Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Media
2.2. Propolis
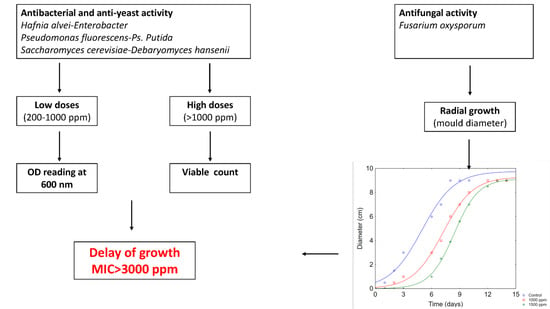
2.3. Growth Index of Bacteria and Yeasts
2.4. Viable Count of Bacteria and Yeasts
2.5. Antifungal Activity
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buchta, V.; Černý, J.; Opletalová, V. In vitro antifungal activity of propolis samples of Czech and Slovak origin. Cent. Eur. J. Biol. 2011, 6, 160–166. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Benhanifia, M.; Shimomura, K.; Tsuchiya, I.; Inui, S.; Kumazawa, S.; Mohamed, W.M.; Boukraa, L.; Sakharkar, M.K.; Benbarek, H. Chemical composition and antimicrobial activity of propolis collected from some localities of Western Algeria. Acta Aliment Hung. 2014, 43, 482–488. [Google Scholar] [CrossRef]
- Nedji, N.; Loucif-Ayad, W. Antimicrobial activity of Algerian propolis in foodborne pathogens and its quantitative chemical composition. Asian Pac. J. Trop. Dis. 2014, 4, 433–437. [Google Scholar] [CrossRef]
- Özcan, M.; Ünver, A.; Ceylan, D.A.; Yetiflir, R. Inhibitory effect of pollen and propolis extracts. Nahrung 2004, 48, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Seibert, J.B.; Bautista-Silva, J.P.; Amparo, T.R.; Petit, A.; Pervier, P.; Dos Santos Almeida, J.C.; Azevedo, M.C.; Silveira, B.M.; Brandão, G.C.; de Souza, G.H.B.; et al. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2019, 287, 61–67. [Google Scholar] [CrossRef]
- Silva, F.R.G.; Matias, T.M.S.; Souza, L.I.O.; Matos-Rocha, T.J.; Fonseca, S.A.; Mousinho, K.C.; Santos, A.F. Phytochemical screening and in vitro antibacterial, antifungal, antioxidant and anitumor activities of the red própolis Alagoas. Braz. J. Biol. 2019, 79, 452–459. [Google Scholar] [CrossRef]
- Soylu, E.M.; Özdemir, A.E.; Ertürk, E.; Sahinler, N. Antifungal activity of propolis against postharvest disease agent Penicillium digitatum. Asian J. Chem. 2008, 24, 4823–4830. [Google Scholar]
- Anjum, S.I.; Ullah, A.; Ali Kan, K.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Kubilene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barcauskaite, K.; Kuilius, R.; Kasparavicine, G.; Savickas, A. Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Compl. Alt. Med. 2015, 15, 156. [Google Scholar] [CrossRef]
- Bodini, R.B.; Sobral, P.J.A.; Favaro-Trindade, C.S.; Carvalho, R.A. Properties of gelatin-based films with added ethanol–propolis extract. LWT-Food Sci. Technol. 2011, 51, 104–110. [Google Scholar] [CrossRef]
- Dias, L.G.; Pereira, A.N.; Estevinho, L.M. Comparative study of different Portuguese samples of propolis: Pollinic, sensorial, physicochemical, microbiological characterization and antibacterial activity. Food Chem. Toxicol. 2012, 50, 4246–4253. [Google Scholar] [CrossRef] [PubMed]
- Grange, J.M.; Davey, R.W. Antibacterial properties of propolis (bee glue). J. Roy. Soc. Med. 1990, 83, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, C.S.; Kim, B.H.; Ro, S.B.; Lim, Y.K.; Park, S.N.; Cho, E.; Ko, J.H.; Kwon, S.S.; Ko, Y.M.; et al. Antimicrobial effect of Korean propolis against the mutans streptococci isolated from Korean. J. Microbiol. 2011, 49, 161–164. [Google Scholar] [CrossRef]
- Mascheroni, E.; Figoli, A.; Musatti, A.; Limbo, S.; Drioli, E.; Suevo, R.; Talarico, S.; Rollini, M. An alternative encapsulation approach for production of active chitosan-propolis beads. Int. J. Food Sci. Technol. 2014, 49, 1401–1407. [Google Scholar] [CrossRef]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food Agric. 2011, 91, 2363–2370. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W.; Sanguandeekul, R. Antioxidant and antimicrobial properties of Thai propolis extracted using ethanol aqueous solution. Int. J. Food Sci. Technol. 2013, 48, 22–27. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Inhibition of Alicyclobacillus acidoterrestris spores by natural compounds. Int. J. Food Sci Technol. 2008, 43, 1271–1275. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Ficelo, S.; Corbo, M.R.; Sinigaglia, M. Bioactivity of grapefruit extract against Pseudomonas spp. J. Food Process. Preserv. 2010, 34, 495–507. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Perricone, M.; Cannarsi, M.; Corbo, M.R.; Sinigaglia, M. Technological and spoiling characteristics of the yeast microflora isolated from Bella di Cerignola table olives. Int. J. Food Sci. Technol. 2009, 44, 2198–2207. [Google Scholar] [CrossRef]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. INT. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef]
- Sinigaglia, M.; Corbo, M.R.; Ciccarone, C. Influence of temperature, pH and water activity on ‘‘in vitro’’ inhibition of Penicillium glabrum (Wehmer) Westling by yeasts. Microbiol. Res. 1998, 153, 137–143. [Google Scholar] [CrossRef]
- Dantigny, P.; Nanguy, S.P.-M.; Judet-Correia, D.; Bensoussan, M. A new model for germination of fungi. Int. J. Food Microbiol. 2011, 146, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Mutlu Sariguzel, F.; Berk, E.; Koc, A.N.; Sav, H.; Demir, G. Antifungal activity of propolis against yeasts isolated from blood culture: In vitro evaluation. J. Clin. Lab. Anal. 2016, 30, 513–516. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial activities of european propolis collected from various geographic origins alone and in combination with antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Andreani, A.A.; Fasolato, L. Pseudomonas and related genera. In The Microbiological Quality of Food. Foodborne Spoilers; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 25–59. [Google Scholar]
- Perricone, M.; Gallo, M.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Yeasts. In The Microbiological Quality of food. Foodborne Spoilers; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 121–132. [Google Scholar]
- Nwakanma, C.; Unachukwu, M. Molds. In The Microbiological Quality of Food. Foodborne Spoilers; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 133–150. [Google Scholar]
- Bevilacqua, A.; Campaniello, D.; Sinigaglia, M.; Ciccarone, C.; Corbo, M.R. Sodium benzoate and citrus extract increase the effect of homogenization towards spores of Fusarium oxysporum in pineapple juice. Food Control 2012, 28, 199–204. [Google Scholar] [CrossRef]
- De Castro, P.A.; Savoldi, M.; Bonatto, D.; Barros, M.H.; Goldman, M.H.; Berretta, A.A.; Goldman, G.H. Molecular characterization of propolis-induced cell death in Saccharomyces cerevisiae. Eukaryot. Cell 2011, 10, 398–411. [Google Scholar] [CrossRef]
- Sidra, Z.T. Propolis effect of the Iraqi (Iraqi propolis) in some types of yeasts that cause damage to fruit juices. DJPS 2010, 6, 227–239. [Google Scholar]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Zokaityte, E.; Dauksiene, A.; Jagminas, P.; Klupsaite, D.; Bliznikas, S.; Ruzauskas, M. Variations of the antimicrobial, antioxidant, sensory attributes and biogenic amines content in Lithuania-derived bee products. LWT-Food Sci. Technol. 2020, 118, 108793. [Google Scholar] [CrossRef]
- Daikh, A.; Segueni, N.; Dogan, N.M.; Arslan, S.; Mutlu, D.; Kivrak, I.; Akkal, S.; Rhouati, S. Comparative study of antibiofilm, cytotoxic activity and chemical composition of Algerian propolis. J. Apicult. Res. 2020, 59, 2. [Google Scholar] [CrossRef]
- de Mélo Silva, I.S.; do Amorim Costa Gaspar, L.M.; Rocha, A.M.O.; da Costa, L.P.; Tada, D.B.; Franceschi, E.; Padilha, F.F. Encapsulation of red propolis in polymer nanoparticles for the destruction of pathogenic biofilms. AAPS PharmSciTech 2010, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Letullier, C.; Manduchet, A.; Dlalah, N.; Hugou, M.; Georgé, S.; Sforcin, J.M.; Cardinault, N. Comparison of the antibacterial efficiency of propolis samples from different botanical and geographic origins with and without standardization. J. Apicult. Res. 2020, 59, 1. [Google Scholar] [CrossRef]
- Seidel, V.; Peyfoon, E.; Watson, D.G.; Fearnley, J. Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother. Res. 2008, 22, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef]
- Speranza, B.; Corbo, M.R. Essential oils for preserving perishable foods: Possibilities and limitations. In Application of Alternative Food Preservation Technologies to Enhance Food Safety and Stability; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Bentham Science: Sharjah, United Arab Emirates, 2010; pp. 35–57. [Google Scholar]
- Meto, A.; Colombari, B.; Meto, A.; Boaretto, G.; Pinetti, D.; Marchetti, L.; Benvenuti, S.; Pellati, F.; Blasi, E. Propolis affects Pseudomonas aeruginosa growth, biofilm formation, eDNA release and phenazine production: Potential involvement of polyphenols. Microorganisms 2020, 8, 243. [Google Scholar] [CrossRef]
- Speranza, B.; Racioppo, A.; Sinigaglia, M.; Corbo, M.R.; Bevilacqua, A. Use of Central Composite Design in food microbiology: A case study on the effects of secondary phenolis on lactic acid bacteria from olives. Int. J. Food Sci. Nutr. 2015, 66, 520–525. [Google Scholar] [CrossRef]
- Ôzcan, M. Antifungal properties of propolis. Grasas Aceites 1999, 50, 395–398. [Google Scholar] [CrossRef]
- Ahmed, S.D.; Mohanad, A.K.; Zaid, N.H. Study antifungal activity of ethanol extract propolis against Fusarium oxysporum fungi. J. Res. Diyala Humanit. 2008, 31, 93–105. [Google Scholar]
- Shehu, A.; Ismail, S.; Rohin, M.A.K.; Harun, A.; Aziz, A.A.; Haque, M. Antifungal Properties of Malaysian tualang honey and stingless bee propolis against Candida albicans and Cryptococcus neoformans. J. Appl. Pharm. 2016, 6, 44–50. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef]
- Maskeroni, E.; Guillard, V.; Nalin, F.; Mora, L.; Piergiovanni, L. Diffusivity of propolis compounds in polylactic acid polymer for the development of anti-microbial packaging films. J. Food Eng. 2010, 98, 294–301. [Google Scholar] [CrossRef]
- Rizzolo, A.; Bianchi, G.; Povolo, M.; Migliori, C.A.; Contarini, G.; Pelizzola, V.; Cattaneo, T.M.P. Volatile compound composition and antioxidant activity of cooked ham slices packed in propolis-based active packaging. Food Packag. Shelf Life 2016, 8, 41–49. [Google Scholar] [CrossRef]




| Target | Source |
|---|---|
| Pseudomonas putida (PSE8) | Wild strain isolated from mozzarella cheese |
| Pseudomonas fluorescens (PSE5) | Wild strain isolated from mozzarella cheese |
| Hafnia alvei (COL8) | Wild strain isolated from mozzarella cheese |
| Enterobacter spp. (COL9) | Wild strain isolated from mozzarella cheese |
| Lactobacillus plantarum (L12) | Wild strain isolated from sourdough |
| Lactobacillus plantarum DSM1055 * | Collection strain |
| Debaryomyces hansenii DSM3428 * | Collection strain |
| Saccharomyces cerevisiae EC1118 ** | Commercial wine strain |
| Fusarium oxysporum DSM2018 * | Collection strain |
| Microorganism | Time | δ | p | Δ25 | R2 |
|---|---|---|---|---|---|
| D. hansenii | 48 h | 0.94 ± 0.11 | 0.65 ± 0.09 | 23.50 | 0.935 |
| S. cerevisiae | 24 h | 645.73 ± 69.04 | 9.20 ± 2.10 | /† | 0.977 |
| 48 h | - | - | - | - | |
| Ps. fluorescens | 48 h | 3.07 ± 0.22 | 0.74 ± 0.05 | 76.75 | 0.909 |
| Ps. putida | 48 h | 7.44 ± 1.25 | 0.84 ± 0.01 | 186.00 | 0.962 |
| H. alvei | 48 h | 0.01 ± 0.01 | 0.29 ± 0.01 | <10 | 0.990 |
| Enterobacter sp. | 48 h | 0.04 ± 0.01 | 0.42 ± 0.03 | <10 | 0.941 |
| Propolis Amount | τ | R2 |
|---|---|---|
| 0 (control) | 4.98 ± 0.44a | 0.985 |
| 1000 ppm | 7.20 ± 0.11b | 0.997 |
| 1500 ppm | 8.40 ± 0.06c | 0.999 |
| 2000 ppm | 8.33 ± 0.08c | 0.998 |
| 2500 ppm | 8.42 ± 0.08c | 0.998 |
| 3000 ppm | 8.29 ± 0.09c | 0.997 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruzzi, L.; Rosaria Corbo, M.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods 2020, 9, 559. https://doi.org/10.3390/foods9050559
Petruzzi L, Rosaria Corbo M, Campaniello D, Speranza B, Sinigaglia M, Bevilacqua A. Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods. 2020; 9(5):559. https://doi.org/10.3390/foods9050559
Chicago/Turabian StylePetruzzi, Leonardo, Maria Rosaria Corbo, Daniela Campaniello, Barbara Speranza, Milena Sinigaglia, and Antonio Bevilacqua. 2020. "Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi" Foods 9, no. 5: 559. https://doi.org/10.3390/foods9050559
APA StylePetruzzi, L., Rosaria Corbo, M., Campaniello, D., Speranza, B., Sinigaglia, M., & Bevilacqua, A. (2020). Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods, 9(5), 559. https://doi.org/10.3390/foods9050559