Influence of Alkaline Treatment on Structural Modifications of Chlorophyll Pigments in NaOH—Treated Table Olives Preserved without Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Preparation of Samples
2.2. Chemicals and Standards
2.3. Pigment Extraction
2.4. Pigment Analysis by HPLC
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- López-López, A.; Montaño, A.; Garrido–Fernández, A. Nutrient profiles of commercial table olives: Proteins and vitamins. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2010; Volume Chapter 74, pp. 705–714. [Google Scholar]
- López–López, A.; Montaño, A.; Garrido–Fernández, A. Nutrient profiles of commercial table olives: Fatty acids, sterols, and fatty alcohols. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2010; Volume Chapter 75, pp. 715–724. [Google Scholar]
- Boskou, D.; Camposeo, S.; Clodoveo, M.L. Table olives as sources of bioactive compounds. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Urbana, IL, USA, 2015; Chapter 8; pp. 217–259. [Google Scholar]
- Charoenprasert, S.; Mitchel, A. Factors influencing phenolic compounds in table olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef]
- Gandul–Rojas, B.; Roca, M.; Gallardo–Guerrero, L. Chlorophylls and carotenoids in food products from olive tree. In Products from Olive Tree; Boskou, D., Ed.; InTech: Rijeka, Croatia, 2016; Volume Chapter 5, pp. 67–97. ISBN 978-953-51-4806-7. [Google Scholar] [CrossRef] [Green Version]
- Romero, C.; García, A.; Medina, E.; Ruíz–Méndez, M.V.; de Castro, A.; Brenes, M. Triterpenic acids in table olives. Food Chem. 2010, 118, 670–674. [Google Scholar] [CrossRef]
- Fundación Dieta Mediterránea. Mediterranean Diet Pyramid: A lifestyle for Today. Available online: https://dietamediterranea.com/piramidedm/piramide_INGLES.pdf. (accessed on 1 April 2020).
- IOC (International Olive Council). World Olive Oil and Table Olives Figures [Internet]. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 1 April 2020).
- Rejano, L.; Montaño, A.; Casado, F.J.; Sánchez, A.H.; de Castro, A. Table olives: Varieties and variations. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2010; Volume Chapter 1, pp. 5–15. [Google Scholar]
- Sánchez–Gómez, A.H.; García–García, P.; Rejano–Navarro, L. Elaboration of table olives. Grasas y Aceites 2006, 57, 86–94. [Google Scholar] [CrossRef] [Green Version]
- IOC (International Olive Oil Council). Trade Standard Applying to Table Olives. Standard COI/OT/NC. No.1/2004. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 1 April 2020).
- Rejano Navarro, L.; Sánchez Gómez, A.H.; Vega Macías, V. New trends on the alkaline treatment–cocido– of Spanish or Sevillian Style green table olives. Grasas y Aceites 2008, 59, 197–204. [Google Scholar]
- Lanza, B. Nutritional and sensory quality of table olives. In Olive Germplasm–The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; Muzzalupo, I., Ed.; IntechOpen: Rijeka, Croatia, 2012; Volume Chapter 16, p. 2840. Available online: https://www.intechopen.com/books/olive-germplasm-the-olive-cultivation-table-olive-and-olive-oil-industry-in-italy/nutritional-and-sensory-quality-of-table-olives (accessed on 1 April 2020). [CrossRef] [Green Version]
- Catania, P.; Alleri, M.; Martorana, A.; Settanni, L.; Moschetti, G.; Vallone, M. Investigation of a tunnel pasteurizer for “Nocellara del Belice” table olives processed according to the “Castelvetrano method”. Grasas y Aceites 2014, 65, e049. [Google Scholar]
- De Lorenzo, C.; González, M.; Iglesias, G.; Lázaro, E.; Valiente, E.; Blázquez, N. La aceituna de Campo Real; Consejería de Medio Ambiente, Ed.; Dirección General de Educación y Protección Ambiental: Madrid, España, 2000; Volume Chapter 3, pp. 25–37. [Google Scholar]
- Luh, B.S.; Ferguson, L.; Kader, A.; Barret, D. Processing California olives. In Olive Production Manual; Sibbett, G.S., Ferguson, L., Eds.; University of California Agriculture and Natural Resources: Davis, CA, USA, 2005; pp. 145–153, Publication 3353. [Google Scholar]
- Brenes, M.; García, P. Elaboración de aceitunas denominadas “Green ripe olives” con variedades españolas. Grasas y Aceites 2005, 56, 188–191. [Google Scholar]
- Bianchi, G. Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 2003, 105, 229–242. [Google Scholar] [CrossRef]
- Jiménez, A.; Guillén, R.; Sánchez, C.; Fernández–Bolaños, J.; Heredia, A. Changes in the texture and cell wall polysaccharides of olive fruit during ‘Spanish green olive’ processing. J. Agric. Food Chem. 1995, 43, 2240–2246. [Google Scholar] [CrossRef]
- Jaramillo Carmona, S.; de Castro, A.; Rejano Navarro, L. Traditional process of green table olives. Rationalization of alkaline treatment. Grasas y Aceites 2011, 62, 375–382. [Google Scholar] [CrossRef] [Green Version]
- García–Serrano, P.; Sánchez, A.H.; Romero, C.; García–García, P.; de Castro, A.; Brenes, M. Processing of table olives with KOH and characterization of the wastewaters as potential fertilizer. Sci. Total Environ. 2019, 676, 834–839. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; de Castro, A.; Brenes, M.; García, A. Inhibitors of lactic acid fermentation in Spanish–style green olive brines of the Manzanilla variety. Food Chem. 2008, 110, 932–937. [Google Scholar] [CrossRef]
- de Castro, A.; Sánchez, A.H.; Cortés–Delgado, A.; López–López, A.; Montaño, A. Effect of Spanish–style processing steps and inoculation with Lactobacillus pentosus starter culture on the volatile composition of cv. Manzanilla green olives. Food Chem. 2019, 271, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Alexandraki, V.; Georgalaki, M.; Papadimitriou, K.; Anastasiou, R.; Zoumpopoulou, G.; Chatzipavlidis, I.; Papadelli, M.; Vallis, N.; Moschochoritis, K.; Tsakalidou, E. Determination of triterpenic acids in natural and alkaline–treated Greek table olives throughout the fermentation process. LWT Food Sci. Technol. 2014, 58, 609–613. [Google Scholar] [CrossRef]
- García, P.; Romero, C.; Brenes, B. Bioactive substances in black ripe olives produced in Spain and the USA. J. Food Comp. Anal. 2018, 66, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, E.; Gandul–Rojas, B.; Romero, C.; Brenes, M.; Gallardo–Guerrero, L. Composition of pigments and colour changes in green table olives related to processing type. Food Chem. 2015, 66, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Gandul–Rojas, B.; Gallardo–Guerrero, L. Pigment changes during processing of green table olive specialities treated with alkali and without fermentation. Food Res. Int. 2014, 65, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Mínguez–Mosquera, M.I.; Garrido–Fernández, J.; Gandul–Rojas, B. Quantification of pigments in fermented manzanilla and hojiblanca olives. J. Agric. Food Chem. 1990, 38, 1662–1666. [Google Scholar] [CrossRef]
- Mínguez–Mosquera, M.I.; Gandul–Rojas, B.; Mínguez–Mosquera, J. Mechanism and kinetic of the degradation of chlorophylls during the processing of green table olives. J. Agric. Food Chem. 1994, 42, 1089–1095. [Google Scholar] [CrossRef]
- Mínguez–Mosquera, M.I.; Gandul–Rojas, B. Mechanism and kinetic of the degradation of carotenoids during the processing of green table olives. J. Agric. Food Chem. 1994, 42, 1551–1554. [Google Scholar] [CrossRef]
- Mínguez–Mosquera, M.I.; Gallardo–Guerrero, L. Anomalous transformation of chloroplastic pigments in Gordal variety olives during processing for table olives. J. Food Prot. 1995, 58, 1241–1248. [Google Scholar] [CrossRef]
- Gandul–Rojas, B.; Gallardo–Guerrero, L. Pigment changes during preservation of green table olive specialities treated with alkali and without fermentation: Effect of thermal treatments and storage conditions. Food Res. Int. 2018, 108, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Aparicio–Ruiz, R.; Riedl, K.M.; Schwartz, S.J. Identification and quantification of metallo–chlorophyll complexes in bright green table olives by high–performance liquid chromatography–mass spectrometry quadrupole/time–of–flight. J. Agric. Food Chem. 2011, 59, 11100–11108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandul–Rojas, B.; Roca, M.; Gallardo–Guerrero, L. Detection of the colour adulteration of green table olives with copper chlorophyllin complexes (E–141ii colorant). LWT Food Sci. Technol. 2012, 46, 311–318. [Google Scholar] [CrossRef]
- Negro, C.; De Bellis, L.; Sabella, E.; Nutricati, E.; Luvisi, A.; Micelli, A. Detection of not allowed food-coloring additives (copper chlrophyllin-copper sulfate) in green table olives sold on the Italian market. Adv. Hort. Sci. 2017, 31, 225–233. [Google Scholar]
- Petigara Harp, B.; Scholl, P.F.; Gray, P.J.; Delmonte, P. Quantitation of copper chlorophylls in green table olives by ultra-high-performance liquid chromatography with inductively coupled plasma isotope dilution mass spectrometry. J. Chromatogr. A 2020, 1620, 461008. [Google Scholar] [CrossRef]
- Schiedt, H.; Liaaen–Jensen, S. Isolation and analysis. In Carotenoids. Isolation and Analysis; Britton, G., Liaaen–Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1995; Volume 1A, pp. 81–108. [Google Scholar]
- Rincón–Llorente, B.; De la Lama–Calvente, D.; Fernández–Rodríguez, M.J.; Borja–Padilla, R. Table olive waste water: Problem, treatments and future strategy. A review. Front. Microbiol. 2018, 9, 1641. [Google Scholar] [CrossRef] [Green Version]
- Gallardo–Guerrero, L.; Gandul–Rojas, B.; Mínguez–Mosquera, M.I. Physico–chemical conditions modulating the pigment profile in fresh fruit (Olea europaea var. Gordal) and favoring interaction between oxidized chlorophylls and endogenous Cu. J. Agric. Food Chem. 2007, 55, 1823–1831. [Google Scholar] [CrossRef]
- Mínguez–Mosquera, M.I.; Gandul–Rojas, B.; Gallardo–Guerrero, L.; Roca, M.; Jarén–Galán, M. Chlorophylls. In Methods of Analysis for Functional Foods and Nutraceuticals, 2nd ed.; Hurst, W.J., Ed.; CRC Press, LLC: Boca Raton, FL, USA, 2008; Volume Chapter 7, pp. 337–400. [Google Scholar]
- Pérez–Gálvez, A.; Viera, I.; Roca, M. Chemistry in the bioactivity of chlorophylls: An overview. Curr. Med. Chem. 2017, 24, 4515–4536. [Google Scholar]
- Gandul–Rojas, B.; Roca, M.; Mínguez–Mosquera, M.I. Chlorophyll and carotenoid degradation mediated by thylakoid–associated peroxidative activity in olives (Olea europaea) cv. Hojiblanca. J. Plant Physiol. 2004, 161, 499–507. [Google Scholar] [CrossRef]
- Sievers, G.; Hynninen, P.H. Thin–layer chormatography of chlorophyll and their derivatives on cellulose layers. J. Chromatogr. 1977, 134, 359–364. [Google Scholar] [CrossRef]
- Mínguez–Mosquera, M.I.; Garrido–Fernández, J. Chlorophyll and carotenoid presence in olive fruit (Olea europaea, L.). J. Agric. Food Chem. 1989, 37, 1–7. [Google Scholar] [CrossRef]
- Mínguez–Mosquera, M.I.; Gandul–Rojas, B.; Montaño–Asquerino, A.; Garrido–Fernández, J. Determination of chlorophylls and carotenoids by high–performance liquid chromatography during olive lactic fermentation. J. Chromatogr. 1991, 585, 259–266. [Google Scholar] [CrossRef]
- Mínguez–Mosquera, M.I.; Gandul–Rojas, B. High–performance liquid chromatographic study of alkaline treatment of chlorophyll. J. Chromatogr. A 1995, 690, 161–176. [Google Scholar] [CrossRef]
- Schwartz, S.J.; Lorenzo, T.V. Chlorophylls in foods. Crit. Rev. Food Sci. Nutr. 1990, 29, 1–17. [Google Scholar] [CrossRef]
- Tarrado–Castellarnau, M.; Domínguez–Ortega, J.M.; Tarrado–Castellarnau, A.; Pleite–Gutiérrez, R. Study of the heat transfer during the alkaline treatment in the processing of Spanish style green table olives. Grasas y Aceites 2013, 64, 415–424. [Google Scholar]
- Louda, J.W.; Mongkhonsri, P.; Bake, E.W. Chlorophyll degradation during senescence and death-III: 3–10 yr experiments, implications for ETIO series generation. Org. Geochem. 2011, 42, 688–699. [Google Scholar] [CrossRef]
- Viera, I.; Chen, K.; Ríos, J.J.; Benito, I.; Pérez-Galvez, A.; Roca, M. First-pass metabolism of chlorophylls in mice. Mol. Nutr. Food Res. 2018, 62, 1800562. [Google Scholar] [CrossRef]
- Mínguez–Mosquera, M.I.; Gandul–Rojas, B.; Gallardo–Guerreo, L. Measurement of chlorophyllase activity in olive fruit (Olea europaea). J. Biochem. 1994, 116, 263–268. [Google Scholar] [CrossRef]
- Lee, G.C.; Chepyshko, H.; Chen, H.H.; Chu, C.C.; Chou, Y.F.; Akoh, C.C.; Shaw, J.F. Genes and biochemical characterization of three novel chlorophyllase isozymes from brassica oleracea. J. Agric. Food Chem. 2010, 5, 8651–8657. [Google Scholar] [CrossRef]



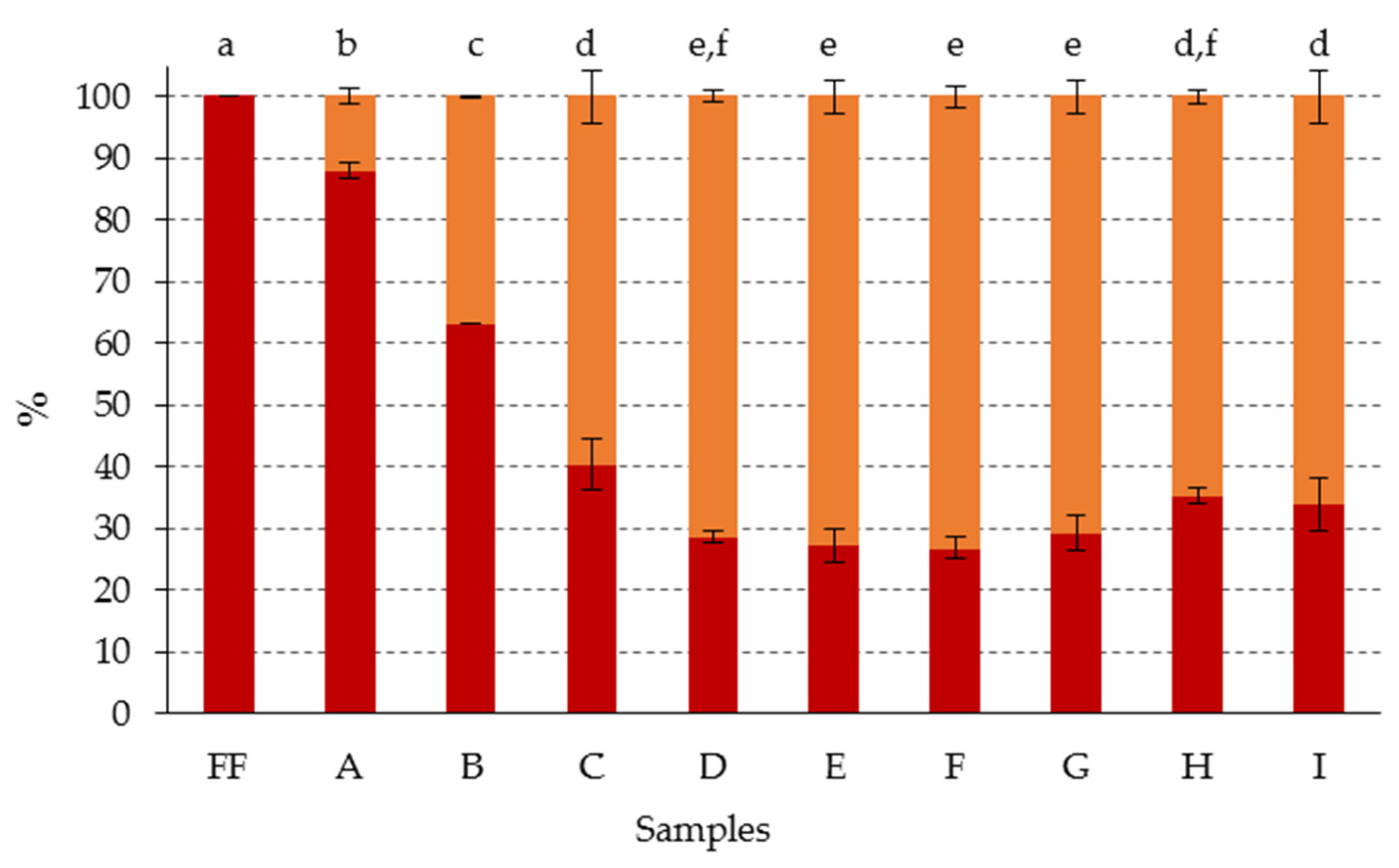
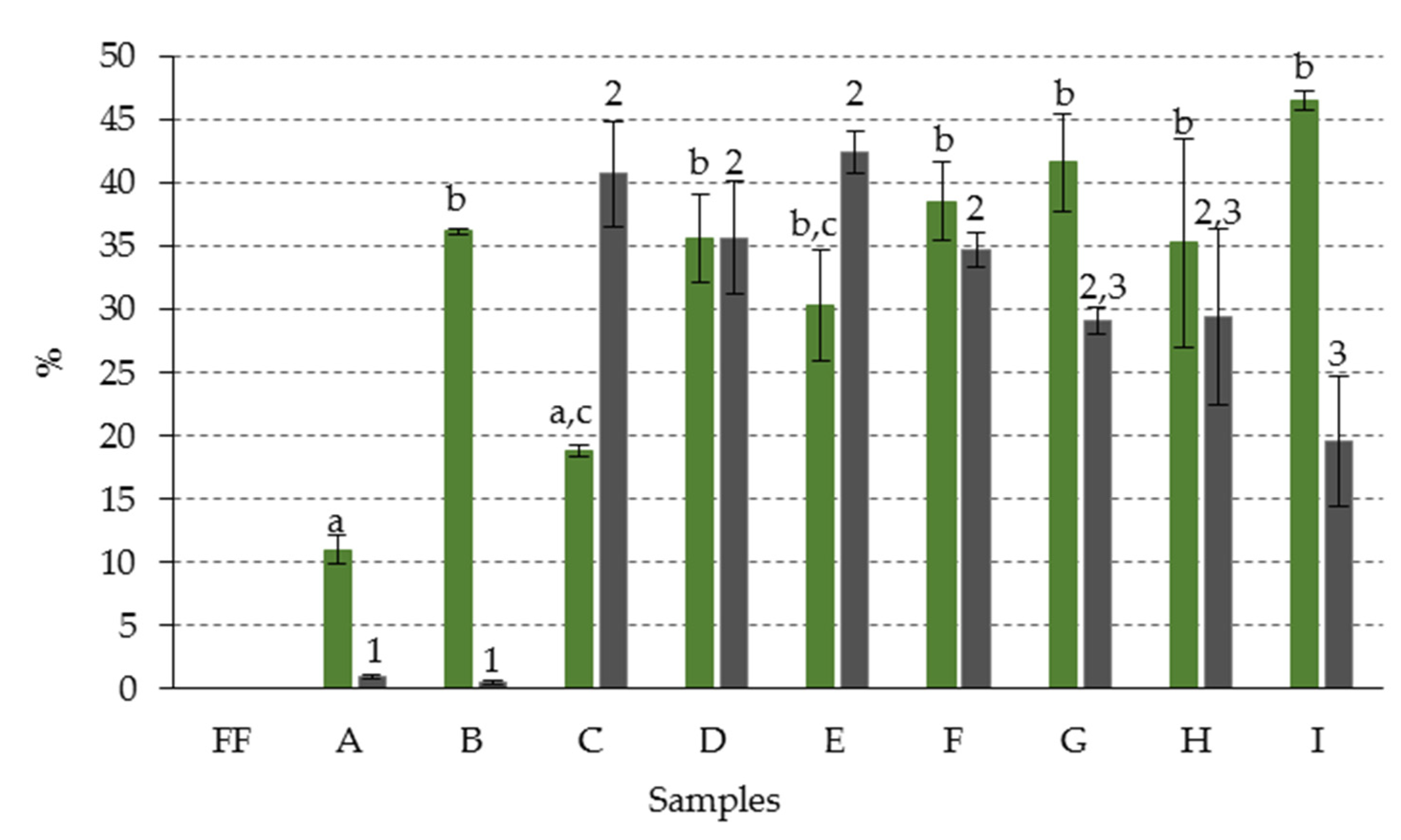


| Sample Code | A | B | C | D | E | F | G | H | I |
|---|---|---|---|---|---|---|---|---|---|
| [NaOH] (%w/v) | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Time (h) | 7 | 6 | 5 | 4 | 3 | 3 | 3 | 3 | 3 |
| Pigments | Peak | tr 1 | kc’ 2 | Spectroscopic Characteristics | |
|---|---|---|---|---|---|
| Absorption Maxima (nm) | |||||
| No | Soret | Q 3 | |||
| Series a | |||||
| Pheophorbide a | 1 | 7.6 | 2.7 | 410 | 666 |
| Pyropheophorbide a | 2 | 8.9 | 3.3 | 410 | 666 |
| Mg-152-Me-phytyl-chlorin e6 ester | 5 | 14.9 | 6.2 | 416 | 638 |
| 152-Me-phytyl-chlorin e6 ester | 6 | 15.1 | 6.3 | 400 | 662 |
| Chlorophyll a | 9 | 18.8 | 8.1 | 432 | 666 |
| Chlorophyll a’ | 9’ | 19.6 | 8.5 | 432 | 666 |
| 132-OH-chlorophyll a | 10 | 20.2 | 8.8 | 434 | 664 |
| Mg-152-Me-phytyl-isochlorin e4 ester | 11 | 21.0 | 9.2 | 416 | 638 |
| 152-Me-phytyl-isochlorin e4 ester | 13 | 26.1 | 11.7 | 400 | 662 |
| Pheophytin a | 14 | 26.3 | 11.8 | 410 | 666 |
| Pheophytin a’ | 14’ | 26.8 | 12.0 | 410 | 666 |
| Pyropheophytin a | 15 | 29.6 | 13.4 | 410 | 666 |
| Series b | |||||
| Mg-152-Me-phytyl-rhodin g7 ester | 3 | 13.1 | 5.4 | 450 | 626 |
| 152-Me-phytyl-rhodin g7 ester | 4 | 14.0 | 5.8 | 426 | 650 |
| Chlorophyll b | 7 | 16.4 | 7.0 | 466 | 650 |
| Chlorophyll b’ | 7’ | 16.9 | 7.2 | 466 | 650 |
| 132-OH-chlorophyll b | 8 | 17.6 | 7.5 | 466 | 646 |
| Pheophytin b | 12 | 24.0 | 10.7 | 436 | 654 |
| Pigment | Fresh Fruit | Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | ||
| Chlorophyll a | 223.70 ± 13.17 | 115.60 ± 3.50 | 30.74 ± 5.43 | 5.10 ± 1.41 | 0.32 ± 0.08 | 0.56 ± 0.04 | 0.66 ± 0.02 | 0.34 ± 0.06 | n. q. | n. q. |
| Chlorophyll a’ | 0.82 ± 0.07 | 31.95 ± 1.93 | 7.84 ± 1.52 | 0.70 ± 0.28 | 0.56 ± 0.34 | 1.34 ± 0.95 | 1.06 ± 0.08 | 1.78 ± 0.08 | n. q. | n. q. |
| 132-OH-chlorophyll a | 0.43 ± 0.06 | n. q. | n. q. | n. q. | 0.70 ± 0.63 | n. q. | 1.96 ± 0.28 | n. q. | 1.36 ± 0.97 | n. q. |
| Mg-152-Me-phytyl-chlorin e6 ester | 1.46 ± 0.13 | 0.41 ± 0.29 | n. q. | 7.89 ± 0.43 | 7.64 ± 3.04 | 16.18 ± 3.52 | 14.22 ± 0.64 | 6.70 ± 0.46 | 6.78 ± 1.28 | |
| Mg-152-Me-phytyl-isochlorin e4 ester | 2.91 ± 0.39 | 1.19 ± 0.35 | 3.54 ± 2.22 | 9.87 ± 1.07 | 11.68 ±2.62 | 14.80 ± 2.50 | 10.88 ± 1.58 | 5.64 ± 6.06 | 1.82 ± 1.29 | |
| Pheophytin a | 2.74 ± 0.25 | 49.43 ± 5.66 | 58.86 ± 1.10 | 4.36 ± 0.73 | 1.83 ± 0.22 | 1.84 ± 0.23 | 0.97 ± 0.22 | 1.83 ± 0.46 | 2.14 ± 0.44 | 1.00 ± 0.41 |
| Pheophytin a’ | 11.52 ± 0.81 | 12.73 ± 1.18 | 2.69 ± 0.30 | n. q. | n. q. | n. q. | n. q. | n. q. | n. q. | |
| Pyropheophytin a | 7.30 ± 0.34 | 26.44 ± 2.08 | 23.87 ± 0.73 | 18.82 ± 0.06 | 23.00 ± 1.94 | 18.74 ± 0.28 | 15.46 ± 1.04 | 9.94 ± 0.78 | 7.04 ± 0.40 | |
| 152-Me-phytyl-chlorin e6 ester | 19.94 ± 1.44 | 64.72 ± 1.78 | 13.45 ± 1.49 | 15.26 ± 1.56 | 17.54 ± 2.28 | 14.32 ± 2.00 | 11.96 ± 1.28 | 5.66 ± 1.62 | 5.80 ± 0.94 | |
| 152-Me-phytyl-isochlorin e4 ester | n. q. | n. q. | 34.94 ± 5.83 | 19.70 ± 2.70 | 34.16 ± 9.56 | 18.84 ± 0.66 | 11.58 ± 0.72 | 6.56 ± 1.92 | 5.10 ± 0.60 | |
| Pheophorbide a | 0.20 ± 0.03 | n. q. | 0.49 ± 0.12 | n. q. | n. q. | n. q. | n. q. | n. q. | n. q. | n. q. |
| Pyropheophorbide a | n. q. | 1.83 ± 0.37 | n. q. | 0.76 ± 0.44 | 0.44 ± 0.08 | 0.86 ± 0.48 | 1.60 ± 0.00 | 0.78 ± 0.24 | 3.98 ± 0.98 | |
| Total series a | 227.90 ± 6.44 | 240.10 ± 2.52 | 205.25 ± 2.06 | 88.65 ± 2.36 | 75.72 ± 2.60 | 98.20 ± 4.98 | 88.39 ± 1.62 | 69.65 ± 0.89 | 38.78 ± 2.37 | 31.52 ± 0.91 |
| Chlorophyll b | 52.99 ± 3.88 | 33.46 ± 1.92 | 11.14 ± 0.28 | 0.95 ± 0.05 | n. q. | 1.24 ± 0.60 | n. q. | n. q. | n. q. | n. q. |
| Chlorophyll b’ | 0.97 ± 0.07 | 13.11 ± 0.39 | 5.20 ± 1.31 | 0.62 ± 0.11 | n. q. | n. q. | 0.20 ± 0.30 | n. q. | n. q. | n. q. |
| 132-OH-chlorophyll b | n. q. | n. q. | n. q. | n. q. | 1.22 ± 0.40 | 1.22 ± 0.42 | 1.60 ± 0.74 | 1.56 ± 0.02 | 0.48 ± 0.02 | n. q. |
| Mg-152-Me-phytyl-rhoding7 ester | 9.91 ± 0.64 | 11.85 ± 0.22 | 3.03 ± 0.48 | 6.12 ± 0.94 | 7.62 ± 1.68 | 6.86 ± 1.18 | 5.90 ± 1.02 | 2.26 ± 0.88 | 3.82 ± 0.42 | |
| Pheophytin b | n. q. | 1.01 ± 1.43 | n. q. | n. q. | n. q. | n. q. | n. q. | n. q. | n. q. | |
| 152-Me-phytyl-rhodin g7 ester | 1.44 ± 0.20 | 12.14 ± 1.01 | 1.31 ± 0.04 | 0.24 ± 0.16 | n. q. | n. q. | n. q. | n. q. | n. q. | |
| Total series b | 53.96 ± 2.44 | 57.92 ± 1.04 | 41.34 ± 0.99 | 5.91 ± 0.25 | 7.58 ± 0.60 | 10.08 ± 1.06 | 8.66 ± 0.82 | 7.46 ± 0.72 | 2.74 ± 0.62 | 3.82 ± 0.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlanga-Del Pozo, M.; Gallardo-Guerrero, L.; Gandul-Rojas, B. Influence of Alkaline Treatment on Structural Modifications of Chlorophyll Pigments in NaOH—Treated Table Olives Preserved without Fermentation. Foods 2020, 9, 701. https://doi.org/10.3390/foods9060701
Berlanga-Del Pozo M, Gallardo-Guerrero L, Gandul-Rojas B. Influence of Alkaline Treatment on Structural Modifications of Chlorophyll Pigments in NaOH—Treated Table Olives Preserved without Fermentation. Foods. 2020; 9(6):701. https://doi.org/10.3390/foods9060701
Chicago/Turabian StyleBerlanga-Del Pozo, Marta, Lourdes Gallardo-Guerrero, and Beatriz Gandul-Rojas. 2020. "Influence of Alkaline Treatment on Structural Modifications of Chlorophyll Pigments in NaOH—Treated Table Olives Preserved without Fermentation" Foods 9, no. 6: 701. https://doi.org/10.3390/foods9060701
APA StyleBerlanga-Del Pozo, M., Gallardo-Guerrero, L., & Gandul-Rojas, B. (2020). Influence of Alkaline Treatment on Structural Modifications of Chlorophyll Pigments in NaOH—Treated Table Olives Preserved without Fermentation. Foods, 9(6), 701. https://doi.org/10.3390/foods9060701






