014, 54, 708–733. [Google Scholar] [CrossRef]
- Sueyoshi, K.; Kitagawa, F.; Otsuka, K. Effect of a low-conductivity zone on field-amplified sample stacking in microchip micellar electrokinetic chromatography. Anal. Sci. 2013, 29, 133–138. [Google Scholar] [CrossRef]
- Breadmore, M.C.; Tubaon, R.M.; Shallan, A.I.; Phung, S.C.; Keyon, A.S.A.; Gstoettenmayr, D.; Prapatpong, P. Recent advances in enhancing the sensitivity of electrophoresis and electrochromatography in capillaries and microchips (2012–2014). Electrophoresis 2015, 36, 36–61. [Google Scholar] [CrossRef]
- Cui, Y.L.; Zhao, J.; Zhou, J.; Tan, G.Y.; Zhao, Q.Y.; Zhang, Y.H.; Wang, B.M.; Jiao, B.N. Development of a sensitive monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for analysing nobiletin in citrus and herb samples. Food Chem. 2019, 293, 144–150. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhang, S.P.; Hong, Z.S.; Lin, Y.Y.; Dai, H. A mimotope peptide-based dual-signal readout competitive enzyme-linked immunoassay for non-toxic detection of zearalenone. J. Mater. Chem. B 2019, 7, 6972–6980. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.C.; Shi, J.; Zhang, J.; Zhao, K.; Deng, A.P.; Li, J.G. Multiple signal amplification chemiluminescence immunoassay for chloramphenicol using functionalized SiO2 nanoparticles as probes and resin beads as carriers. Spectrochim. Acta. A 2019, 222, 117177. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, X.; Chen, L.J.; Qian, H.L.; Wang, W.L.; Yang, C.; Yan, X.P. p-Bromophenol-enhanced bienzymatic chemiluminescence competitive immunoassay for ultrasensitive determination of aflatoxin B-1. Anal. Chem. 2019, 91, 13191–13197. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.J.; Xu, Y.; Ma, B.; Cui, H.F.; Sun, C.X.; Zhang, M.Z. Carboxyl-functionalized, europium nanoparticle-based fluorescent immunochromatographic assay for sensitive detection of citrinin in monascus fermented food. Toxins 2019, 11, 605. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, K.K.; Gao, B.; Wu, Y.Q.; Huang, X.L.; Lai, W.H.; Xiong, Y.H.; Liu, Y. Fluorescence immunoassay through histone-ds-poly(AT)-templated copper nanoparticles as signal transductors for the sensitive detection of salmonella choleraesuis in milk. J. Dairy Sci. 2019, 102, 6047–6055. [Google Scholar] [CrossRef]
- Singh, G.; Brady, B.; Koerner, T.; Becalski, A.; Zhao, T.; Feng, S.; Godefroy, S.B.; Huet, A.C.; Delahaut, P. Development of a highly sensitive competitive indirect enzyme-linked immunosorbent assay for detection of acrylamide in foods and water. Food Anal. Method 2014, 7, 1298–1304. [Google Scholar] [CrossRef]
- Wu, J.; Shen, Y.D.; Lei, H.T.; Sun, Y.M.; Yang, J.Y.; Xiao, Z.L.; Wang, H.; Xu, Z.L. Hapten synthesis and development of a competitive indirect enzyme-linked immunosorbent assay for acrylamide in food samples. J. Agr. Food Chem. 2014, 62, 7078–7084. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Song, S.S.; Liu, L.Q.; Kuang, H.; Xu, C.L. An indirect competitive enzyme-linked immunosorbent assay for acrylamide detection based on a monoclonal antibody. Food Agric. Immunol. 2016, 27, 796–805. [Google Scholar] [CrossRef]
- Assaat, L.D.; Saepudin, E.; Soejoedono, R.D.; Adji, R.S.; Poetri, O.N.; Ivandini, T.A. Production of a polyclonal antibody against acrylamide for immunochromatographic detection of acrylamide using strip tests. J. Anim. Vet. Adv. 2019, 6, 366–375. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, L.H.; Ma, Y.; Qiao, X.G.; Xu, Z.X. Study on a biomimetic enzyme-linked immunosorbent assay method for rapid determination of trace acrylamide in French fries and cracker samples. J. Sci. Food Agric. 2014, 94, 102–108. [Google Scholar] [CrossRef]
- Sarkar, D.; Xie, X.J.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-effect transistor for next-generation label-free biosensors. ACS Nano. 2014, 8, 3992–4003. [Google Scholar] [CrossRef]
- Hu, Q.Q.; Wang, R.H.; Wang, H.; Slavik, M.F.; Li, Y.B. Selection of acrylamide-specific aptamers by a quartz crystal microbalance combined SELEX method and their application in rapid and specific detection of acrylamide. Sens. Actuators B Chem. 2018, 273, 220–227. [Google Scholar] [CrossRef]
- Bhadani, S.N.; Prasad, Y.K.; Kundu, S. Electrochemical and chemical polymerization of acrylamide. J. Polym. Sci. Pol. Chem. 1980, 18, 1459–1469. [Google Scholar] [CrossRef]
- Casella, I.G.; Pierri, M.; Contursi, M. Determination of acrylamide and acrylic acid by isocratic liquid chromatography with pulsed electrochemical detection. J. Chromatogr. A 2006, 1107, 198–203. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Hitchman, M.L. Determination of pesticides using electrochemical biosensors. Trac-Trends Anal. Chem. 2015, 14, 1311–1328. [Google Scholar] [CrossRef]
- Wu, D.; Du, D.; Lin, Y. Recent progress on nanomaterial-based biosensors for veterinary drug residues in animal-derived food. Trends Anal. Chem. 2016, 83, 95–101. [Google Scholar] [CrossRef]
- Pandey, C.M.; Malhotra, B.D. Nanomaterials for Biosensors; Biosensors: Basel, Switzerland, 2018; pp. 1–74. [Google Scholar]
- Rasooly, A.; Herold, K.E. Biosensors for the analysis of food-and waterborne pathogens and their toxins. J. Aoac. Int. 2019, 89, 873–883. [Google Scholar] [CrossRef]
- Shiddiky, M.J.A.; Torriero, A.A.J. Application of ionic liquids in electrochemical sensing systems. Biosens. Bioelectron. 2011, 26, 1775–1787. [Google Scholar] [CrossRef]
- Batra, B.; Lata, S.; Pundir, C.S. Construction of an improved amperometric acrylamide biosensor based on hemoglobin immobilized onto carboxylated multi-walled carbon nanotubes/iron oxide nanoparticles/chitosan composite film. Bioproc. Biosyst. Eng. 2013, 36, 1591–1599. [Google Scholar] [CrossRef]
- Garabagiu, S.; Mihailescu, G. Simple hemoglobin-gold nanoparticles modified electrode for the amperometric detection of acrylamide. J. Electroanal. Chem. 2011, 659, 196–200. [Google Scholar] [CrossRef]
- Sayyad, A.S.; Balakrishnan, K.; Ci, L.; Kabbani, A.T.; Vajtai, R.; Ajayan, P.M. Synthesis of iron nanoparticles from hemoglobin and myoglobin. Nanotechnology 2012, 23, 1–5. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; Qiao, M. Enhancing sensitivity of hemoglobin-based electrochemical biosensor by using protein conformational intermediate. Sens. Actuators B-Chem. 2015, 22, 1694–1699. [Google Scholar] [CrossRef]
- Yadav, N.; Chhillar, A.K.; Pundir, C.S. Preparation, characterization and application of haemoglobin nanoparticles for detection of acrylamide in processed foods. Int. J. Biol. Macromol. 2018, 107, 1000–1013. [Google Scholar] [CrossRef]
- Asnaashari, M.; Kenari, R.E.; Farahmandfar, R.; Abnous, K.; Taghdisi, S.M. An electrochemical biosensor based on hemoglobin-oligonucleotides-modified electrode for detection of acrylamide in potato fries. Food Chem. 2019, 271, 54–61. [Google Scholar] [CrossRef]
- Bucur, M.P.; Bucur, B.; Radu, G.L. Simple, selective and fast detection of acrylamide based on glutathione S-transferase. RSC Adv. 2018, 8, 23931–23936. [Google Scholar] [CrossRef]
- Sumner, S.C.; MacNeela, J.P.; Fennell, T.R. Characterization and quantitation of urinary metabolites of [1,2,3-13C]acrylamide in rats and mice using 13C nuclear magnetic resonance spectroscopy. Chem. Res. Toxicol. 1992, 5, 81–89. [Google Scholar] [CrossRef]
- Hartmann, E.C.; Bolt, H.M.; Drexler, H.; Angerer, J. N-Acetyl-S-(1-carbamoyl-2-hydroxy-ethyl)-l-cysteine (iso-GAMA) a further product of human metabolism of acrylamide: Comparison with the simultaneously excreted other mercaptuic acids. Arch. Toxicol. 2009, 83, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E.C.; Latzin, J.M.; Schindler, B.K.; Koch, H.M.; Angerer, J. Excretion of 2,3-dihydroxy- propionamide (OH-PA), the hydrolysis product of glycidamide, in human urine after single oral dose of deuterium-labeled acrylamide. Arch. Toxicol. 2011, 85, 601–606. [Google Scholar] [CrossRef]
- Luo, Y.S.; Long, T.Y.; Shen, L.C.; Huang, S.L.; Chiang, S.Y.; Wu, K.Y. Synthesis, characterization and analysis of the acrylamide- and glycidamide-glutathione conjugates. Chem. Biol. Interact. 2015, 237, 38–46. [Google Scholar] [CrossRef]
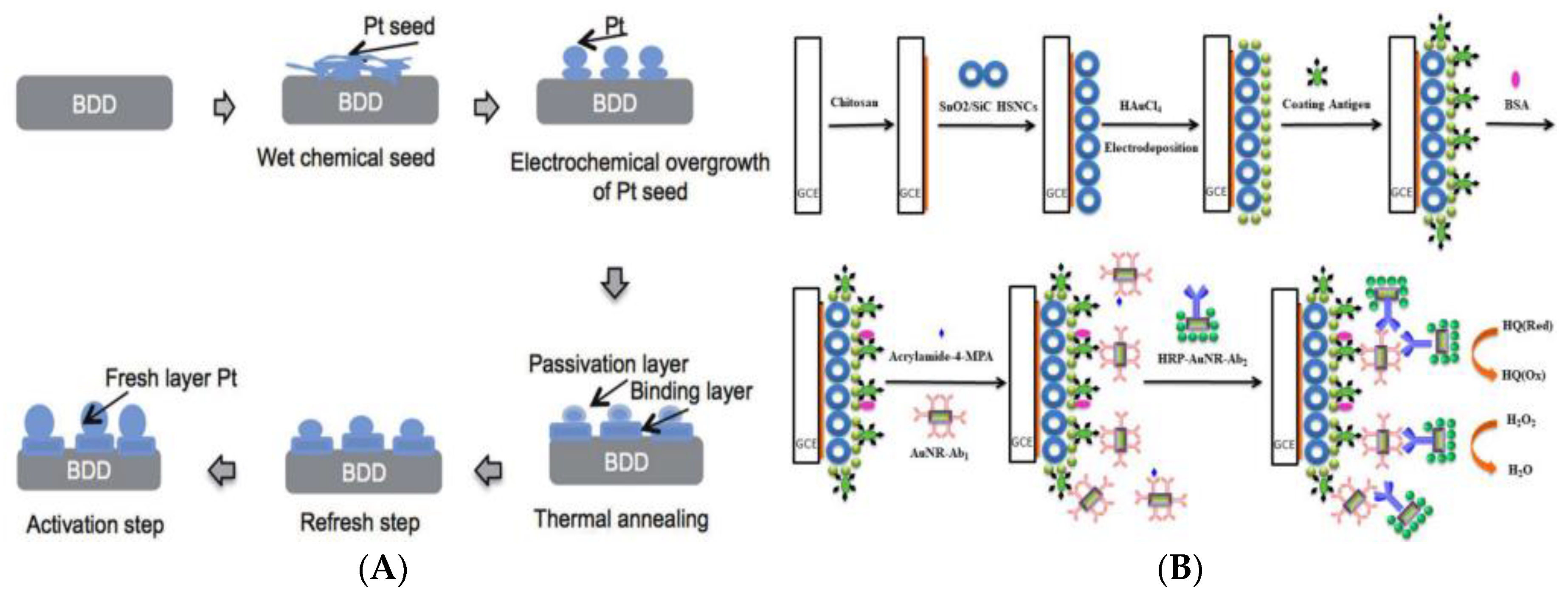
- Wulandari, R.; Ivandini, T.A.; Irkham; Saepudin, E.; Einaga, Y. Modification of boron-doped diamond electrodes with platinum to increase the stability and sensitivity of haemoglobin-based acrylamide sensors. Sens. Mater. 2019, 31, 1105–1117. [Google Scholar]
- Wu, M.F.; Wang, Y.; Li, S.; Dong, X.X.; Yang, J.Y.; Shen, Y.D.; Wang, H.; Sun, Y.M.; Lei, H.T.; Xu, Z.L. Ultrasensitive immunosensor for acrylamide based on chitosan/SnO2-SiC hollow sphere nanochains/gold nanomaterial as signal amplification. Anal. Chim. Acta 2019, 1049, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Rivas, G.A.; Rubianes, M.D.; Rodriguez, M.C.; Ferreyra, N.E.; Luque, G.L.; Pedano, M.L.; Miscoria, S.A.; Parrado, C. Carbon nanotubes for electrochemical biosensing. Talanta 2007, 74, 291–307. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Jeon, J.Y.; Ha, T.J. Carbon nanotubes: An effective platform for biomedical electronics. Biosens. Bioelectron. 2020, 150, 111919. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, L.G.; Wang, Y.L.; Shi, X.B.; Liu, Y.; Yang, Y.; He, Z. Electrochemical sensor based on imprinted sol-gel polymer on Au NPs-MWCNTs-CS modified electrode for the determination of acrylamide. Food Anal. Method. 2016, 9, 114–121. [Google Scholar] [CrossRef]
- Batra, B.; Lata, S.; Sharma, M.; Pundir, C.S. An acrylamide biosensor based on immobilization of hemoglobin onto multiwalled carbon nanotube/copper nanoparticles/polyaniline hybrid film. Anal. Biochem. 2013, 433, 210–217. [Google Scholar] [CrossRef]
- Varmira, K.; Abdi, O.; Gholivand, M.B.; Goicoechea, H.C.; Jalalvand, A.R. Intellectual modifying a bare glassy carbon electrode to fabricate a novel and ultrasensitive electrochemical biosensor: Application to determination of acrylamide in food samples. Talanta 2018, 176, 509–517. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Materials for fluorescence-based optical chemical sensors. J. Mater. Chem. 2005, 15, 2657–2669. [Google Scholar] [CrossRef]
- Dandin, M.; Abshire, P.; Smela, E. Optical filtering technologies for integrated fluorescence sensors. Lab Chip 2007, 7, 955–977. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lee, H.L.; Yadav, K.; Lin, K.C.; Lin, Y.T.; Chang, A.H.H. Silicon quantum dots based fluorescence turn-on metal ion sensors in live cells. ACS Appl. Mater. Inter. 2016, 8, 3953–23962. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Yang, Y.W. Tetraphenylethylene-interweaving conjugated macrocycle polymer materials as two-photon fluorescence sensors for metal ions and organic molecules. Adv. Mater. 2018, 30, 1800177. [Google Scholar] [CrossRef]
- Al-Zahrani, F.A.M.; El-Shishtawy, R.M.; Asiri, A.M.; Al-Soliemy, A.M.; Abu Mellah, K.; Ahmed, N.S.E.; Jedidi, A. A new phenothiazine-based selective visual and fluorescent sensor for cyanide. BMC Chem. 2020, 14, 2. [Google Scholar] [CrossRef]
- Kong, Y.L.; Cheng, Q.; He, Y.; Ge, Y.L.; Zhou, J.G.; Song, G.W. A dual-modal fluorometric and colorimetric nanoprobe based on graphitic carbon nitrite quantum dots and Fe(II)-bathophenanthroline complex for detection of nitrite in sausage and water. Food Chem. 2020, 312, 126089. [Google Scholar] [CrossRef]
- Huang, B.H.; Shen, S.S.; Wei, N.; Guo, X.F.; Wang, H. Fluorescence biosensor based on silicon quantum dots and 5,5’-dithiobis-(2-nitrobenzoic acid) for thiols in living cells. Spectrochim. Acta A 2020, 229, 117972. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z.F. A novel fluorescence enhanced route to detect copper(II) by click chemistry-catalyzed connection of Au@SiO2 and carbon dots. Sens. Actuators B-Chem. 2016, 233, 426–430. [Google Scholar] [CrossRef]
- Mani, N.P.; Ganiga, M.; Cyriac, J. MoS2 nanohybrid as a fluorescence sensor for highly selective detection of dopamine. Analyst 2018, 143, 1691–1698. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, L.; Liang, R.P.; Qiu, J.D. Rapid detection of mercury ions based on nitrogen doped graphene quantum dots accelerating formation of manganese porphyrin. ACS Sens. 2018, 3, 1040–1047. [Google Scholar] [CrossRef]
- Hu, Q.Q.; Xu, X.H.; Li, Z.M.; Zhang, Y.; Wang, J.P.; Fu, Y.C.; Li, Y.B. Detection of acrylamide in potato chips using a fluorescent sensing method based on acrylamide polymerization-induced distance increase between quantum dots. Biosens. Bioelectron. 2014, 54, 64–71. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Bai, L.; Jiang, Y.H.; Qiu, J.; Meng, M.J.; Liu, Z.C.; Ni, L. A molecularly imprinted polymer placed on the surface of graphene oxide and doped with Mn(II)-doped ZnS quantum dots for selective fluorometric determination of acrylamide. Microchim. Acta 2018, 185, UNSP 48. [Google Scholar] [CrossRef]
- Asnaashari, M.; Kenari, R.E.; Farahmandfar, R.; Taghdisi, S.M.; Abnous, K. Fluorescence quenching biosensor for acrylamide detection in food products based on double-stranded DNA and gold nanoparticles. Sens. Actuators B-Chem. 2018, 265, 339–345. [Google Scholar] [CrossRef]
- Amit, N.; Anupam, B.; Shweta, P. Gold nanoparticle induced enhancement of molecular fluorescence for Zn2+ detection in aqueous niosome solution. In Proceedings of the 13th International Conference on Fibre Optics & Photonics, Kanpur, India, 4–8 December 2016; Optical Society of America: Washington, DC, USA, 2016. Paper W2D.1. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Liu, K.; Yang, J.; Hong, L.; Xie, X.; Wang, S. Review of Research into the Determination of Acrylamide in Foods. Foods 2020, 9, 524. https://doi.org/10.3390/foods9040524
Pan M, Liu K, Yang J, Hong L, Xie X, Wang S. Review of Research into the Determination of Acrylamide in Foods. Foods. 2020; 9(4):524. https://doi.org/10.3390/foods9040524
Chicago/Turabian StylePan, Mingfei, Kaixin Liu, Jingying Yang, Liping Hong, Xiaoqian Xie, and Shuo Wang. 2020. "Review of Research into the Determination of Acrylamide in Foods" Foods 9, no. 4: 524. https://doi.org/10.3390/foods9040524
APA StylePan, M., Liu, K., Yang, J., Hong, L., Xie, X., & Wang, S. (2020). Review of Research into the Determination of Acrylamide in Foods. Foods, 9(4), 524. https://doi.org/10.3390/foods9040524