Effect of Blanching Plus Fermentation on Selected Functional Properties of Mealworm (Tenebrio molitor) Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Processing
2.2. Characterization of Mealworm Powders
2.2.1. Crude Protein, Crude Fat and Dry Matter Content
2.2.2. pH, Bulk Density and Colour Measurement
2.3. Analysis of the Techno-Functional Properties of the Powders
2.3.1. Water and Oil Binding Capacity
2.3.2. Foaming Capacity and Stability
2.3.3. Emulsion Capacity and Stability
2.4. Analysis of the Protein Properties of the Powders
2.4.1. pH Dependent Protein Solubility
2.4.2. Quantification of Free Amino Groups
2.4.3. Protein Molecular Weight Distribution
2.4.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Mealworm Powders
3.2. Impact of Fermentation on the Techno-Functional Properties of the Powders
3.2.1. Water and Oil Binding Capacity
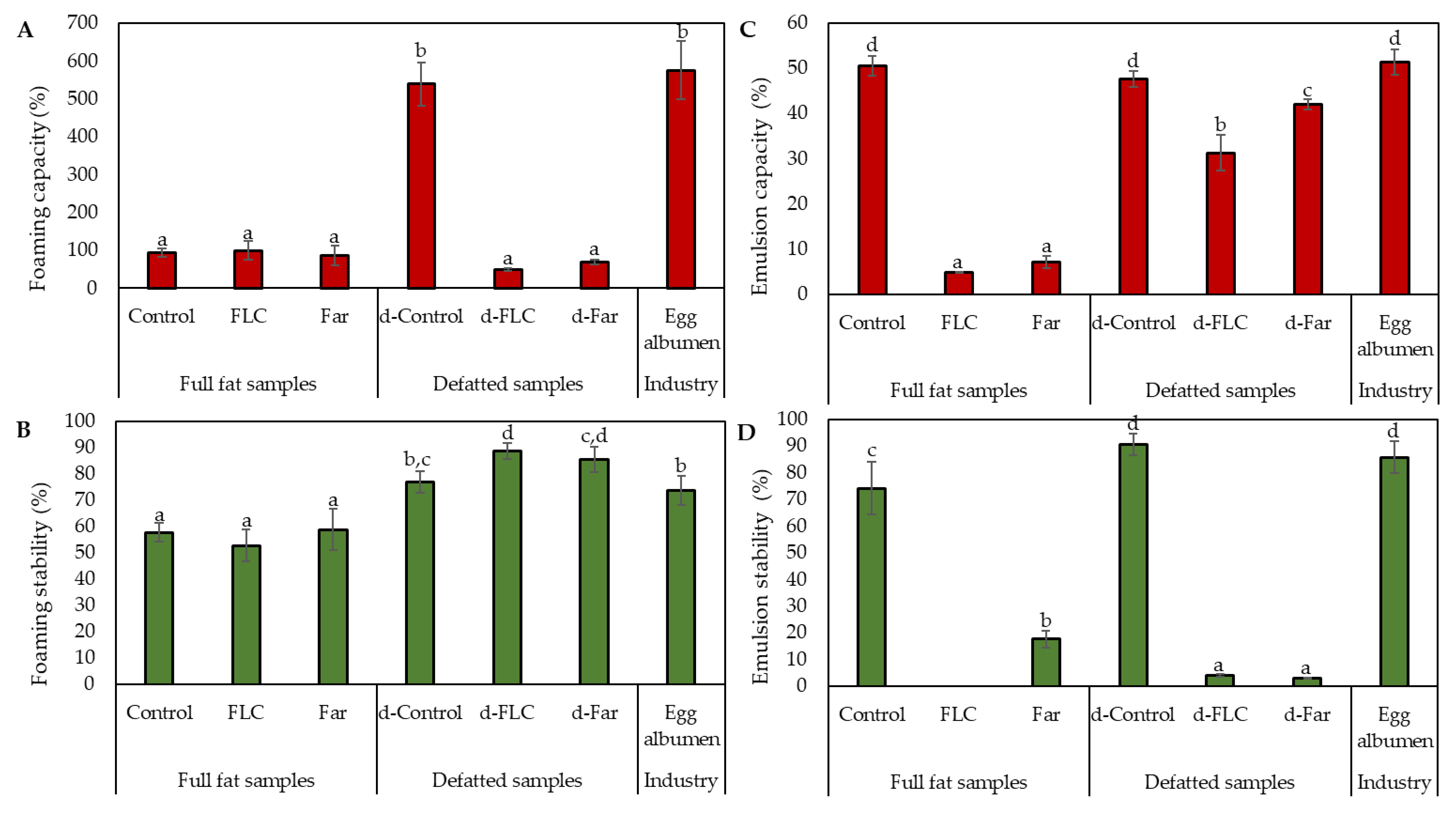
3.2.2. Foaming Capacity and Stability
3.2.3. Emulsion Capacity and Stability
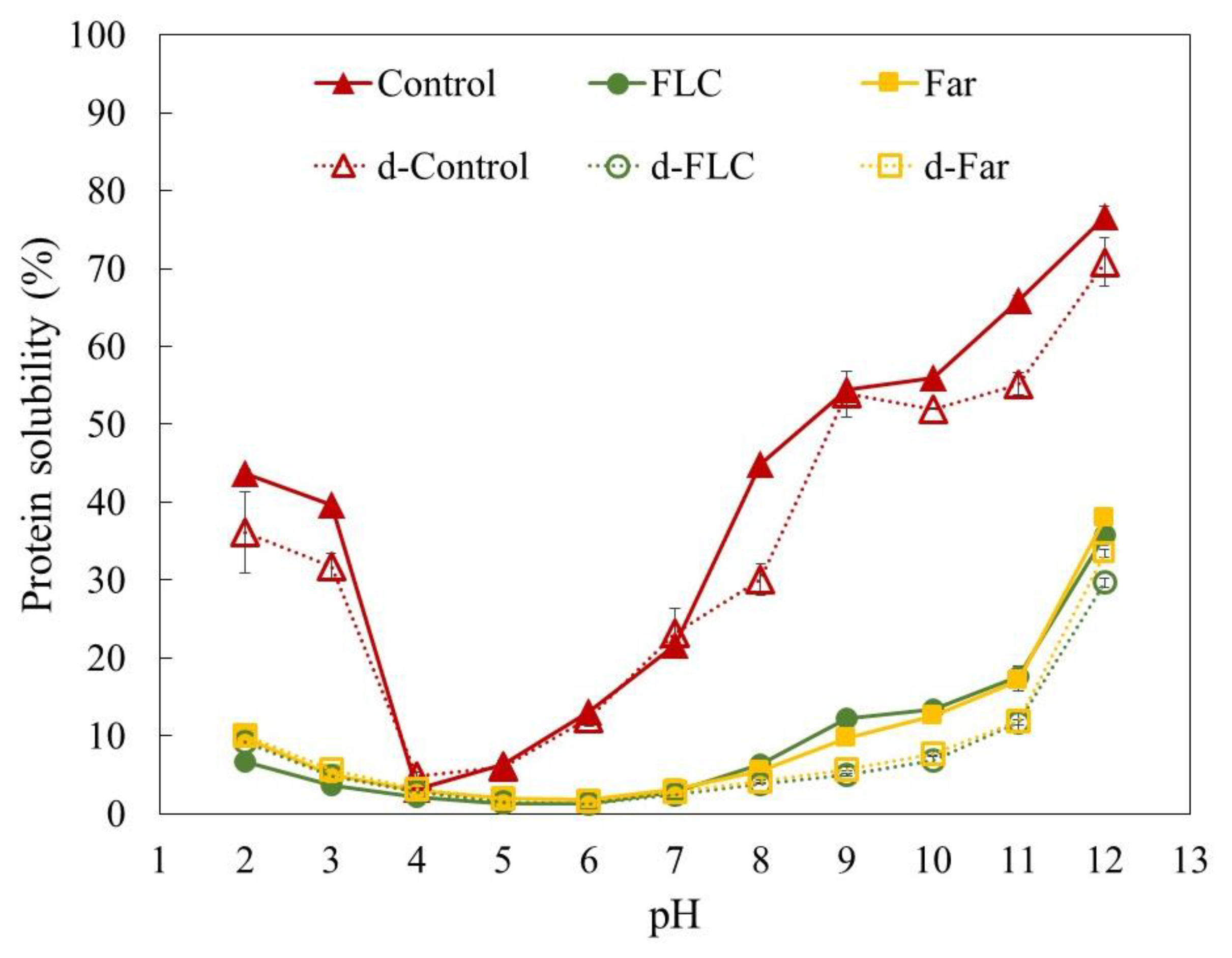
3.2.4. pH Dependent Protein Solubility
3.2.5. Quantification of Free Amino Groups and Protein Molecular Weight Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A.; et al. Addition of olive pomace to feeding substrate affects growth performance and nutritional value of mealworm (Tenebrio molitor L.) larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef] [Green Version]
- van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell-Platt, G. Fermented Foods of the World: A Dictionary and Guide; Butterworths: London, UK, 1987. [Google Scholar]
- Cho, J.H.; Zhao, H.L.; Kim, J.S.; Kim, S.H.; Chung, C.H. Characteristics of fermented seasoning sauces using Tenebrio molitor larvae. Innov. Food Sci. Emerg. Technol. 2018, 45, 186–195. [Google Scholar] [CrossRef]
- Patrignani, F.; Del Duca, S.; Vannini, L.; Rosa, M.; Schlüter, O.; Lanciotti, O. Potential of Yarrowia lipolytica and Debaryomyces hansenii strains to produce high quality food ingredients based on cricket powder. LWT 2020, 119, 108866. [Google Scholar] [CrossRef]
- De Smet, J.; Lenaerts, S.; Borremans, A.; Scholliers, J.; van Der Borght, M.; van Campenhout, L. Stability assessment and laboratory scale fermentation of pastes produced on a pilot scale from mealworms (Tenebrio molitor). LWT 2019, 102, 113–121. [Google Scholar] [CrossRef]
- Borremans, A.; Crauwels, S.; Vandeweyer, D.; Smets, R.; Verreth, C.; van Der Borght, M.; van Campenhout, L. Comparison of six commercial meat starter cultures for the fermentation of yellow mealworm (Tenebrio molitor) paste. Microorganisms 2019, 7, 540. [Google Scholar] [CrossRef] [Green Version]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2. [Google Scholar] [CrossRef]
- Kim, H.W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of functional properties of edible insects and protein preparations thereof. LWT Food Sci. Technol. 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow mealworm protein for food purposes—Extraction and functional properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef] [Green Version]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Broyard, C.; Gaucheron, F. Modifications of structures and functions of caseins: A scientific and technological challenge. Dairy Sci. Technol. 2015, 95, 831–862. [Google Scholar] [CrossRef]
- Borremans, A.; Lenaerts, S.; Crauwels, S.; Lievens, B.; van Campenhout, L. Marination and fermentation of yellow mealworm larvae (Tenebrio molitor). Food Control. 2018, 92, 47–52. [Google Scholar] [CrossRef]
- VDLUFA Methodenbuch III VDLUFA-Verlag (Ed.) Band III Die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Bonn, Germany, 1976; Volume 3, p. 2190. [Google Scholar]
- American Oil Chemists’ Society. 2005 American Oil Chemists’ Society Official Methods and Recommended Practices of the AOCS American Oil Chemists’ Society; AOCS: Champaign, IL, USA, 2005. [Google Scholar]
- Wang, J.C.; Kinsella, J.E. Functional property of novel protein alfalfa protein. J. Food Sci. 1976, 4, 286–296. [Google Scholar] [CrossRef]
- Lenaerts, S.; van Der Borght, M.; Callens, A.; van Campenhout, L. Suitability of microwave drying for mealworms (Tenebrio molitor) as alternative to freeze drying: Impact on nutritional quality and colour. Food Chem. 2018, 254, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Çabuk, B.; Nosworthy, M.G.; Stone, A.K.; Korber, D.R.; Tanaka, T.; House, J.D.; Nickerson, M.T. Effect of fermentation on the protein digestibility and levels of non-nutritive compounds of pea protein concentrate. Food Technol. Biotechnol. 2018, 56, 257–264. [Google Scholar] [CrossRef]
- Ojokoh, A.O.; Fayemi, O.E.; Ocloo, F.C.K.; Nwokolo, F.I. Effect of fermentation on proximate composition, physicochemical and microbial characteristics of pearl millet (Pennisetum glaucum (L.) R. Br.) and Acha (Digitaria exilis (Kippist) Stapf) flour blends. J. Agric. Biotech. Sustain. Dev. 2015, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Klupsaite, D.; Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Maknickiene, Z.; Liutkute, G. The influence of lactic acid fermentation on functional properties of narrow-leaved lupine protein as functional additive for higher value wheat bread. LWT 2017, 75, 180–186. [Google Scholar] [CrossRef]
- Omowaye-Taiwo, O.A.; Fagbemi, T.N.; Ogunbusola, E.M.; Badejo, A.A. Effect of germination and fermentation on the proximate composition and functional properties of full-fat and defatted cucumeropsis mannii seed flours. J. Food Sci. Technol. 2015, 52, 5257–5263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogodo, A.C.; Ugbogu, O.C.; Onyeagba, R.A.; Okereke, H.C. In-vitro starch and protein digestibility and proximate composition of soybean flour fermented with lactic acid bacteria (LAB) consortia. Agric. Nat. Resour. 2018, 52, 503–509. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference _E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Malomo, S.A.; He, R.; Aluko, R.E. Structural and functional properties of hemp seed protein products. J. Food Sci. 2014, 79. [Google Scholar] [CrossRef] [PubMed]
- Akpossan, R.; Digbeu, Y.; Koffi, M.; Kouadio, J.; Dué, E.; Kouamé, P. Protein Fractions and Functional Properties of Dried Imbrasia oyemensis Larvae Full-Fat and Defatted Flours. Int. J. Biochem. Res. Rev. 2015, 5, 116–126. [Google Scholar] [CrossRef]
- Lampart-Szczapa, E.; Konieczny, P.; Nogala-Kałucka, M.; Walczak, S.; Kossowska, I.; Malinowska, M. Some functional properties of lupin proteins modified by lactic fermentation and extrusion. Food Chem. 2006, 96, 290–296. [Google Scholar] [CrossRef]
- Gong, S.; Xie, F.; Lan, X.; Zhang, W.; Gu, X.; Wang, Z. Effects of Fermentation on Compositions, Color, and Functional Properties of Gelatinized Potato Flours. J. Food Sci. 2019, 85, 57–64. [Google Scholar] [CrossRef]
- Ogodo, A.C.; Ugbogu, O.C.; Onyeagba, R.A. Variations in the functional properties of soybean flour fermented with lactic acid bacteria. Int. J. Biol. Biomed. Eng. 2018, 12, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, J.; Ji, D.; Lee, C. Effects of Heating Time and Temperature on Functional Properties of Proteins of Yellow Mealworm Larvae (Tenebrio molitor L.). Food Sci. Anim. Resour. 2019, 39, 296–308. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Schiffler, B.; Bernhardt, R. Effect of fermentation on the functional properties of sorghum flour. Food Chem. 2005, 92, 1–5. [Google Scholar] [CrossRef]
- Weng, T.M.; Chen, M.T. Changes of protein in Natto (a fermented soybean food) affected by fermenting time. Food Sci. Technol. Res. 2010, 16, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Paraman, I.; Hettiarachchy, N.S.; Schaefer, C.; Beck, M.I. Hydrophobicity, solubility, and emulsifying properties of enzyme-modified rice endosperm protein. Cereal Chem. 2007, 84, 343–349. [Google Scholar] [CrossRef]
- Tavakoli, M.; Habibi Najafi, M.B.; Mohebbi, M. Effect of the milk fat content and starter culture selection on proteolysis and antioxidant activity of probiotic yogurt. Heliyon 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Xia, W.; Gao, P.; Xu, Y.; Jiang, Q. Proteolysis during fermentation of Suanyu as a traditional fermented fish product of China. Int. J. Food Prop. 2017, 20, S166–S176. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zheng, X. A primary study on texture modification and proteolysis of mao-tofu during fermentation. Afr. J. Biotechnol. 2009, 8, 2294–2300. [Google Scholar] [CrossRef]
- Rahali, V.; Chobert, J.M.; Haertlé, T.; Guéguen, J. Emulsification of chemical and enzymatic hydrolysates of β-lactoglobulin: Characterization of the peptides adsorbed at the interface. Nahr. Food 2000, 44, 89–95. [Google Scholar] [CrossRef]
- Razali, A.N.; Amin, A.M.; Sarbon, N.M. Antioxidant activity and functional properties of fractionated cobia skin gelatin hydrolysate at different molecular weight. Int. Food Res. J. 2015, 22, 651–660. [Google Scholar]
- Petsko, G.A.; Ringe, D. Protein Structure and Function; New Science Press Ltd.: London, UK, 2004; pp. 1–47. [Google Scholar]



| Powder | DM [g/100g as is] | CP [g/100 g DM] | CF [g/100 g DM] | pH [-] | BD [g/mL] | BI [-] | ΔE 1 |
|---|---|---|---|---|---|---|---|
| Control | 96.26 ± 0.43 c | 49.68 ± 0.02 c | 16.61 ± 0.23 c | 6.28 ± 0.05 d | 0.35 ± 0.00 a | 64.77 ± 0.13 b | - |
| FLC | 95.76 ± 0.13 c | 42.60 ± 0.02 a | 21.05 ± 0.43 e | 4.60 ± 0.04 a | 0.40 ± 0.01 b,c | 72.30 ± 0.42 d | 2.35 ± 0.04 a |
| Far | 96.08 ± 0.10 c | 44.55 ± 0.04 b | 20.20 ± 0.31 d | 4.64 ± 0,04 a,b | 0.41 ± 0.01 c | 68.24 ± 1.08 c | 2.16 ± 0.19 a |
| d-Control | 94.35 ± 0.21 b | 67.89 ± 0.02 e | 3.37 ± 0.10 a | 6.28 ± 0.02 d | 0.39 ± 0.01 | 28.17 ± 0.78 a | - |
| d-FLC | 92.19 ± 0.07 a | 61.94 ± 0.02 d | 5.13 ± 0.21 b | 4.72 ± 0.02 b,c | 0.49 ± 0.01 d | 28.11 ± 2.45 a | 1.76 ± 0.60 a |
| d-Far | 92.21 ± 0.07 a | 61.86 ± 0.04 d | 3.17 ± 0.15 a | 4.75 ± 0.02 c | 0.49 ± 0.01 d | 30.25 ± 0.50 a | 1.97 ± 0.58 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borremans, A.; Bußler, S.; Sagu, S.T.; Rawel, H.; Schlüter, O.K.; Leen, V.C. Effect of Blanching Plus Fermentation on Selected Functional Properties of Mealworm (Tenebrio molitor) Powders. Foods 2020, 9, 917. https://doi.org/10.3390/foods9070917
Borremans A, Bußler S, Sagu ST, Rawel H, Schlüter OK, Leen VC. Effect of Blanching Plus Fermentation on Selected Functional Properties of Mealworm (Tenebrio molitor) Powders. Foods. 2020; 9(7):917. https://doi.org/10.3390/foods9070917
Chicago/Turabian StyleBorremans, An, Sara Bußler, Sorel Tchewonpi Sagu, Harshadrai Rawel, Oliver K. Schlüter, and Van Campenhout Leen. 2020. "Effect of Blanching Plus Fermentation on Selected Functional Properties of Mealworm (Tenebrio molitor) Powders" Foods 9, no. 7: 917. https://doi.org/10.3390/foods9070917






