Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Coffee Silverskin and Spent Coffee Ground Sample
2.3. Preparation of CS and SCG Extracts
2.4. Total Phenolic and Flavonoid Content
2.5. Bioactive Compound Quantification by HPLC-MS/MS
2.6. Determination of Antioxidant and Enzyme Inhibitory Effects
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Profile
3.1.1. Total Phenolic and Flavonoid Content
3.1.2. Content of 25 Bioactive Compounds in CS and SCG Extracts
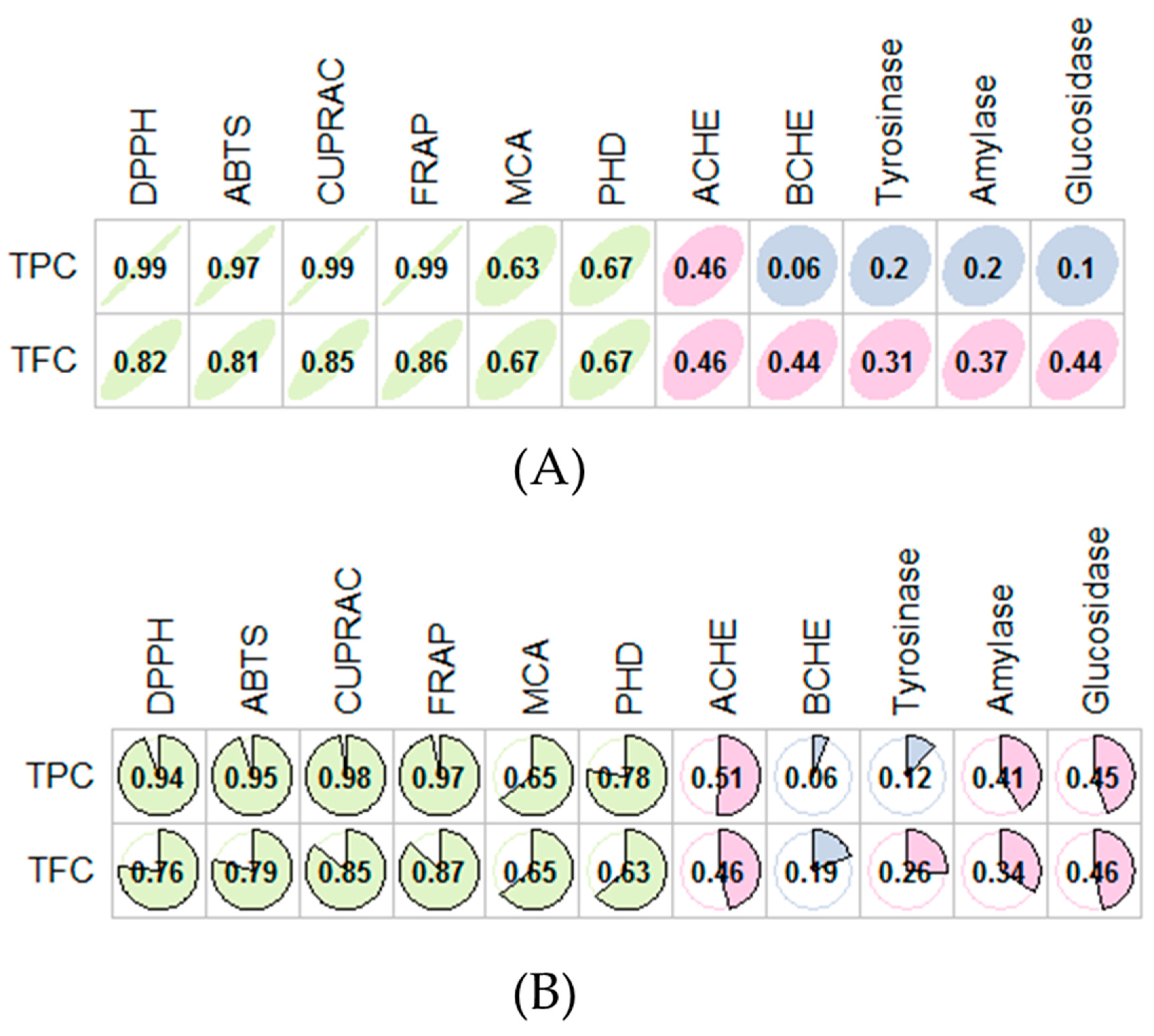
3.2. Antioxidant Capacity
3.3. Enzyme Inhibitory Effects
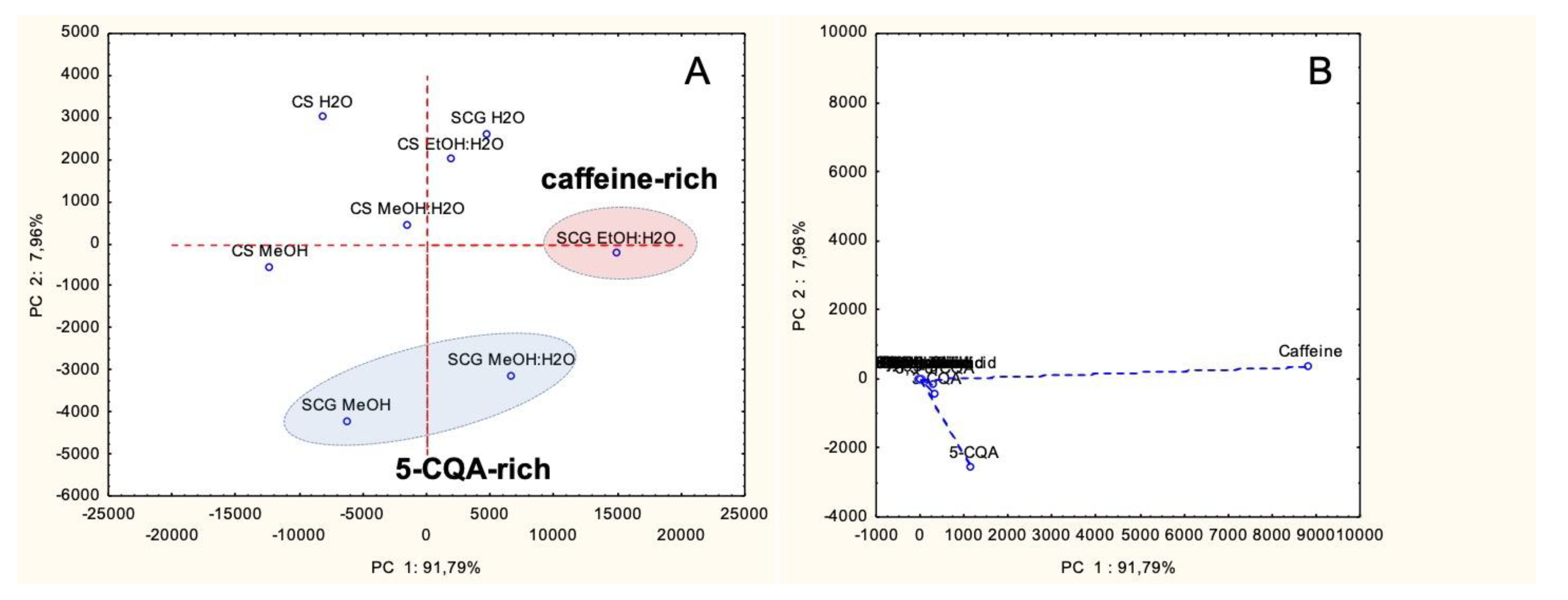
3.4. Discriminant Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef] [PubMed]
- International Coffee Organization (ICO). Statistics. Trade Statistics Tables. World coffee consumption. Available online: http://www.ico.org/prices/new-consumption-table.pdf (accessed on 29 April 2020).
- Iriondo-DeHond, A.; Garcia, N.A.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Escobar, F.V.; Blanch, G.P.; Andres, M.S.; Rodríguez, S.S.-F.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Malara, A.; Paone, E.; Frontera, P.; Bonaccorsi, L.; Panzera, G.; Mauriello, F. Sustainable Exploitation of Coffee Silverskin in Water Remediation. Sustainability 2018, 10, 3547. [Google Scholar] [CrossRef] [Green Version]
- dos Santos Polidoro, A.; Scapin, E.; Lazzari, E.; Silva, A.N.; dos Santos, A.L.; Caramão, E.B.; Jacques, R.A. Valorization of coffee silverskin industrial waste by pyrolysis: From optimization of bio-oil production to chemical characterization by GC× GC/qMS. J. Anal. App. Pyrolysis 2018, 129, 43–52. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Martorell, P.; Genovés, S.; Ramón, D.; Stamatakis, K.; Fresno, M.; Molina, A.; Del Castillo, M.D. Coffee silverskin extract protects against accelerated aging caused by oxidative agents. Molecules 2016, 21, 721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 109128. [Google Scholar] [CrossRef]
- Severini, C.; Caporizzi, R.; Fiore, A.G.; Ricci, I.; Onur, O.M.; Derossi, A. Reuse of spent espresso coffee as sustainable source of fibre and antioxidants. A map on functional, microstructure and sensory effects of novel enriched muffins. LWT 2020, 119, 108877. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recy. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Kamgang Nzekoue, F.; Khamitova, G.; Angeloni, S.; Sempere, A.N.; Tao, J.; Maggi, F.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G. Spent coffee grounds: A potential commercial source of phytosterols. Food Chem. 2020, 126836. [Google Scholar] [CrossRef]
- Bessada, S.M.; Alves, R.C.; Oliveira, P.P.; Beatriz, M. Coffee silverskin: A review on potential cosmetic applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.S.; Mansour, A.F.; Kamil, M.M.; Hussein, A.M. Formulation of nutraceutical biscuits based on dried spent coffee grounds. Int. J. Pharmacol. 2018, 14, 584–594. [Google Scholar] [CrossRef]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Vecchia, S.B.D.; Zeppa, G. Coffee silverskin as nutraceutical ingredient in yogurt: Its effect on functional properties and its bioaccessibility. J. Sci. Food Agri. 2019, 99, 4267–4275. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Huamán-Castilla, N.; Vargas-González, M.; Pedreschi, F.; Pérez-Correa, J.R. The Antioxidant and Safety Properties of Spent Coffee Ground Extracts Impacted by the Combined Hot Pressurized Liquid Extraction–Resin Purification Process. Molecules 2018, 23, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crop. Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Van Camp, J.; Alasalvar, C.; Elmore, S.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids-interaction testing in model solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef]
- Pohanka, M.; Dobes, P. Caffeine inhibits acetylcholinesterase, but not butyrylcholinesterase. Int. J. Mol. Sci. 2013, 14, 9873–9882. [Google Scholar] [CrossRef]
- Orhan, I.E. Enzyme Inhibitors as the Attractive Targets for the Treatment of Various Diseases. Curr. Med. Chem. 2019, 26, 3206–3207. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, S.; Nzekoue, F.K.; Navarini, L.; Sagratini, G.; Torregiani, E.; Vittori, S.; Caprioli, G. An analytical method for the simultaneous quantification of 30 bioactive compounds in Spent Coffee Ground by HPLC-MS/MS. J. Mass Spectrom. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Regazzoni, L.; Saligari, F.; Marinello, C.; Rossoni, G.; Aldini, G.; Carini, M.; Orioli, M. Coffee silver skin as a source of polyphenols: High resolution mass spectrometric profiling of components and antioxidant activity. J. Funct. Foods 2016, 20, 472–485. [Google Scholar] [CrossRef]
- Mirón-Mérida, V.A.; Yáñez-Fernández, J.; Montañez-Barragán, B.; Huerta, B.E.B. Valorization of coffee parchment waste (Coffea arabica) as a source of caffeine and phenolic compounds in antifungal gellan gum films. LWT 2019, 101, 167–174. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Monente, C.; Ludwig, I.A.; Irigoyen, A.; De Penña, M.P.; Cid, C. Assessment of total (free and bound) phenolic compounds in spent coffee extracts. J. Agric. Food Chem. 2015, 63, 4327–4334. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Panda, S.S.; Hall, C.D. Quinine conjugates and quinine analogues as potential antimalarial agents. Eur. J. Med. Chem. 2015, 97, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Caprioli, G.; Ricciutelli, M.; Maggi, F.; Marín, R.; Vittori, S.; Sagratini, G. Comparative HPLC/ESI-MS and HPLC/DAD study of different populations of cultivated, wild and commercial Gentiana lutea L. Food Chem. 2015, 174, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Belhaoues, S.; Amri, S.; Bensouilah, M. Major phenolic compounds, antioxidant and antibacterial activities of Anthemis praecox Link aerial parts. S. Afr. J. Bot. 2020, 131, 200–205. [Google Scholar] [CrossRef]
- Ghafoor, K.; Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Ahmed, I.A.M. Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger (Zingiber officinale) rhizome as affected by drying methods. LWT 2020, 109354. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Camilo, C.J.; Nonato, C.D.F.A.; Rodrigues, F.F.G.; Menezes, I.R.A.; Ribeiro-Filho, J.; Xiao, J.; Souza, M.M.d.A.; da Costa, J.G.M. Influence of seasonal variation on phenolic content and in vitro antioxidant activity of Secondatia floribunda A. DC. (Apocynaceae). Food Chem. 2020, 315, 126277. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med. Sci. Monit. 2003, 9, BR325–BR330. [Google Scholar] [PubMed]
- Ganzon, J.G.; Chen, L.G.; Wang, C.C. 4-O-Caffeoylquinic acid as an antioxidant marker for mulberry leaves rich in phenolic compounds. J. Food Drug Anal. 2018, 26, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Tellone, E.; Galtieri, A.; Giardina, B.; Russo, A.; Bellocco, E.; Barreca, D.; Ficarra, S. Antioxidant Activity of Caffeine: A Focus on Human Red Blood Cells and Correlations with Several Neurodegenerative Disorders. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 835–842. [Google Scholar]
- Hung, T.M.; Na, M.; Thuong, P.T.; Su, N.D.; Sok, D.; Song, K.S.; Seong, Y.H.; Bae, K. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Wang, J.Y.; Nemzer, B. Antioxidant and antiradical activity of coffee. Antioxidants 2013, 2, 230–245. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Sieniawska, E.; Sinan, K.I.; Picot-Allain, M.C.N.; Yerlikaya, S.; Baloglu, M.C.; Altunoglu, Y.C.; Senkardes, I.; Rengasamy, K.R.; Zengin, G. Utilisation of Rhododendron luteum Sweet bioactive compounds as valuable source of enzymes inhibitors, antioxidant, and anticancer agents. Food Chem. Toxicol. 2020, 135, 111052. [Google Scholar] [CrossRef]
- Marwah, R.G.; Fatope, M.O.; Al Mahrooqi, R.; Varma, G.B.; Al Abadi, H.; Al-Burtamani, S.K.S. Antioxidant capacity of some edible and wound healing plants in Oman. Food Chem. 2007, 101, 465–470. [Google Scholar] [CrossRef]
- Uysal, S.; Ugurlu, A.; Zengin, G.; Baloglu, M.C.; Altunoglu, Y.C.; Mollica, A.; Custodio, L.; Neng, N.R.; Nogueira, J.M.; Mahomoodally, M.F. Novel in vitro and in silico insights of the multi-biological activities and chemical composition of Bidens tripartita L. Food Chem Toxicol. 2018, 111, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Fuster, V.; Frazer, J.; Snair, M.; Vedanthan, R.; Dzau, V.; Abbam, G.; Batson, A.; Burkle, F.; Chin, L.; Ferguson, S.; et al. The future role of the United States in global health: Emphasis on cardiovascular disease. J. A. Coll. Cardiol. 2017, 70, 3140–3156. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, D. Global health: Current issues, future trends and foreign policy. Clin. Med. 2009, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.L.; Halim, S.; Gurudas, S.; Sivaprasad, S.; Owens, D.R. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res. Clin. Pract. 2019, 107840. [Google Scholar] [CrossRef]
- Vyas, S.; Kothari, S.L.; Kachhwaha, S. Nootropic Medicinal Plants: Therapeutic Alternatives for Alzheimer’s disease. J. Herb. Med. 2019, 100291. [Google Scholar] [CrossRef]
- Rauf, A.; Jehan, N. Natural products as a potential enzyme inhibitors from medicinal plants. In Enzyme Inhibitors and Activators; InTech: Rijeka, Croatia, 2017; pp. 165–177. [Google Scholar]
- Mishra, P.; Kumar, A.; Panda, G. Anti-cholinesterase hybrids as multi-target-directed ligands against Alzheimer’s disease (1998–2018). Bioorg. Med. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol 2019. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Diabetes mellitus and nature’s pharmacy of putative antidiabetic plants. J. Her. Med. 2019, 15, 100230. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Oh, H.N.; Park, D.H.; Park, J.Y.; Song, S.Y.; Lee, S.H.; Yoon, G.; Moon, H.S.; Oh, D.-S.; Rhee, S.H.; Yoon, I.S.; et al. Tyrosinase Inhibition Antioxidant Effect and Cytotoxicity Studies of the Extracts of Cudrania tricuspidata Fruit Standardized in Chlorogenic Acid. Molecules 2019, 24, 3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioproc. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Conde, T.; Mussatto, S.I. Isolation of polyphenols from spent coffee grounds and silverskin by mild hydrothermal pretreatment. Prep. Biochem. Biotechnol. 2016, 46, 406–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, E.O.; Demirtaş, C.Y.; Paşaoğlu, Ö.T. The effects of caffeine on the renal antioxidant activity in rats/Ratlarda böbrek antioksidan aktivitesi üzerine kafeinin etkileri. Turk. J. Biochem. 2016, 41, 216–222. [Google Scholar] [CrossRef]
- Liu, L.; Nagai, I.; Gao, Y.; Matsushima, Y.; Kawai, Y.; Sayama, K. Effects of catechins and caffeine on the development of atherosclerosis in mice. Biosci. Biotechnol. Biochem. 2017, 81, 1948–1955. [Google Scholar] [CrossRef] [Green Version]
- Bernatova, I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Lai, C.Q.; Liu, D. Dietary Epicatechin, A Novel Anti-aging Bioactive Small Molecule. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]

| Extracts | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg RE/g) | |
|---|---|---|---|
| SCG | EtOH:H2O (70:30) | 93.55 ± 0.65 a | 4.37 ± 0.02 a |
| MeOH | 63.25 ± 0.10 abc | 4.37 ± 0.16 a | |
| MeOH:H2O (50:50) | 93.26 ± 0.14 ab | 3.39 ± 0.27 ab | |
| H2O | 56.86 ± 0.16 abcd | 2.37 ± 0.10 abc | |
| CS | EtOH:H2O (70:30) | 25.34 ± 0.44 cd | 0.18 ± 0.05 c |
| MeOH | 35.68 ± 1.80 bcd | 2.32 ± 0.12 abc | |
| MeOH:H2O (50:50) | 25.02 ± 0.37 d | 0.86 ± 0.08 bc | |
| H2O | 20.49 ± 0.27 d | 0.75 ± 0.06 c |
| Spent Coffee Ground Extracts (µg g−1) | Silverskin Extracts (µg g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Analytes 1 | MeOH | H2O | MeOH:H2O (50:50) | EtOH:H2O (70:30) | MeOH | H2O | MeOH:H2O (50:50) | EtOH:H2O (70:30) |
| 1 | Shikimic acid | n.d.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2 | Gallic acid | 42.45 ± 3.20 | 53.42 ± 4.10 | 51.90 ± 3.95 | 57.47 ± 5.03 | 32.65 ± 2.92 | 38.16 ± 1.58 | 43.25 ± 2.97 | 44.21 ± 3.45 |
| 3 | 5-CQA 2 | 9786.20 ± 815.36 | 4660.85 ± 254.32 | 10,613.61 ± 901.24 | 8620.83 ± 756.31 | 5436.78 ± 278.35 | 2589.36 ± 132.69 | 5896.45 ± 214.35 | 4789.35 ± 297.63 |
| 4 | (+)-Catechin | 1.23 ± 0.10 | 0.85 ± 0.06 | 1.56 ± 0.12 | 1.37 ± 0.10 | n.d. | n.d. | n.d. | n.d. |
| 5 | Del-3,5-diglu 2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 6 | 3-CQA 2 | 3377.82 ± 156.32 | 2091.89 ± 154.78 | 2736.86 ± 203.12 | 3777.65 ± 306.69 | 2598.32 ± 178.35 | 1549.55 ± 115.21 | 2657.15 ± 199.78 | 2698.32 ± 237.78 |
| 7 | Caffeine | 32,838.17 ± 2569.11 | 44,755.37 ± 3251.45 | 45,857.23 ± 4016.98 | 54,440.27 ± 4875.19 | 27,365.14 ± 1023.65 | 31,968.12 ± 1548.47 | 38,214.36 ± 1689.53 | 41,877.13 ± 2356.30 |
| 8 | Cya-3-glu 2 | 1.56 ± 0.12 | 0.93 ± 0.08 | 1.65 ± 0.13 | 1.89 ± 0.15 | n.d. | n.d. | n.d. | n.d. |
| 9 | Vanillic acid | 86.61 ± 5.36 | 95.28 ± 8.21 | 140.38 ± 10.32 | 119.39 ± 9.96 | 96.23 ± 5.68 | 105.87 ± 9.23 | 116.98 ± 9.65 | 132.65 ± 10.65 |
| 10 | Caffeic acid | 67.98 ± 4.12 | 118.03 ± 8.65 | 156.60 ± 9.21 | 204.95 ± 15.45 | 45.32 ± 2.41 | 98.36 ± 5.68 | 142.36 ± 11.32 | 157.65 ± 10.56 |
| 11 | (-)-Epicatechin | n.d. | n.d. | n.d. | n.d. | 123.65 ± 10.63 | n.d. | 102.98 ± 8.65 | n.d. |
| 12 | Syringic acid | 33.65 ± 1.23 | 50.83 ± 4.56 | 50.26 ± 5.21 | 94.20 ± 8.32 | n.d. | 42.36 ± 2.63 | 45.69 ± 3.58 | 85.64 ± 6.82 |
| 13 | p-Coumaric acid | 7.10 ± 0.65 | 10.62 ± 1.03 | 13.30 ± 1.22 | 22.09 ± 1.98 | 5.46 ± 0.36 | 9.65 ± 0.79 | 10.23 ± 0.95 | 15.78 ± 1.03 |
| 14 | Ferulic acid | 55.36 ± 4.32 | 65.31 ± 5.36 | 125.36 ± 9.56 | 136.98 ± 11.54 | 38.69 ± 2.32 | 62.65 ± 4.01 | 97.69 ± 6.41 | 145.32 ± 10.85 |
| 15 | 3,5-diCQA 2 | 879.32 ± 65.32 | 852.36 ± 75.75 | 753.84 ± 54.21 | 1194.62 ± 98.96 | 115.63 ± 11.21 | 125.65 ± 8.95 | 358.97 ± 22.41 | 459.47 ± 49.8 |
| 16 | Quinine | 1.23 ± 0.09 | 1.56 ± 0.12 | 2.12 ± 0.19 | 3.65 ± 0.25 | 0.68 ± 0.02 | 0.71 ± 0.02 | 0.87 ± 0.03 | 0.93 ± 0.03 |
| 17 | Naringin | 0.36 ± 0.02 | 0.52 ± 0.04 | 0.31 ± 0.02 | 0.51 ± 0.03 | n.d. | 0.45 ± 0.03 | n.d. | 0.39 ± 0.02 |
| 18 | Rutin | 2.36 ± 0.15 | 3.54 ± 0.26 | 5.23 ± 0.47 | 9.65 ± 0.87 | 1.25 ± 0.06 | 2.65 ± 0.13 | 6.35 ± 0.54 | 10.65 ± 0.85 |
| 19 | Hyperoside | 0.65 ± 0.05 | 0.32 ± 0.02 | 0.54 ± 0.03 | 0.87 ± 0.06 | 0.56 ± 0.03 | 0.40 ± 0.02 | 0.59 ± 0.04 | 0.52 ± 0.02 |
| 20 | Trans-cin acid 2 | 1.98 ± 0.12 | 2.35 ± 0.20 | 3.21 ± 0.31 | 4.95 ± 0.36 | 1.23 ± 0.04 | 2.56 ± 0.13 | 2.65 ± 0.14 | 4.87 ± 0.24 |
| 21 | Resveratrol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 22 | Kae-3-glu 2 | 0.59 ± 0.03 | 0.47 ± 0.02 | 1.40 ± 0.09 | 1.89 ± 0.10 | 0.65 ± 0.04 | 0.36 ± 0.01 | 0.78 ± 0.05 | 1.26 ± 0.10 |
| 23 | Quercitrin | 0.43 ± 0.03 | 0.13 ± 0.01 | 0.62 ± 0.05 | 0.83 ± 0.07 | 0.36 ± 0.02 | 0.12 ± 0.01 | 0.41 ± 0.03 | 0.59 ± 0.05 |
| 24 | Quercetin | 3.74 ± 0.29 | 3.20 ± 0.28 | 3.76 ± 0.31 | 3.67 ± 0.33 | 3.12 ± 0.25 | 3.56 ± 0.25 | 3.42 ± 0.28 | 3.06 ± 0.20 |
| 25 | Isogentisin | 2.54 ± 0.19 | 1.05 ± 0.08 | 1.09 ± 0.09 | 0.99 ± 0.07 | 1.95 ± 0.15 | 0.95 ± 0.08 | 1.36 ± 0.11 | 1.10 ± 0.09 |
| Total Compounds | 47,191.31 ± 3058.21 | 52,768.88 ± 3658.54 | 60,520.82 ± 4536.41 | 68,698.72 ± 5012.36 | 35,867.67 ± 2898.32 | 36,601.49 ± 3025.45 | 47,702.54 ± 3987.36 | 50,428.89 ± 4023.74 | |
| Extracts | DPPH (mg TE g−1) | ABTS (mg TE g−1) | CUPRAC (mg TE g−1) | FRAP (mg TE g−1) | Chel. ab. 2 (mg EDTAE g−1) | Pho. 2 (mmol TE g−1) | |
|---|---|---|---|---|---|---|---|
| SCG | EtOH:H2O | 296.78 ± 7.08 a | 276.19 ± 9.65 a | 501.85 ± 10.16 a | 277.15 ± 3.22 a | 12.82 ± 0.25 c | 1.80 ± 0.05 a |
| MeOH | 156.13 ± 0.23 abcd | 136.11 ± 13.35 abcd | 280.96 ± 1.56 abc | 158.75 ± 0.90 ab | 16.45 ± 0.42 ab | 1.49 ± 0.09 abc | |
| MeOH:H2O | 274.44 ± 4.29 ab | 218.75 ± 6.88 ab | 446.39 ± 7.24 ab | 235.47 ± 4.98 ab | 19.47 ± 1.32 a | 1.54 ± 0.10 abc | |
| H2O | 163.72 ± 2.21 abc | 164.70 ± 1.55 abc | 246.90 ± 3.68 abcd | 143.61 ± 0.31 abc | 13.88 ± 0.26 bc | 1.03 ± 0.01 cd | |
| CS | EtOH:H2O | 45.63 ± 0.11 cde | 73.66 ± 1.43 cde | 90.50 ± 0.78 cde | 45.76 ± 0.30 cd | 7.66 ± 0.71 d | 1.39 ± 0.08 bcd |
| MeOH | 48.73 ± 0.09 bcde | 95.05 ± 0.04 bcde | 110.97 ± 0.87 bcde | 65.28 ± 1.91 bcd | 16.56 ± 0.45 ab | 1.60 ± 0.07 ab | |
| MeOH:H2O | 36.62 ± 0.58 de | 63.50 ± 1.65 de | 77.23 ± 1.24 de | 45.83 ± 0.19 bcd | 12.52 ± 2.96 c | 0.85 ± 0.02 cd | |
| H2O | 29.63 ± 0.14 e | 54.41 ± 0.76 e | 56.96 ± 0.74 e | 37.04 ± 0.20 d | 3.76 ± 0.31 e | 0.68 ± 0.01 d |
| Extracts | AChE Inhibition (mg GALAE g−1) | BChE Inhibition (mg GALAE g−1) | Tyrosinase Inhibition (mg KAE g−1) | Amylase Inhibition (mmol ACAE g−1) | Glucosidase Inhibition (mmol ACAE g−1) | |
|---|---|---|---|---|---|---|
| SCG | EtOH:H2O | 2.42 ± 0.01 a | 2.07 ± 0.27 abc | 44.73 ± 0.51 bcd | 0.42 ± 0.01 ab | 3.80 ± 0.18 abc |
| MeOH | 2.57 ± 0.05 a | 9.07 ± 0.04 a | 58.42 ± 0.46 a | 0.40 ± 0.02 abc | 8.11 ± 0.03 a | |
| MeOH:H2O | 1.96 ± 0.03 ab | 1.73 ± 0.06 bc | 49.24 ± 0.71 abc | 0.20 ± 0.01 bcd | 1.21 ± 0.09 abcd | |
| H2O | 1.39 ± 0.06 bc | 0.29 ± 0.04 c | Ni | 0.12 ± 0.01 cd | 0.47 ± 0.05 bcd | |
| CS | EtOH:H2O | 2.36 ± 0.03 a | 2.78 ± 0.22 ab | 45.84 ± 0.35 abcd | 0.41 ± 0.01 ab | 4.07 ± 0.28 ab |
| MeOH | 1.35 ± 0.08 ce | 4.81 ± 0.19 a | 60.43 ± 0.18 a | 0.46 ± 0.01 a | 7.95 ± 0.05 a | |
| MeOH:H2O | 1.64 ± 0.01 abc | 2.19 ± 0.05 abc | 52.66 ± 0.31 ab | 0.18 ± 0.01 bcd | Ni | |
| H2O | 1.37 ± 0.04 bc | 0.72 ± 0.06 c | Ni | 0.05 ± 0.01 d | Ni |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin. Foods 2020, 9, 713. https://doi.org/10.3390/foods9060713
Zengin G, Sinan KI, Mahomoodally MF, Angeloni S, Mustafa AM, Vittori S, Maggi F, Caprioli G. Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin. Foods. 2020; 9(6):713. https://doi.org/10.3390/foods9060713
Chicago/Turabian StyleZengin, Gokhan, Kouadio Ibrahime Sinan, Mohamad Fawzi Mahomoodally, Simone Angeloni, Ahmed M. Mustafa, Sauro Vittori, Filippo Maggi, and Giovanni Caprioli. 2020. "Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin" Foods 9, no. 6: 713. https://doi.org/10.3390/foods9060713
APA StyleZengin, G., Sinan, K. I., Mahomoodally, M. F., Angeloni, S., Mustafa, A. M., Vittori, S., Maggi, F., & Caprioli, G. (2020). Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin. Foods, 9(6), 713. https://doi.org/10.3390/foods9060713










