New Liquid Source of Antioxidant Phenolic Compounds in the Olive Oil Industry: Alperujo Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Standard Compounds
2.3. Determination of Insoluble Solids
2.4. Determination of Fat in the Separated Solid and Liquid
2.5. Determination of pH and the Amount of Dry Matter Content of the Samples
2.6. Phenolic Extracts
2.7. Determination of Single Phenols by HPLC
2.8. Determination of Total Phenolics by the Folin-Ciocalteu Method
2.9. Colorimetric Determination of Total Sugars in the Extracts
2.10. Determination of Antioxidant Activity In Vitro
2.10.1. Iron Reduction Power
2.10.2. Anti-radical Activity:2,2-diphenyl-1-picrylhydrazyl (DPPH Assay)
2.10.3. Determination of Antioxidant Activity in Oil: Rancimat Method
2.11. Statistical Analysis
3. Results and Discussion
3.1. Raw Material Analysis
3.2. Phenolic Extracts
Characterization of Phenolic Extracts
3.3. Antioxidant Activity
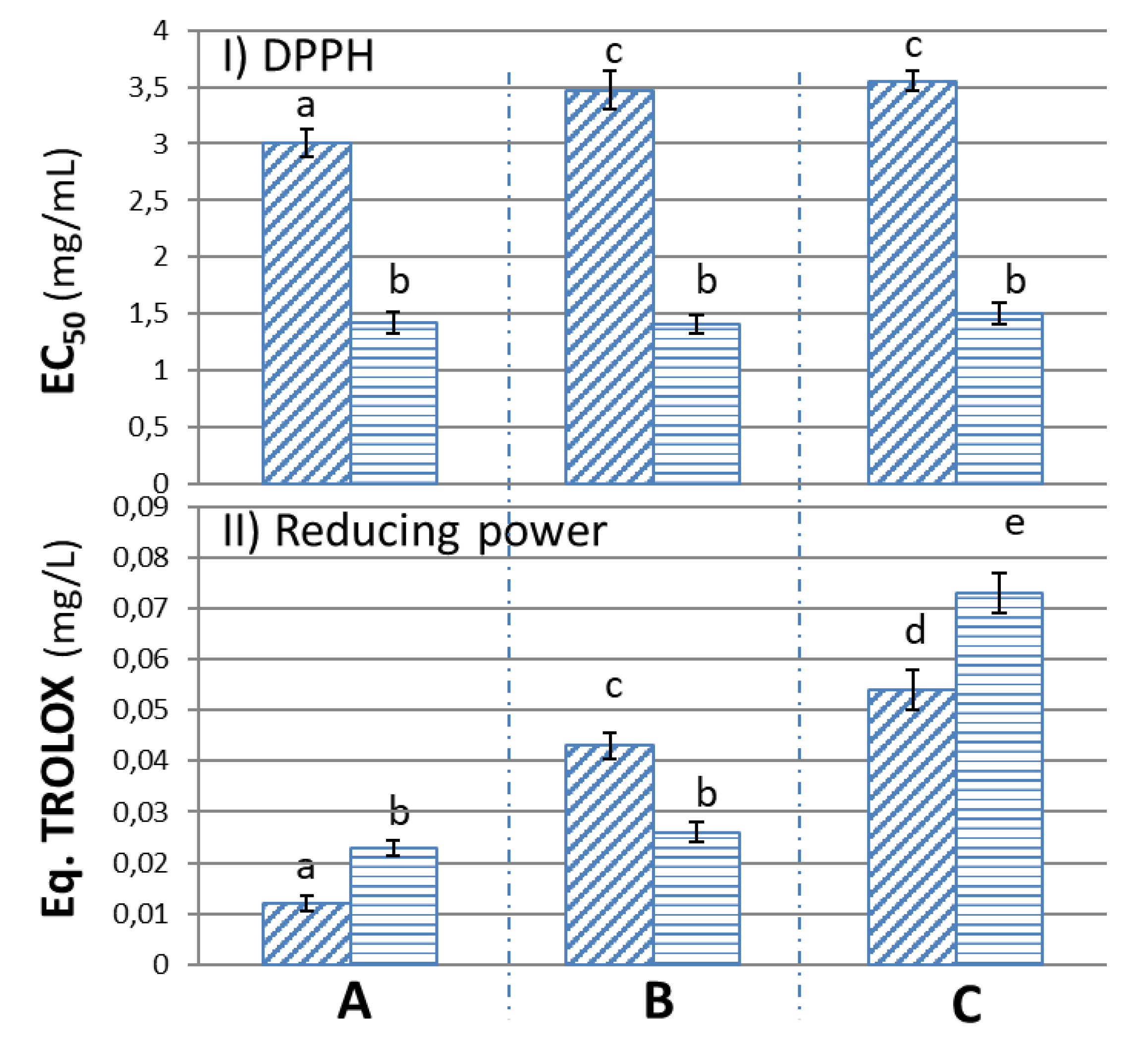
3.3.1. Anti-Radical Potential (DPPH)
3.3.2. Reducing Power (RP)
3.3.3. Lipid Oxidation (Rancimat Test)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Olive Oil Production Cost Study. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/INTERNATIONAL-OLIVE-OIL-PRODUCTION-COSTS-STUDY-.pdf (accessed on 18 January 2020).
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Lama-Muñoz, A.; Rubio-Senent, F.; Bermúdez-Oria, A.; Fernández-Bolaños, J.; Fernández Prior, A.; Rodríguez-Gutiérrez, G. The use of industrial thermal techniques to improve the bioactive compounds extraction and the olive oil solid waste utilization. Innovative. Innov. Food Sci. Emerg. Technol. 2019, 55, 11–17. [Google Scholar] [CrossRef]
- Gomez-de la Cruz, F.J.; Casanova-Pelaez, P.J.; Palomar-Carnicero, J.M.; Cruz-Peragon, F. Characterization and analysis of the drying real process in an industrial olive-oil mill waste rotary dryer: A case of study in Andalusia. Appl. Therm. Eng. 2017, 116, 1–10. [Google Scholar] [CrossRef]
- Zrncevic, S. Evaluation of olive oil mill wastewater. HrvatskeVode 2018, 26, 75–90. [Google Scholar]
- Pantziaros, A.G.; Trachili, X.A.; Zentelis, A.D.; Sygouni, V.; Paraskeva, C.A. A new olive oil production scheme with almost zero wastes. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Guillén, R.; Jiménez, A. Extraction of interesting organic compounds from olive oil waste. Grasas Y Aceites 2006, 57, 95–106. [Google Scholar] [CrossRef]
- Rodríguez, G.; Fernández-Bolaños, J.; Rodríguez, R.; Guillén, R.; Jiménez, A. Antioxidant activity of effluents during the purification of hydroxytyrosol and 3,4-dihydroxyphenylglycol from olive oil waste. Eur. Food Res. Technol. 2007, 224, 733–741. [Google Scholar] [CrossRef]
- Visioli, F.; Davalos, A.; Hazas, M.C.L.; Crespo, M.C.; Tome-Carneiro, J. An overview of the pharmacology of olive oil and its active ingredients. Br. J. Pharmacol. 2020, 177, 1316–1330. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.G.; López, O.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Hydroxytyrosol and derivatives: Isolation, synthesis, and biological properties. Curr. Org. Chem. 2008, 12, 442–463. [Google Scholar] [CrossRef]
- Britton, J.; Davis, R.; O’Connor, K.E. Chemical, physical and biotechnological approaches to the production of the potent antioxidant hydroxytyrosol. Appl. Microbiol. Biotechnol. 2019, 103, 5957–5974. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Duthie, G.G.; Wood, S.; Morrice, P.; Nicol, F.; Reid, M.; Cantlay, L.L.; Kelder, T.; Fernández-Bolaños, J.; de Roos, B. Alperujo extract, hydroxytyrosol and 3,4-dihydroxyphenylglycol are bioavailable and have antioxidant properties in vitamin-E deficient rats–a proteomics and network analysis approach. Mol. Nutr. Food. Res. 2012, 56, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Lama-Muñoz, A.; Rubio-Senent, F.; Bermúdez-Oria, A.; Fernández-Prior, A.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Synergistic effect of 3,4-dihydroxyphenylglycol with hydroxytyrosol and α-tocopherol on the Rancimat oxidative stability of vegetable oils. Innov. Food Sci. Emerg. Technol. 2019, 51, 100–106. [Google Scholar] [CrossRef]
- Kielbasa, W.; Lobo, E. Pharmacodynamics of Norepinephrine Reuptake Inhibition: Modeling the Peripheral and Central Effects of Atomoxetine, Duloxetine, and Edivoxetine on the Biomarker 3,4-Dihydroxyphenylglycol in Humans. J. Clin. Pharmacol. 2015, 55, S80. [Google Scholar] [CrossRef]
- Sanchez-Fidalgo, S.; Villegas, I.; Aparicio-Soto, M.; Cardeno, A.; Rosillo, M.A.; González-Benjumea, A.; Marset, A.; López, O.; Maya, I.; Fernández-Bolaños, J.G.; et al. Effects of dietary virgin olive oil polyphenols: Hydroxytyrosyl acetate and 3, 4-dihydroxyphenylglycol on DSS-induced acute colitis in mice. J. Nutr. Biochem. 2015, 26, 513–520. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Fernández-Bolaños, J.; García-Borrego, A.; Espejo-Calvo, J.A.; Rojano-Delgado, A.M.; Fernández-Prior, M.A. Uso Del 3,4-dihidroxifenilglicol (DHFG) Como Fitorregulador. Spanish National Patent No. P201631404, 3 November 2016. [Google Scholar]
- Medina, E.; Romero, C.; Brenes, M. Residual Olive Paste as a Source of Phenolic Compounds and Triterpenic Acids. Eur. J. Lipid Sci. Technol. 2018, 120, 1700368. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutiérrez, G.; Fernández-Bolaños, J.; Borja, R. Phenols recovery after steam explosion of Olive Mill Solid Waste and its influence on a subsequent biomethanization process. Bioresour. Technol. 2017, 20, 169–178. [Google Scholar] [CrossRef]
- Fernández-BolañosGuzmán, J.; Rodríguez Gutiérrez, G.; Lama Muñoz, A.; Senent Rubio, F.; Fernández-BolañosGuzmán, J.M.; Maya Castilla, I.; López López, O.; Marset Castro, A. Method for Obtaining Hydroxytyrosol Extract, Mixture of Hydroxytyrosol and 3,4-Dihydroyphenylglycol Extract, and Hydroxytyrosol Acetate Extract, from by-Products of the Olive Tree, and the Purification Thereof; International publication number WO 2013/007850; WIPO: Geneva, Switzerland, 2014. [Google Scholar]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Chemical screening of olive biophenol extracts by hyphenated liquid chromatography. Anal. Chim. 2007, 603, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Witham, F.H.; Blaydes, D.F.; Devlin, R.M. Experiment in Plant Physiology; Van Nostrand Reinhold Co.: New York, NY, USA, 1971; p. 245. [Google Scholar]
- Psarra, E.; Makris, D.P.; Kallithraka, S.; Kefalas, P. Evaluation of the antiradical and reducing properties of selected Greek whitewines: Correlation with polyphenolic composition. J. Sci. Food Agric. 2002, 82, 1014–1020. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Rodríguez-Gutiérrez, G.; Jaramillo-Carmona, S.; Espejo-Calvo, J.A.; Rodríguez-Arcos, R.; Fernández-Bolaños, J.; Guillén-Bejarano, R.; Jiménez-Araujo, A. Extraction method on phytochemical composition and antioxidant activity of high dietary fibre powders obtained from asparagus byproducts. Food Chem. 2009, 116, 484–490. [Google Scholar]
- Yoshida, H.; Kondo, I.; Kajimoto, G. Participation of free fatty acids in the oxidation of purified soybean oil during microwave heating. JAOCS 1992, 69, 1136–1140. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Kalogerakis, N. Treatment of olive mill effluents Part I. Organic matter degradation by chemical and biological processes an overview. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef]
- Saez, L.; Pérez, J.; Martínez, J. Low molecular weight phenolics attenuation during simulated treatment of wastewaters from olive oil mills in evaporation ponds. Water Res. 1992, 26, 1261–1266. [Google Scholar]
- Kavvadias, V.; Doula, M.K.; Komnitsas, K.; Liakopoulou, N. Disposal of olive oil mill wastes in evaporation ponds: Effects on soil properties. J. Hazard. Mater. 2010, 182, 144–155. [Google Scholar] [PubMed]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; De Martino, A.; Arienzo, M. Recovery of the metal polymeric organic fraction (polymerin) from olive oil mill waste waters. J. Agric. Food Chem. 2002, 50, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Chemical characterization and properties of a polymeric phenolic fraction obtained from olive oil waste. Food Res. Int. 2013, 54, 2122–2129. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Munoz, A.; Fernández-Bolaños, J. New phenolic compounds hydrothermally extracted from the olive oil by-product alperujo and their antioxidative activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar] [CrossRef] [PubMed]


| Chemical Parameters | Different Heights of the Pond | |||
|---|---|---|---|---|
| A (Upper) | B (Medium) | C (Down) | ||
| Suspended solid (g/L) | 1.71 ± 0.06c * | 2.23 ± 0.10b | 8.49 ± 0.38a | |
| Fat concentration (g/L) | ||||
| Fat in solid | 0.15 ± 0.03c | 0.19 ± 0.02b | 0.77 ± 0.13a | |
| Fat in water | 0.59 ± 0.11b | 0.77 ± 0.07a | 0.70 ± 0.06b | |
| Total fat | 0.74 ± 0.14c | 0.96 ± 0.09b | 1.47 ± 0.19a | |
| Phenolic compounds (g/L) | ||||
| Total phenols | 3.74 ± 0.41a | 3.91 ± 0.17a | 4.11 ± 0.22a | |
| Individual phenolic | 3,4-dihydroxyphenylglycol | 0.11 ± 0.02a | 0.12 ± 0.01a | 0.12 ± 0.01a |
| Hydroxytyrosol | 1.12 ± 0.09a | 1.27 ± 0.04a | 1.21 ± 0.01a | |
| Tyrosol | 0.27 ± 0.04a | 0.33 ± 0.03a | 0.31 ± 0.03a | |
| Chemical Parameters of the Extracts | Different Depths of the Pond | |||
|---|---|---|---|---|
| A (Upper) | B (Medium) | C (Down) | ||
| Ethyl acetate extract | ||||
| g of extract/L of alperujo water | 10.65 ± 1.43b * | 12.95 ± 2.02ab | 14.44 ± 1.77a | |
| Sugars | Total sugar (g/g of dry extract) | 0.23 ± 0.01a | 0.19 ± 0.01a | 0,19 ± 0,02a |
| % referred to dry matter | 2.29 | 1.93 | 1,94 | |
| Phenolic | Total phenols | 0.19 ± 0.07a | 0.17 ± 0.03b | 0,17 ± 0,04b |
| (g/g of dry extract) | ||||
| % referred to dry matter | 19.42 | 17.21 | 17,12 | |
| Individual phenolic(mg/g of dry extract) | DHPG | 2.71 ± 0.30a | 2.04 ± 0.05b | 1,21 ± 0,12c |
| HT | 52.91 ± 3.14a | 48.37 ± 4.16a | 53,23 ± 1,77a | |
| Ty | 24.04 ± 2.33a | 18.19 ± 2.07a | 20,23 ± 3,91a | |
| Chromatographic extract | ||||
| g of extract/L of alperujo water | 3.39 ± 0.12 | 4.05 ± 0.06 | 3.65 ± 0.19 | |
| Sugars | Total sugar (g/g of dry extract) | 0.33 ± 0.02a | 0.28 ± 0.03a | 0.34 ± 0.04a |
| % referred to dry matter | 3.31 | 2.83 | 3.35 | |
| Phenolic | Total phenols (g/g of dry extract) | 0.33 ± 0.04a | 0.26 ± 0.02b | 0.26 ± 0.03b |
| % referred to dry matter | 33.44 | 26.18 | 26.25 | |
| Individual phenolic (mg/g of dry extract) | DHPG | 0.64 ± 0.02a | 0.53 ± 0.06b | 1.10 ± 0.11b |
| HT | 64.89 ± 3.01a | 89.27 ± 5.45b | 82.84 ± 4.67b | |
| Ty | 34.38 ± 2.18a | 31.11 ± 2.50a | 32.84 ± 3.07a | |
| Retention Time (min) | Compounds | Molecular Weight | λmax (nm) | m/z − |
|---|---|---|---|---|
| 10.20 | Hydroxytyrosol | 154 | 214, 234, 278 | 153, 123 |
| 14.25 | Tyrosol | 138 | 200, 218, 275 | 137 |
| 15.41 | Elenolic acid derivative | 242 | 230 | 241, 237, 135 |
| 16.80 | Tyrosol derivative | 186 | 275 | 185, 151, 137 |
| 18.10 | Vanillic acid | 168 | 200, 218, 255, 298 | 167, 108, 45 |
| 21.23 | Acido 4-Hidroxibenzoico | 336 | 278 | 335, 215, 153, 125 |
| 23.60 | Hydroxytyrosol derivative | 234 | 278 | 233, 151, 123 |
| 24.78 | 3,4-Dihydroxyphenylacetic acid derivative | 168 | 214, 234, 278 | 151, 123, 109, 59 |
| 25.53 | 4 metilcatecol | 124 | 236 | 123, 107, 69 |
| 27.05 | Hydroxytyrosol derivative | 211 | 280 | 210,151, 123, 59, |
| 29.35 | Luteolin-7-O-Rutinoside | 594 | 200, 254, 349 | 593, 447, 285, 151 |
| 30.13 | Verbascoside | 624 | 198, 328 | 623, 461, 161 |
| 31.92 | Oleuropein aglycone derivative | 378 | 200, 222, 280 | 377, 225, 123 |
| 32.58 | Oleuropein derivative 1 | 538 | 214, 234, 278 | 537, 377, 287, 257, 211, 123 |
| 34.09 | Oleuropein derivative 2 | 378 | 280 | 377, 361, 313, 180, 151 |
| 36.02 | Oleuropein derivative 3 | 538 | 280 | 537, 403, 385, 223, 151, 123 |
| 38.07 | Unidentified | 630 | 280 | 629, 303, 187, 123 |
| 39.68 | Unidentified | 630 | 280 | 629, 303, 185, 183, 139 |
| 39.97 | Unidentified | 630 | 280 | 629, 305, 186,185 |
| 42.51 | Unidentified | 810 | 280 | 809, 613, 563, 359, 195, 123,153 |
| 42.97 | Unidentified | 810 | 280 | 809, 323, 195, 125, |
| 45.00 | Unidentified | 810 | 280 | 329, 307, 185, 125 |
| 46.23 | Unidentified | 810 | 280 | 329, 285, 187, 153 |
| Rancimat Test | Concentration (mg/L) | Induction Time (h) | ΔIT (%) | |
|---|---|---|---|---|
| Control (without extracts) | 0 | 7.30 ± 0.03 b * | - | |
| Solvent extract | 760 | 9.23 ± 0.18 a | 23.4 | |
| Main phenols | DHPG | 1.5 | ||
| HT | 39.1 | |||
| Ty | 15.8 | |||
| Resin extract | 760 | 9.85 ± 0.17 a | 34.9 | |
| Main phenols | DHPG | 0.6 | ||
| HT | 60.0 | |||
| Ty | 24.9 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Prior, M.Á.; Fatuarte, J.C.P.; Oria, A.B.; Viera-Alcaide, I.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. New Liquid Source of Antioxidant Phenolic Compounds in the Olive Oil Industry: Alperujo Water. Foods 2020, 9, 962. https://doi.org/10.3390/foods9070962
Fernández-Prior MÁ, Fatuarte JCP, Oria AB, Viera-Alcaide I, Fernández-Bolaños J, Rodríguez-Gutiérrez G. New Liquid Source of Antioxidant Phenolic Compounds in the Olive Oil Industry: Alperujo Water. Foods. 2020; 9(7):962. https://doi.org/10.3390/foods9070962
Chicago/Turabian StyleFernández-Prior, María África, Juan Carlos Pérez Fatuarte, Alejandra Bermúdez Oria, Isabel Viera-Alcaide, Juan Fernández-Bolaños, and Guillermo Rodríguez-Gutiérrez. 2020. "New Liquid Source of Antioxidant Phenolic Compounds in the Olive Oil Industry: Alperujo Water" Foods 9, no. 7: 962. https://doi.org/10.3390/foods9070962
APA StyleFernández-Prior, M. Á., Fatuarte, J. C. P., Oria, A. B., Viera-Alcaide, I., Fernández-Bolaños, J., & Rodríguez-Gutiérrez, G. (2020). New Liquid Source of Antioxidant Phenolic Compounds in the Olive Oil Industry: Alperujo Water. Foods, 9(7), 962. https://doi.org/10.3390/foods9070962







