Determination of Organosulfides from Onion Oil
Abstract
1. Introduction
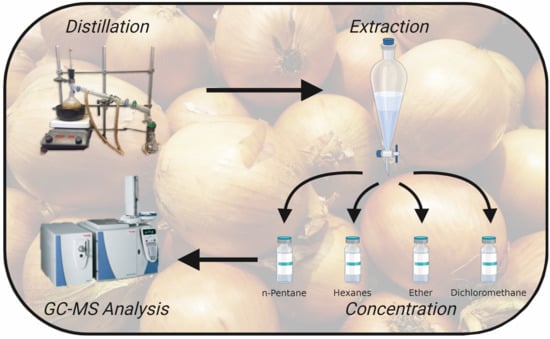
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sample Preparation
2.3. GC-MS Method & Compound Identification
2.4. Statistical Analysis
3. Results and Discussion
3.1. Organosulfide Determination and Quantitation in Steam Distilled Onion Oil
3.2. Solvent Extraction Efficiency
3.2.1. Dichloromethane Extraction
3.2.2. Diethyl Ether Extraction
3.2.3. n-Pentane Extraction
3.2.4. Hexanes Extraction
3.3. Solvent Extraction
3.4. Organosulfide Profile of Steam Distilled Onion Oil
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-Alk(En)Yl Cysteine Sulfoxide Metabolites in the Genus Allium: The Chemistry of Potential Therapeutic Agents. Nat. Prod. Rep. 2005, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Adams, T.B. GRAS Flavoring Substances 22. Flavor. Subst. 2005, 22, 24–62. [Google Scholar]
- Khalifa, E.S.; Hala, A.O. Onion Juice (Allium Cepa L.), a New Topical Treatment for Alopecia Areata. J. Dermatol. 2002, 29, 343–346. [Google Scholar]
- Gao, Y.; Wu, S.; Sun, Y.; Cong, R.; Xiao, J.; Ma, F. Effect of Freeze Dried, Hot Air Dried and Fresh Onions on the Composition of Volatile Sulfocompounds in Onion Oils. Dry. Technol. 2019, 37, 1427–1440. [Google Scholar] [CrossRef]
- Mondy, N.; Naudin, A.; Christides, J.P.; Mandon, N.; Auger, J. Comparison of GC-MS and HPLC for the Analysis of Allium Volatiles. Chromatographia 2001, 53, S356–S360. [Google Scholar] [CrossRef]
- Boelens, M.H.; de Valois, P.J.; Wobben, H.J.; van der Gen, A. Volatile Flavor Compounds from Onion. J. Agric. Food Chem. 1971, 19, 984–991. [Google Scholar] [CrossRef]
- D’auria, M.; Racioppi, R. HS-SPME-GC-MS Analysis of Onion (Allium Cepa L.) and Shallot (Allium Ascalonicum L.). Food Res. 2017, 1, 161–165. [Google Scholar] [CrossRef]
- Wardencki, W.; Michulec, M.; Curyło, J. A Review of Theoretical and Practical Aspects of Solid-Phase Microextraction in Food Analysis. Int. J. Food Sci. Technol. 2004, 39, 703–717. [Google Scholar] [CrossRef]
- Falaki, F. Sample Preparation Techniques for Gas Chromatography. In Gas Chromatography—Derivatization, Sample Preparation, Application; IntechOpen: London, UK, 2019; pp. 1–30. [Google Scholar] [CrossRef]
- Tocmo, R.; Lin, Y.; Huang, D. Effect of Processing Conditions on the Organosulfides of Shallot (Allium Cepa L. Aggregatum Group). J. Agric. Food Chem. 2014, 62, 5296–5304. [Google Scholar] [CrossRef]
- Abe, K.; Hori, Y.; Myoda, T. Volatile Compounds of Fresh and Processed Garlic (Review). Exp. Ther. Med. 2020, 19, 1585–1593. [Google Scholar] [CrossRef]
- Lanzotti, V. The Analysis of Onion and Garlic. J. Chromatogr. A 2006, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Augusti, K.T. Therapeutic Values of Onion (Allium Cepa L.) and Garlic (Allium Sativum L.). Indian J. Helminthol. 1996, 34, 634–640. [Google Scholar]
- Tocmo, R.; Wu, Y.; Liang, D.; Fogliano, V.; Huang, D. Boiling Enriches the Linear Polysulfides and the Hydrogen Sulfide-Releasing Activity of Garlic. Food Chem. 2017, 221, 1867–1873. [Google Scholar] [CrossRef]
- Kuo, M.-C. Volatile Constituents of Solvent Extracts of Welsh Onions (Allium Fistulosum L. Variety Maichuon) and Scallions (A. Fistulosum L. Variety Caespitosum). J. Agric. Food Chem. 1992, 40, 1906–1910. [Google Scholar] [CrossRef]
- Calvey, E.M.; Matusik, J.E.; White, K.D.; Betz, J.M.; Block, E.; Littlejohn, M.H.; Naganathan, S.; Putman, D. Off-Line Supercritical Fluid Extraction of Thiosulfinates from Garlic and Onion. J. Agric. Food Chem. 1994, 42, 1335–1341. [Google Scholar] [CrossRef]
- Kubec, R.; Drhová, V.; Velíšek, J. Thermal Degradation of S -Methylcysteine and Its Sulfoxide Important Flavor Precursors of Brassica and Allium Vegetables. J. Agric. Food Chem. 1998, 46, 4334–4340. [Google Scholar] [CrossRef]
- Zalepugin, D.Y.; Tilkunova, N.A.; Chernyshova, I.V. Stability of Thiosulfinates from Garlic (Allium Sativum L.) Supercritical Extracts in Polar and Nonpolar Solvents. Russ. J. Phys. Chem. B 2015, 9, 1032–1042. [Google Scholar] [CrossRef]
- Masood, F.; Malik, A. Cytotoxic and Genotoxic Potential of Tannery Waste Contaminated Soils. Sci. Total Environ. 2013, 444, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.; Sharma, A. Phytoconstituents and Therapeutic Potential of Allium Cepa Linn.—A Review. Pharmacogn. Rev. 2009, 3, 170–180. [Google Scholar]
- Ramirez, D.A.; Locatelli, D.A.; González, R.E.; Cavagnaro, P.F.; Camargo, A.B. Analytical Methods for Bioactive Sulfur Compounds in Allium: An Integrated Review and Future Directions. J. Food Compos. Anal. 2016, 61, 4–19. [Google Scholar] [CrossRef]
- Arnault, I.; Mondy, N.; Cadoux, F.; Auger, J. Possible Interest of Various Sample Transfer Techniques for Fast Gas Chromatography–Mass Spectrometric Analysis of True Onion Volatiles. J. Chromatogr. A 2000, 896, 117–124. [Google Scholar] [CrossRef]
- Shen, V.K.; Siderius, D.W.; Krekelberg, W.P.; Hatch, H.W. (Eds.) NIST Standard Reference Simulation Website, NIST Standard Reference Database Number 173; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017; p. 20899. Available online: http://doi.org/10.18434/T4M88Q (accessed on 21 May 2020).
- Schreyen, L.; Dirinck, P.; Van Wassenhove, F.; Schamp, N. Analysis of Leek Volatiles by Headspace Condensation. J. Agric. Food Chem. 1976, 24, 1147–1152. [Google Scholar] [CrossRef]
- Garbuzov, V.G.; Misharina, T.A.; Aerov, A.F.; Golovnya, R.V. Gas chromatographic retention indices for sulphur(II)-containing organic substances. J. Anal. Chem. USSR (Engl. Transl.) 1985, 40, 576–586. [Google Scholar]
- Liguori, L.; Califano, R.; Albanese, D.; Raimo, F.; Crescitelli, A.; Di Matteo, M. Chemical Composition and Antioxidant Properties of Five White Onion (Allium Cepa L.) Landraces. J. Food Qual. 2017, 2017. [Google Scholar] [CrossRef]
- Calvo-Gómez, O.; Morales-López, J.; López, M.G. Solid-Phase Microextraction-Gas Chromatographic-Mass Spectrometric Analysis of Garlic Oil Obtained by Hydrodistillation. J. Chromatogr. A 2004, 1036, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.H.; Wu, C.M.; Rosen, R.T.; Hartman, T.G.; Ho, C.T. Volatile Compounds Generated from Thermal Degradation of Alliin and Deoxyalliin in an Aqueous Solution. J. Agric. Food Chem. 1994, 42, 146–153. [Google Scholar] [CrossRef]
- Mochizuki, E.; Yamamoto, T.; Komiyama, Y.; Nakazawa, H. Identification of Allium Products Using Flame Photometric Detection Gas Chromatography and Distribution Patterns of Volatile Sulfur Compounds. J. Agric. Food Chem. 1998, 46, 5170–5176. [Google Scholar] [CrossRef]
- Farkas, P.; Hradsky, P.; Kovae, M. Novel Flavour Components Identified in the Steam Distillate of Onion Oil. Zeitschrift Leb. Forsch. 1992, 195, 95–110. [Google Scholar]
- Pino, J.A.; Fuentes, V.; Correa, M.T. Volatile constituents of Chinese chive (Allium tuberosum Rottl. ex Sprengel) and Rakkyo (Allium chinense G. Don). J. Agric. Food Chem. 2001, 49, 1328–1330. [Google Scholar] [CrossRef]
- Chen, C.W.; Ho, C.T. Thermal Degradation of Allyl Isothiocyanate in Aqueous Solution. J. Agric. Food Chem. 1998, 46, 220–223. [Google Scholar] [CrossRef]
- Kuo, M.C.; Ho, C.T.; Chien, M. Novel Polysulfides Identified in the Volatile Components from Welsh Onions (Allium Fistulosum L. Var. Maichuon) and Scallions (Allium Fistulosum L. Var. Caespitosum). J. Agric. Food Chem. 1990, 38, 1378–1381. [Google Scholar] [CrossRef]
- Brodnitz, M.H.; Pollock, C.L.; Vallon, P.P. Flavor Components of Onion Oil. J. Agric. Food Chem. 1969, 17, 760–763. [Google Scholar] [CrossRef]
- Oser, B.L.; Ford, R.A. GRAS Flavoring Substances 7. Recent Progress in the Consideration of Flavoring Ingredients under the Food Additives Amendment. Food Technol. 1973, 27, 56–57. [Google Scholar]
- Munday, R.; Manns, E. Comparative Toxicity of Prop (En) Yl Disulfides Derived from Alliaceae: Possible Involvement of 1 -Propenyl Disulfides in Onion-Induced Hemolytic Anemia. J. Agric. Food Chem. 1994, 42, 959–962. [Google Scholar] [CrossRef]
- Harvey, J.W.; Rackear, D. Experimental Onion-Induced Hemolytic Anemia in Dogs. Vet. Pathol. 1985, 22, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, D.S.; Howell, M.E.; Combs, J.J.; Hinman, D.D. Hematologic Effects and Feeding Performance in Cattle Fed Cull Domestic Onions (Alluim Cepa). J. Am. Vet. Med. Assoc. 1992, 200, 1090–1094. [Google Scholar] [PubMed]
- IARC. IARC Monographs Vol. 71 Dichloromethane; IARC: Lyon, France, 1996. [Google Scholar]
- Yu, T.H.; Wu, C.M.; Ho, C.T. Meat-Like Flavor Generated from Thermal Interactions of Glucose and Alliin or Deoxyalliin. J. Agric. Food Chem. 1994, 42, 1005–1009. [Google Scholar] [CrossRef]
- Iranshahi, M. A Review of Volatile Sulfur-Containing Compounds from Terrestrial Plants: Biosynthesis, Distribution and Analytical Methods. J. Essent. Oil Res. 2012, 24, 393–434. [Google Scholar] [CrossRef]
- Hemandez-Molinar, E. An Improved Solvent Extraction of Onion Oil. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1981. [Google Scholar]
- Tocmo, R.; Lai, A.N.; Wu, Y.; Liang, D.; Fogliano, V.; Huang, D. Organosulphide Profile and Hydrogen Sulphide-Releasing Activity of Garlic Fermented by Lactobacillus Plantarum. J. Funct. Foods 2017, 30, 254–259. [Google Scholar] [CrossRef]
- Publication of NEG and NIOSH Basis for an Occupational Health Standard: Ethyl Ether; MMWR. Morbidity and Mortality Weekly Report; U.S. Department of Health & Human Services: Washington, DC, USA, 1994; p. 267.
- Block, E.; Putman, D.; Zhao, S.-H. Allium Chemistry: GC-MS Analysis of Thiosulfinates and Relation Compounds from Onion, Leek, Scallion, Shallot, Chive and Chinese Chive. J. Agric. Food Chem. 1992, 40, 2431–2438. [Google Scholar] [CrossRef]
- Colina-Coca, C.; González-Peña, D.; Vega, E.; De Ancos, B.; Sánchez-Moreno, C. Novel Approach for the Determination of Volatile Compounds in Processed Onion by Headspace Gas Chromatography-Mass Spectrometry (HS GC-MS). Talanta 2013, 103, 137–144. [Google Scholar] [CrossRef] [PubMed]


| No a | r.t. (min) | Compound Name, Formula | Structure | Probability Score b | m/z (%) c | Identification d |
|---|---|---|---|---|---|---|
| 1 | 7.02 | 3,4-Dimethylthiophene, C6H8S |  | 10.76 | 111(100),112(66),97 (56),50(29), 77(27),59(26), 106(25),113(11) | MS, [4,6,24,25] |
| 2 | 7.87 | 2,4-Dimethyl Thiophene, C6H8S |  | 21.13 | 111(100),112(61),97 (41),74(39), 116(34),50(21), 77(21),113(9) | MS, [4,6] |
| 3 | 9.65 | (E)-1-(Methyldisulfanyl)prop-1-ene, C6H8S2 |  | 32.84 | 72(100),120(74), 72(42),103(27),91 (22),71(21), 104(12),121(4) | MS, [6,26] |
| 4 | 15.73 | (E)-Propenyl Propyl Disulfide, C6H12S2 |  | 46.78 | 106(100),148(52),63 (21),73(20), 72(18),77(16), 59(15),149(5) | MS, [4,6,27] |
| 5 | 16.40 | Dipropyl Disulfide, C6H14S2 |  | 52.04 | 150(100),108(54),65 (17),74(15), 148(14),117(10), 75(10),151(8) | MS, [4,6,28] |
| 6 | 16.95 | Allyl Isobutyl Disulfide, C7H12S2 |  | 49.47 | 73(100),105(42), 148(39),83(15), 71(9),72(6),74(5), 149(3) | MS, [27] |
| 7 | 17.55 | 2-Ethyl-1,3-Dithiane, C6H12S2 |  | 32.08 | 119(100),85(22), 148(21),58(12), 73(10),121(9), 75(6) | MS, [29,30] |
| 8 | 18.31 | 4-Methyl-1,2,3-Trithiolane, C3H3S6 |  | 86.36 | 137(100),73(55), 63(14),139(14), 59(13),154(12), 74(12),95(9) * | MS, [12,13] |
| 9 | 19.97 | 3,4-Dimethyl-2,3-Dihydro-2-Thiophenethiol, C6H10S2 |  | 80.51 | 144(100),111(77),117(49),146(28), 143(18),77(16), 99(16),129(12) | MS |
| 10 | 20.30 | 4H-1,2,3-Trithiin, C3H4S3 |  | 14.51 | 70(100),135(68), 103(59),72(15), 143(14),63(13), 144(12),137(4) | MS, [14,31] |
| 11 | 20.53 | 2-Ethylidene-1,3-Dithiane, C6H10S2 |  | 59.44 | 146(100),71(33), 104(33),103(31), 75(22),113(20), 85(14),147(10) | MS, [15,32] |
| 12 | 21.03 | 1-(Methylthiopropyl) Methyl Disulfide, C5H12S3 |  | 24.54 | 89(100),61(53), 73(19),88(17), 146(14),71(13), 103(13),161(10) * | MS, [5,16,24,25] |
| 13 | 21.33 | Methyl-trans-1-propenyl trisulfide |  | 27.50 | 146(100),152(80),103(77),87(64), 117(58),71(43), 85(37),154(11) | MS, [6,16,28,33,34] |
| 14 | 21.89 | 3-Ethyl-5-methyl-1,2,4-trithiolane, C4H8S3 (isomer of 15) |  | 13.50 | 166(100),101(68),59 (54),63(25), 69(22),168(14),137 (13),102(13) | MS, [17] |
| 15 | 22.22 | 3-Ethyl-5-methyl-1,2,4-trithiolane, C5H10S3 (isomer of 14) |  | 92.55 | 166(100),101(63),58 (51),63(24), 69(21),168(14), 102(13),73(12) | MS |
| 16 | 23.66 | 2-Methyl-4-Methylsulfanyl-2,3-Dihydrothiophene, C6H10S2 |  | 26.86 | 146(100),131(74),71 (41),73(24), 77(22),59(22), 55(21),79(19) | MS |
| 17 | 25.48 | 5-Methyl tetrathiane, C3H6S4 (isomer of 21) |  | 94.32 | 169(100),106(78),63 (36),171(19), 59(14),127(14), 72(12),170(6) | MS, [10,29] |
| 18 | 26.22 | (E)-1-Propenyl Propyl Trisulfide, C6H12S3 (isomer of 19) |  | 65.27 | 180(100),182(73),115(70),75(26), 106(18),58(18), 55(17),181(9) | MS, [6,24] |
| 19 | 26.55 | (Z)-1-Propenyl Propyl Trisulfide, C6H12S3 (isomer of 18) |  | 81.35 | 180(100),115(68),81 (19),74(17), 58(16),106(16), 182(13),181(9) | MS, [6,24] |
| 20 | 26.84 | 3,5-Diethyl-1,2,4-Trithiolane, C6H12S3 |  | 30.68 | 116(100),180(38),74 (32),73(22), 114(12),118(11), 182(6),181(3) | MS, [10] |
| 21 | 27.61 | 5-Methyltetrathiane, C3H6S4 (isomer of 17) |  | 95.88 | 169(100),106(49),127(26),63(20), 171(18),59(9), 73(7),170(6) | MS, [10,25,33] |
| 22 | 29.28 | 1-(Methylthio)Propyl Propyl Disulfide, C7H16S3 (isomer of 23) |  | 31.78 | 89(100),61(47),73 (35),194(14), 55(10),70(8), 88(7),59(6) | MS, [16,18,24,25] |
| 23 | 30.28 | 1-(Methylthio)Propyl Propyl Disulfide (isomer of 22), C7H16S3 |  | 38.72 | 89(100),61(47), 73(35),194(14), 55(10),70(8), 88(7),59(6) | MS, [16, 18,24,25] |
| 24 | 31.72 | 2,3-Dimethyl-5,6-Dithiabicyclo [2.1.1]hexane 5,5-Dioxide, C6H10O2S2 |  | 86.57 | 113(100),99(70), 177(34),79(23), 77(22),111(21), 97(16),65(16) | MS, [15,24] |
| 25 | 32.88 | Hexathiane, S6 |  | 74.50 | 191(100),63(29), 193(28),127(26), 130(4),192(4), 96(4),65(3) | MS, [19,23] |
| 26 | 35.99 | Dipropyl Tetrasulfide, C6H14S4 |  | 82.22 | 214(100),73(34), 108(27),216(17), 75(15),117(14), 171(13),69(11) | MS, [18,25,28] |
| 27 | 36.64 | 3,6-Diethyl-1,2,4,5-Tetrathiane, C6H12S4 (isomer of 28, 29) |  | 35.67 | 73(100),115(91), 147(65),211(29), 74(17),138(14), 81(12),59(9) | MS, [23] |
| 28 | 37.84 | 3,6-Diethyl-1,2,4,5-Tetrathiane, C6H12S4 (isomer of 27, 29) |  | 35.28 | 73(100),115(94), 147(60),211(22), 74(19),81(14), 137(13),180(10) | MS |
| 29 | 37.84 | 3,6-Diethyl-1,2,4,5-Tetrathiane, C6H12S4 (isomer of 27, 28) |  | 35.28 | 73(100),115(94), 147(60),211(22), 74(19),81(14), 137(13),180(10) | MS |
| Organosulfide Content (mg/kg) in Each Solvent | ||||
|---|---|---|---|---|
| No α | DCM | DEE | n-Pentane | Hexanes |
| 1 | 3.03 ± 0.06 a | 2.94 ± 0.04 b | 2.90 ± 0.01 a | n.d. |
| 2 | 3.36 ± 0.17 a | n.d. | n.d. | n.d. |
| 3 | 3.98 ± 0.45 a | n.d. | 3.70 ± 0.29 b | n.d. |
| I.S. | 5.29 ± 1.09 a | 5.08 ± 0.26 b | 5.13 ± 0.56 c | 4.96 ± 0.08 d |
| 4 | 3.27 ± 0.02 a | 3.71 ± 1.1 b | 3.22 ± 0.03 c | 3.16 ± 0.09 d |
| 5 | 3.32 ± 0.16 a | n.d. | 3.14 ± 0.09 b | 3.10 ± 0.13 c |
| 6 | 4.26 ± 0.62 a | 3.22 ± 0.16 b | 3.82 ± 0.31 c | 3.64 ± 0.49 d |
| 7 | 2.99 ± 0.05 a | n.d. | n.d. | n.d. |
| 8 | 4.30 ± 0.56 a | 3.21 ± 0.15 b | 3.93 ± 0.3 c | 3.55 ± 0.68 d |
| 9 | 3.40 ± 0.21 a | n.d. | 3.21 ± 0.11 b | 3.14 ± 0.17 c |
| 10 | 2.99 ± 0.02 a | 3.14 ± 0.31 b | 2.98 ± 0.02 c | 2.96 ± 0.01 d |
| 11 | 2.93 ± 0.01 a | n.d. | n.d. | n.d. |
| 12 | 3.16 ± 0.14 a | n.d. | n.d. | n.d. |
| 13 | 3.30 ± 0.08 a | n.d. | n.d. | 3.07 ± 0.15 b |
| 14 | 3.08 ± 0.09 a | n.d. | 3.00 ± 0.06 b | 2.96 ± 0.06 c |
| 15 | 3.23 ± 0.19 a | n.d. | 3.10 ± 0.11 b | 3.02 ± 0.09 c |
| 16 | 2.90 ± 0.02 a | n.d. | n.d. | n.d. |
| 17 | 3.06 ± 0.23 a | n.d. | 3.03 ± 0.07 b | 2.98 ± 0.06 c |
| 18 | 5.66 ± 1.35 a | 3.41 ± 0.22 b | 4.06 ± 1.09 b | 4.27 ± 0.84 c |
| 19 | 5.84 ± 1.30 a | 3.56 ± 0.30 a | 4.84 ± 0.74 b | 4.40 ± 0.76 c |
| 20 | 3.61 ± 0.30 a | n.d. | n.d. | 3.26 ± 0.26 |
| 21 | n.d. | 3.17 ± 0.28 a | 3.86 ± 0.14 b | 3.67 ± 0.32 a |
| 22 | 3.23 ± 0.09 a | n.d. | n.d. | 3.02 ± 0.13 b |
| 23 | 2.97 ± 0.05 a | n.d. | n.d. | n.d. |
| 24 | 3.20 ± 0.11 a | n.d. | 3.20 ± 0.10 b | 3.15 ± 0.13 c |
| 25 | n.d. | 3.21 ± 0.07 a | 3.28 ± 0.11 b | 3.24 ± 0.08 c |
| 26 | 3.19 ± 0.16 a | n.d. | 3.07 ± 0.07 b | n.d. |
| 27 | 4.50 ± 0.68 a | n.d. | n.d. | n.d. |
| 28 | 3.20 ± 0.11 a | n.d. | 4.20 ± 0.45 b | n.d. |
| 29 | 5.37 ± 1.14 a | 3.41 ± 0.26 a | 4.60 ± 0.68 b | n.d. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantrell, M.S.; Seale, J.T.; Arispe, S.A.; McDougal, O.M. Determination of Organosulfides from Onion Oil. Foods 2020, 9, 884. https://doi.org/10.3390/foods9070884
Cantrell MS, Seale JT, Arispe SA, McDougal OM. Determination of Organosulfides from Onion Oil. Foods. 2020; 9(7):884. https://doi.org/10.3390/foods9070884
Chicago/Turabian StyleCantrell, Maranda S., Jared T. Seale, Sergio A. Arispe, and Owen M. McDougal. 2020. "Determination of Organosulfides from Onion Oil" Foods 9, no. 7: 884. https://doi.org/10.3390/foods9070884
APA StyleCantrell, M. S., Seale, J. T., Arispe, S. A., & McDougal, O. M. (2020). Determination of Organosulfides from Onion Oil. Foods, 9(7), 884. https://doi.org/10.3390/foods9070884