Bee Pollen Role in Red Winemaking: Volatile Compounds and Sensory Characteristics of Tintilla de Rota Warm Climate Red Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Layout
2.2. Analysis of Volatile Compounds and Odor Activity Values (OAV)
2.3. Sensory Wines Evaluation
2.4. Data Treatment
3. Results and Discussion
3.1. Evaluation of the Effects of the Addition of Pollen on Wine Volatile Compounds and their Corresponding Odorant Activity Values (OAV)
3.1.1. Higher Alcohols and Methanol
3.1.2. Aldehydes
3.1.3. Alcohols
3.1.4. Acids
3.1.5. Esters
3.1.6. C6-Alcohols
3.1.7. Terpenes
3.1.8. Phenols
3.1.9. Thiols, Acetals, and Norisoprenoids
3.1.10. Lactones
3.1.11. Principal Component Analysis (PCA) of Volatile Compounds
3.1.12. Odorant Activity Values (OAV) Analysis.
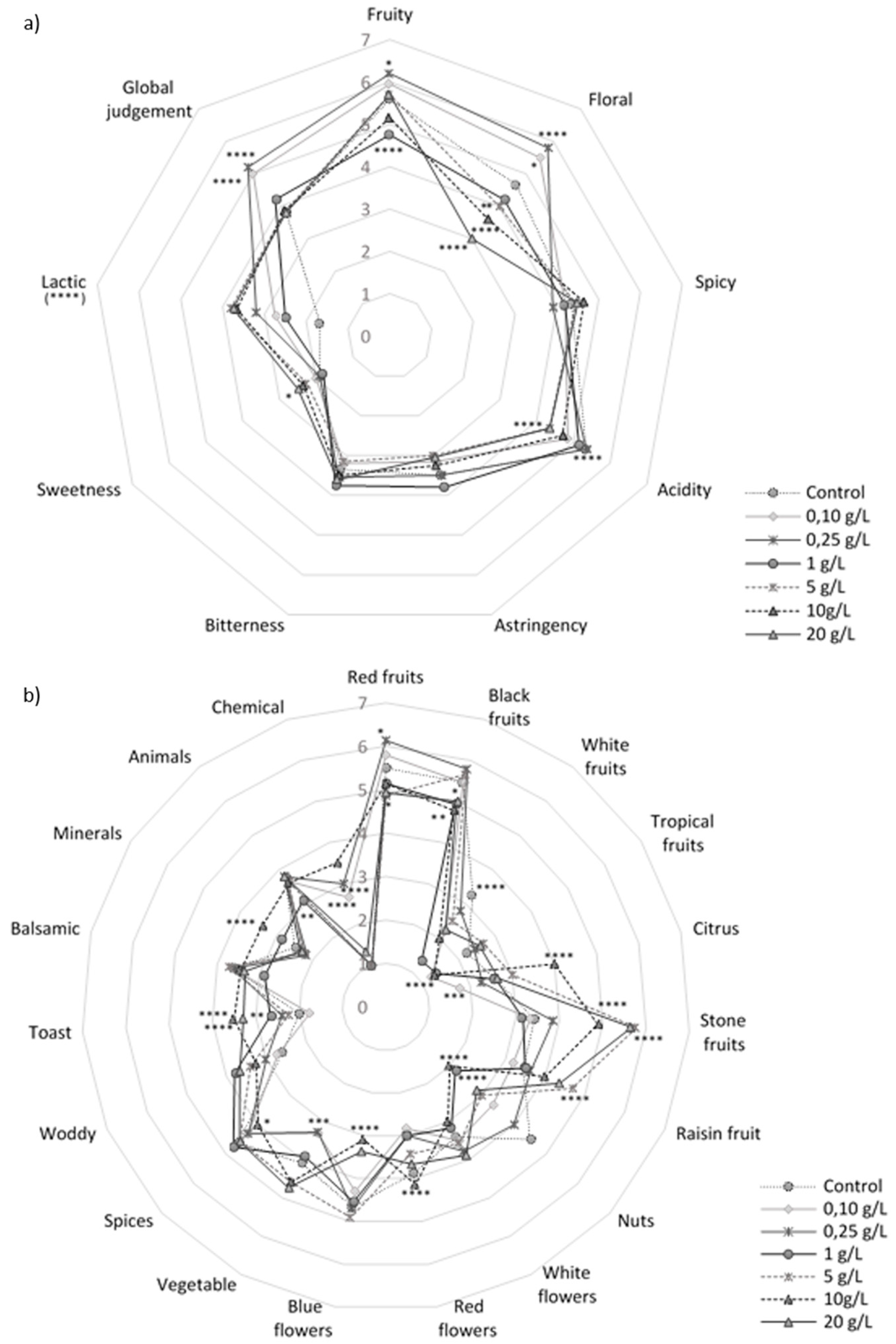
3.2. Sensory Evaluation of the Resulting Wines.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mestre, M.V.; Maturano, Y.P.; Gallardo, C.; Combina, M.; Mercado, L.; Toro, M.E.; Carrau, F.; Vazquez, F.; Dellacassa, E. Impact on sensory and aromatic profile of low ethanol malbec wines fermented by sequential culture of Hanseniaspora uvarum and Saccharomyces cerevisiae native yeasts. Fermentation 2019, 5, 65. [Google Scholar] [CrossRef]
- King, E.S.; Kievit, R.L.; Curtin, C.; Swiegers, J.H.; Pretorius, I.S.; Bastian, S.E.P.; Leigh Francis, I. The effect of multiple yeasts co-inoculations on Sauvignon Blanc wine aroma composition, sensory properties and consumer preference. Food Chem. 2010, 122, 618–626. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Polášková, P.; Herszage, J.; Ebeler, S.E. Wine flavor: Chemistry in a glass. Chem. Soc. Rev. 2008, 37, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- Briche, E.; Beltrando, G.; Somot, S.; Quénol, H. Critical analysis of simulated daily temperature data from the ARPEGE-climate model: Application to climate change in the Champagne wine-producing region. Clim. Chang. 2014, 123, 241–254. [Google Scholar] [CrossRef]
- Sacchelli, S.; Fabbrizzi, S.; Menghini, S. Climate change effects and adaptation strategies in the wine sector: A quantitative literature review. Wine Econ. Policy 2016, 5, 114–126. [Google Scholar] [CrossRef]
- Zamora, F. Dealcoholised wines and low-alcohol wines. In Wine Safety, Consumer Preference, and Human Health; Springer: Cham, Switzerland, 2016; pp. 163–182. [Google Scholar]
- De Orduña, R.M. Climate change associated effects on grape and wine quality and production Food Research International Climate change associated effects on grape and wine quality and production. Food Res. Int. 2017, 43, 1844–1855. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Yeast and Biochemistry of Ethanol Fermentation. In Principles and Practices of Winemaking, 1st ed.; Boulton, R.B., Singleton, V.L., Bisson, L.F., Kunkee, R.E., Eds.; Springer: London, UK, 1996; pp. 141–143. [Google Scholar]
- Webb, L.B.; Whetton, P.H.; Barlow, E.W.R. Modelled impact of future climate change on the phenology of winegrapes in Australia. Aust. J. Grape Wine Res. 2007, 13, 165–175. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Ibarz, M.J.; Cacho, J.; Ferreira, V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005, 89, 163–174. [Google Scholar] [CrossRef]
- Lee, P.R.; Toh, M.; Yu, B.; Curran, P.; Liu, S.Q. Manipulation of volatile compound transformation in durian wine by nitrogen supplementation. Int. J. Food Sci. Technol. 2013, 48, 650–662. [Google Scholar] [CrossRef]
- Trinh, T.T.T.; Woon, W.Y.; Yu, B.; Curran, P.; Liu, S.Q. Effect of L-isoleucine and L-phenylalanine addition on aroma compound formation during longan juice fermentation by a co-culture of Saccharomyces cerevisiae and Williopsis saturnus. S. Afr. J. Enol. Vitic. 2010, 31, 116–124. [Google Scholar] [CrossRef]
- Barth, O.M.; Almeida-Muradian, L.B.; Pamplona, L.C.; Coimbra, S.; Barth, O.M. Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food Compos. Anal. 2005, 18, 105–111. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; Freitas, A.D.S.D.; Barth, O.M.; de Almeida-Muradian, L.B.D.; De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; et al. Phenolic profile by HPLC-MS, biological potential, and nutritional value of a promising food: Monofloral bee pollen. J. Food Biochem. 2018, 42, e12536. [Google Scholar] [CrossRef]
- Human, H.; Nicolson, S.W. Nutritional content of fresh, bee-collected and stored pollen of Aloe greatheadii var. davyana (Asphodelaceae). Phytochemistry 2006, 67, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Paramás, A.M.G.G.; Bárez, J.A.G.; Marcos, C.C.; García-Villanova, R.J.; Sánchez, J.S.; Gonzalez-paramas, A.M.; Garcia-villanova, R.J.; Gonza, A.M.; Paramás, A.M.G.G.; Bárez, J.A.G.; et al. HPLC-fluorimetric method for analysis of amino acids in products of the hive (honey and bee-pollen). Food Chem. 2006, 95, 148–156. [Google Scholar] [CrossRef]
- Dong, J.; Yang, Y.; Wang, X.; Zhang, H. Fatty acid profiles of 20 species of monofloral bee pollen from China. J. Apic. Res. 2017, 54, 503–511. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Dong, J.; Zhang, H. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov. Food Sci. Emerg. Technol. 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Sancho-Galán, P.; Jiménez-Cantizano, A.; Palacios, V. Bee Pollen as Oenological Tool to Carry out Red Winemaking in Warm Climate Conditions. Agronomy 2020, 10, 634. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Effect on White Grape Must of Multiflora Bee Pollen Addition during the Alcoholic Fermentation Process. Molecules 2018, 23, 1321. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Evaluation of the use of multiflora bee pollen on the volatile compounds and sensorial profile of Palomino fino and Riesling white young wines. Food Res. Int. 2018, 105, 197–209. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Use of multiflora bee pollen as a flor velum yeast growth activator in biological aging wines. Molecules 2019, 24, 1763. [Google Scholar] [CrossRef] [PubMed]
- Tronchoni, J.; Curiel, J.A.; Sáenz-Navajas, M.P.; Morales, P.; De-la-Fuente-Blanco, A.; Fernández-Zurbano, P.; Ferreira, V.; Gonzalez, R. Aroma profiling of an aerated fermentation of natural grape must with selected yeast strains at pilot scale. Food Microbiol. 2018, 70, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.A.J.; Bueno, J.E.; Moreno, J.A.J.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- ISO NORM. 3591(1977) Sensory Analysis: Apparatus Wine Tasting Glass; ISO: Geneva, Switzerland, 1977. [Google Scholar]
- Jackson, R.S. Wine Tasting: A Professional Handbook; Academic Press: London, UK, 2009; p. 350. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A. Tratado de Enologia: Microbiología del Vino. Vinificaciones; Mundi-Prensa: Buenos Aires, Argentina, 2003. [Google Scholar]
- Togores, J.H. Tratado de Enología I; Mundi Prensa: Madrid, Spain, 2011. [Google Scholar]
- Lonvaud-Funel, A.; Joyeux, A.; Desens, C. Inhibition of malolactic fermentation of wines by products of yeast metabolism. J. Sci. Food Agric. 1988, 44, 183–191. [Google Scholar] [CrossRef]
- Zeeman, W.; Snyman, J.P.; van Wyk, C.J. The influence of yeast strain and malolactic fermentation on some volatile bouquet substances and on quality of table wines. In Grape and Wine Centennial Symposium; University of California: Davis, CA, USA, 1982. [Google Scholar]
- Laurent, M.-H.; Henick-Kling, T.; Acree, T.E. Changes in he aroma and odor of Chardonnay wine due to malolactic fermentation. Vitic. Enol. Sci. 1994, 49, 3–10. [Google Scholar]
- De Revel, G.; Martin, N.; Pripis-Nicolau, L.; Lonvaud-Funel, A.; Bertrand, A. Contribution to the knowledge of malolactic fermentation influence on wine aroma. J. Agric. Food Chem. 1999, 47, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P.; Cliff, M.; King, M.; Girard, B.; Hall, J.; Reynolds, A. Effect of two commercial malolactic cultures on the chemical and sensory properties of Chancellor wines vinified with different yeasts and fermentation temperatures. Am. J. Enol. Vitic. 2000, 51, 42–48. [Google Scholar]
- Gambaro, A.; Boido, E.; Zlotejablko, A.; Medina, K.; Lloret, A.; Dellacassa, E.; Carrau, F. Effect of malolactic fermentation on the aroma properties of Tannat wine. Aust. J. Grape Wine Res. 2001, 7, 27–32. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef]
- Crouzet, J.; Flanzy, C. Les Enzymes En Oenologie. In Œnologie, Fondements Scientifiques et Technologiques; Tec & doc-Lavoisier: Paris, France, 1998; pp. 362–413. [Google Scholar]
- Cheynier, V.; Schneider, R.; Salmon, J.M.; Fulcrand, H. Chemistry of Wine. In Comprehensive Natural Products II, 1st ed.; Hung-Wen, L., Mander, L., Eds.; Elsevier: Ámsterdam, The Netherlands, 2010; pp. 1119–1172. [Google Scholar]
- Bayonove, C.L.; Baumes, R.L.; Crouzet, J.; Günata, Z. Aromas. In Enología: Fundamentos científicos y tecnológicos, 1st ed.; Flanzy, C., Ed.; Mundi-Prensa: Madrid, Spain, 2003; pp. 137–176. [Google Scholar]
- Campos, M.G.R.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- da Silva, G.R.; da Natividade, T.B.; Camara, C.A.; da Silva, E.M.S.; dos Santos, F.D.A.R.; Silva, T.M.S. Identification of Sugar, Amino Acids and Minerals from the Pollen of Jandaíra Stingless Bees (Melipona subnitida). Food Nutr. Sci. 2014, 05, 1015–1021. [Google Scholar] [CrossRef]
- Bogdanov, S. Pollen: Production, Nutrition and Health : A Review. 2017. Available online: https://www.bee-hexagon.net/pollen/ (accessed on 4 May 2018).
- Palacios, V.; Roldán, A.; Jiménez-Cantizano, A.; Amores-Arrocha, A. Physicochemical and microbiological characterization of the sensory deviation responsible for the origin of the special sherry wines “palo cortado” type. PLoS ONE 2018, 13, e0208330. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic, I. Gas Chromatography and Mass Spectrometry in Honey Fingerprinting: The Occurrence of 3,4-dihydro-3-oxoedulan and (E)-4-(r-1’,t-2’,c-4’-trihdroxy-3’,6’,6’-trimethylcyclohexyl)-but-3-en-2-one. Int. J. Chem. Mol. Eng. 2018, 12, 81526. [Google Scholar]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.S.; Wang, H.; Zhang, L. Impact odorants of Chardonnay dry white wine from Changli County (China). Eur. Food Res. Technol. 2008, 227, 287–292. [Google Scholar] [CrossRef]
| Compounds | Tintilla de Rota Red Wines Pollen Doses | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.1 g/L | 0.25 g/L | 1 g/L | 5 g/L | 10 g/L | 20 g/L | |||||||||||||||
| Higher alcohols | |||||||||||||||||||||
| 2-Propanol | 34,953.9 | ± | 1276.1 a | 36,052.5 | ± | 2210.1 a | 38,842.7 | ± | 4907.9 a | 34,985.5 | ± | 1788.9 a | 36,902.1 | ± | 799.7 a | 34,768.3 | ± | 1495 a | 33,171.9 | ± | 815.5 a |
| n-Propyl alcohol | 27,691.4 | ± | 2165.4 a | 28,078.3 | ± | 3292.1 a,b | 27,692.6 | ± | 1445 a | 33,332.2 | ± | 3069.1 b,c | 29,588.4 | ± | 720.7 a,c | 30,273.1 | ± | 2148.2 a,c | 34,846.2 | ± | 2039.4 c |
| Isobutanol | 31,385.1 | ± | 1539.9 a,b | 31,248.3 | ± | 1983.1 a | 39,121 | ± | 683.8 b | 38,397.8 | ± | 1745.5 a,b | 34,307.7 | ± | 358.1 a,b | 36,460.3 | ± | 1599.1 a,b | 50,569 | ± | 1463.5 c |
| Isoamyl alcohols | 354,323.6 | ± | 16,785.8 a,b | 350,155.6 | ± | 21,853 a,b | 393,105.9 | ± | 5534 a | 354,546.7 | ± | 16,445 a,b | 316,247.7 | ± | 3734.7 b | 347,556.9 | ± | 15,377.3 a,b | 355,663.7 | ± | 10,652.6 a,b |
| Total | 448,353.9 | ± | 21,767.1 | 445,534.8 | ± | 29,338.3 | 498,762.1 | ± | 12,570.7 | 461,262.2 | ± | 23,048.6 | 417,045.8 | ± | 5613.1 | 449,058.5 | ± | 20,619.7 | 474,250.8 | ± | 14,971 |
| % higher alcohols | 77.53% | 76.12% | 77.82% | 74.73% | 70.49% | 71.89% | 67.65% | ||||||||||||||
| Methanol | 32,708.5 | ± | 3833 a,b | 34,834.6 | ± | 3461.2 a,c,d | 27,651.5 | ± | 141.1 b | 40,294 | ± | 5164 c,d,e | 40,450.6 | ± | 1741.7 d,e | 43,161.7 | ± | 5621 e | 33,131.9 | ± | 4624.9 a,b |
| Total | 32,708.5 | ± | 3833 | 34,834.6 | ± | 3461.2 | 27,651.5 | ± | 141.1 | 40,294 | ± | 5164 | 40,450.6 | ± | 1741.7 | 43,161.7 | ± | 5621 | 33,131.9 | ± | 4624.9 |
| % methanol | 5.66% | 5.95% | 4.31% | 6.53% | 6.84% | 6.91% | 4.73% | ||||||||||||||
| Acids | |||||||||||||||||||||
| Butanoic acid | 30 | ± | 0.2 a,b | 33 | ± | 0.7 b | 34.2 | ± | 0.8 c,d | 29.3 | ± | 0.8 a | 32 | ± | 2.5 b,c,d | 29.8 | ± | 0.4 a,b | 29.4 | ± | 0.7 a,b |
| 3-Methyl-butanoic acid | 189 | ± | 1.6 a | 190.4 | ± | 2.7 a | 182.2 | ± | 10.7 a | 188.8 | ± | 13.5 a | 193.4 | ± | 6.8 a | 189.9 | ± | 14.1 a | 184.2 | ± | 7.3 a |
| Hexanoic acid | 556.3 | ± | 42.4 a | 651.6 | ± | 5.8 b | 466.5 | ± | 26.1 c,e,f | 390.4 | ± | 4.5 d | 463.5 | ± | 29.4 e,f | 477.2 | ± | 3.3 f | 770.4 | ± | 23 g |
| Heptanoic acid | 20.7 | ± | 0.7 a | 24 | ± | 0.6 a | 24.7 | ± | 0.5 a | 25.6 | ± | 0.7 a | 27.9 | ± | 0.9 a | 74.8 | ± | 0.9 b | 144.4 | ± | 10.7 c |
| 2-Hexenoic acid | 34 | ± | 0.6 a | 39.4 | ± | 0.6 b,c | 39.9 | ± | 0.3 c | 44.6 | ± | 1.6 d,e | 45.7 | ± | 1.7 e | 53.7 | ± | 4.5 f | 60 | ± | 0.2 g |
| Octanoic acid | 624.4 | ± | 19.8 a | 366.6 | ± | 23.9 b,c | 443.7 | ± | 30.9 c | 648.1 | ± | 37.2 a | 1424.5 | ± | 14 d,e | 1443.3 | ± | 30.6 e | 1253.8 | ± | 39.3 f |
| Nonanoic acid | 87.7 | ± | 2.1 a | 76.4 | ± | 3.4 a | 90.5 | ± | 3.7 a | 116.2 | ± | 1.4 a | 556.2 | ± | 9.7 b | 182.6 | ± | 0.4 c | 184.4 | ± | 11.1 c |
| n-Decanoic acid | 0.19 | ± | 0.01 a | 398.5 | ± | 26.5 b,c,f | 412.4 | ± | 1.3 c,f | 311.9 | ± | 6.9 d | 866.4 | ± | 46.2 e | 440.9 | ± | 3.0 f | 450.3 | ± | 39.6 f |
| 9-Decenoic acid | 21.4 | ± | 1.5 a | 154.3 | ± | 12.4 b | 33.8 | ± | 1.9 c,d | 39.1 | ± | 0.8 d | 80.1 | ± | 1.3 e,g | 52.7 | ± | 2.7 f | 87.9 | ± | 0.6 g |
| Benzoic acid | 84.2 | ± | 5.9 a | 200.8 | ± | 0.1 b | 108.7 | ± | 1.4 a,c | 125 | ± | 5.8 c | 328.8 | ± | 29.8 d | 274 | ± | 3.5 e | 371.3 | ± | 12.2 f |
| Benzeneacetic acid | 40.8 | ± | 0.4 a | 42.8 | ± | 0.8 a,b,c | 41.9 | ± | 1.1 a | 43.9 | ± | 0.5 a,b,c | 46 | ± | 0.7 b,c | 46.4 | ± | 0.7 c | 49.5 | ± | 2.2 c |
| Total | 1688.6 | ± | 75.2 | 2177.7 | ± | 77.5 | 1878.5 | ± | 78.7 | 1962.8 | ± | 73.8 | 4064.5 | ± | 143.1 | 3265.3 | ± | 64.1 | 3585.5 | ± | 147 |
| % acids | 0.29% | 0.37% | 0.29% | 0.32% | 0.69% | 0.52% | 0.51% | ||||||||||||||
| C-6 alcohols | |||||||||||||||||||||
| 1-Hexanol | 493.9 | ± | 9.5 a | 431.1 | ± | 1.7 b,c,d | 436.6 | ± | 19.2 c,d | 433 | ± | 10.2 d | 434.2 | ± | 20.2 e | 520.3 | ± | 1.4 a | 539.5 | ± | 53.1 a |
| (E)-3-Hexen-1-ol | 16.7 | ± | 0.2 a | 29.1 | ± | 0.5 b,c | 30.9 | ± | 0.9 c,d | 33.1 | ± | 0.8 d | 35.7 | ± | 1.7 e | 36.1 | ± | 0.6 e | 37.7 | ± | 1.4 e |
| (Z)-3-Hexen-1-ol | 25.3 | ± | 2.4 a | 30 | ± | 1.4 a,d | 31.8 | ± | 1.0 a | 91.2 | ± | 0.4 b | 101.1 | ± | 6.8 c | 6.2 | ± | 0.3 d | 9.5 | ± | 0.1 a,d |
| Total | 535.9 | ± | 12.1 | 490.3 | ± | 3.6 | 499.2 | ± | 21 | 557.2 | ± | 11.4 | 571 | ± | 28.7 | 562.6 | ± | 2.3 | 586.7 | ± | 54.6 |
| % C-6 alcohols | 0.09% | 0.08% | 0.08% | 0.09% | 0.10% | 0.09% | 0.08% | ||||||||||||||
| Alcoholes | |||||||||||||||||||||
| 3-Penten-2-ol | 103.6 | ± | 4.9 a | 45.1 | ± | 2.4 b,c | 50.6 | ± | 0.1 c | 63.6 | ± | 4.7 d,e,f | 67.2 | ± | 3.9 e,f | 70.9 | ± | 0.1 f | 129.5 | ± | 2.0 g |
| 1-Pentanol | 1358.2 | ± | 53.1 a | 1812.7 | ± | 73.5 b,c,d,e | 1331.8 | ± | 123.8 a | 1746.8 | ± | 14.0 c,d,e | 1847.5 | ± | 17.9 d,e | 1992.8 | ± | 0.5 e | 2925.1 | ± | 187.4 f |
| 3-Ethyl-2-pentanol | 10.1 | ± | 0.3 a | 12.7 | ± | 0.7 b,c | 13.9 | ± | 1.0 c | 16.6 | ± | 0.1 d,e | 17.8 | ± | 0.1 e | 20 | ± | 0.2 f | 21.3 | ± | 1.9 f |
| 4-Methyl-1-pentanol | 13 | ± | 0.7 a | 16 | ± | 0.8 b,c,d | 17.3 | ± | 0.6 c,d | 18.3 | ± | 0.7 d,e | 20.5 | ± | 0.4 e,f | 20.7 | ± | 1.1 f | 25.8 | ± | 1.7 g |
| 3-Methyl-1-pentanol | 290.4 | ± | 8.0 a | 278.7 | ± | 2.9 a | 184.1 | ± | 7.2 b | 320.1 | ± | 20.5 c | 288.6 | ± | 15.5 a | 273.6 | ± | 4.3 a | 110.2 | ± | 8.7 d |
| 3-Ethoxy-1-Propanol | 99.3 | ± | 0.7 a | 100.7 | ± | 0.7 a | 112.4 | ± | 2.8 b,c,e | 118.9 | ± | 1.1 c,d,e,f | 125 | ± | 0.8 d,e,f | 120 | ± | 1.6 e,f | 125.9 | ± | 0.6 f |
| 1-Octanol | 20.8 | ± | 1.0 a | 23 | ± | 0.7 a,b | 24.7 | ± | 0.4 b,c,d,e | 25.5 | ± | 0.1 c,d,e,f | 25.9 | ± | 0.1 d,e,f | 25.8 | ± | 1.6 e,f | 27.6 | ± | 0.7 f |
| 1-Nonanol | 3.8 | ± | 0.3 a | 2.4 | ± | 0.2 b,c,d,e | 2.7 | ± | 0.02 c,d,f | 2.4 | ± | 0.2 d,e | 2.2 | ± | 0.2 e | 2.8 | ± | 0.2 f,g | 3.1 | ± | 0.02 g |
| Benzyl alcohol | 84.5 | ± | 7.1 a | 93.7 | ± | 0.6 a | 133 | ± | 10.6 a,b | 179.7 | ± | 3.8 b | 603.9 | ± | 58.2 c | 248.8 | ± | 2.3 d | 247.7 | ± | 18.8 d |
| Phenylethyl Alcohol | 23,942.5 | ± | 1414.2 a | 23,512 | ± | 235.9 a | 28,318.7 | ± | 302.5 b,c | 24,110.5 | ± | 1481.8 a | 27,830.4 | ± | 2039.4 c | 22,209.8 | ± | 331.3 a | 23,346.3 | ± | 2021.2 a |
| 1 H-Indol-3-ethanol | 862.1 | ± | 25 a | 1313.5 | ± | 10.6 b,c | 1452 | ± | 118.6 c | 1823.4 | ± | 2.6 d,e | 1866.8 | ± | 64.1 e | 452 | ± | 28.7 f | 551.7 | ± | 6.5 f |
| 1-Butanol | 31.2 | ± | 1.6 a | 22.7 | ± | 0.9 a,b | 15.3 | ± | 0.9 b,e | 23.3 | ± | 1.7 a,e | 114.3 | ± | 5.8 c | 44.3 | ± | 1.0 d | 29.7 | ± | 0.8 a |
| 3-Metil-2-buten-1-ol | 41.5 | ± | 1.7 a | 25.8 | ± | 1.2 b | 46.9 | ± | 1.4 a,c | 49.3 | ± | 4.3 c | 57.1 | ± | 2.8 d | 65.5 | ± | 4.1 e | 77.9 | ± | 1.1 f |
| Total | 26,861 | ± | 1518.6 | 27,259 | ± | 331.2 | 31,703.5 | ± | 570 | 28,498.3 | ± | 1535.6 | 32,867.2 | ± | 2209 | 25,547 | ± | 377 | 27,622 | ± | 2251.4 |
| % Alcoholes | 4.64% | 4.66% | 4.95% | 4.62% | 5.56% | 4.09% | 3.94% | ||||||||||||||
| Phenols | |||||||||||||||||||||
| 2,6-di-terc-butil-4-ethylfenol | 23.1 | ± | 0.8 a | 29.1 | ± | 0.5 b,c | 29.8 | ± | 0.4 c | 20.1 | ± | 0.1 d,e,f | 18.6 | ± | 1.4 e,f | 20.8 | ± | 0.9 a,f | 23.3 | ± | 0.1 a |
| 4-Etilfenol | 6.7 | ± | 0.2 a | 8.2 | ± | 0.4 b,e | 9.4 | ± | 0.7 c,d,e | 10.1 | ± | 0.1 d | 8.6 | ± | 0.2 e | 4.9 | ± | 0.5 f | 7.2 | ± | 0.1 a |
| 4-Vinilguaiacol | 33.4 | ± | 1.1 a | 55.7 | ± | 5.3 b | 76.6 | ± | 3.7 c,d | 74.9 | ± | 6.3 d | 23.1 | ± | 0.8 e,f | 23 | ± | 1.4 f | 41.5 | ± | 0.3 g |
| Acetovainillin | 19.7 | ± | 0.7 a | 32.7 | ± | 0.8 b,d,f | 46.2 | ± | 0.5 c | 32.6 | ± | 0.7 d,f | 26.3 | ± | 0.7 e | 20.5 | ± | 1.4 a | 36.3 | ± | 0.7 f |
| Total | 82.9 | ± | 2.9 | 125.6 | ± | 7 | 162 | ± | 5.2 | 137.7 | ± | 7.2 | 76.6 | ± | 3.1 | 69.2 | ± | 4.1 | 108.3 | ± | 1.2 |
| % Phenols | 0.01% | 0.02% | 0.03% | 0.02% | 0.01% | 0.01% | 0.02% | ||||||||||||||
| Terpenes | |||||||||||||||||||||
| Linalool oxide | 50.5 | ± | 0.9 a | 74.2 | ± | 1.8 b | 86.3 | ± | 8.1 c,f | 59.1 | ± | 0.8 d | 50.1 | ± | 3.5 a | 38.2 | ± | 0.6 e | 88.3 | ± | 2.7 f |
| Linalool | 11.5 | ± | 0.5 a | 12.9 | ± | 0.8 a,b | 13.5 | ± | 0.1 b | 16.7 | ± | 0.3 c,e | 11.8 | ± | 0.5 a | 18.4 | ± | 0.8 d | 17 | ± | 0.5 e,d |
| α-Terpieol | 9.7 | ± | 0.2 a | 10.8 | ± | 0.1 a | 15.3 | ± | 0.1 b | 19.1 | ± | 0.9 c,e | 23.2 | ± | 1.4 d | 18.9 | ± | 1.4 e | 34 | ± | 2.4 f |
| (R)-(+)-β-Citronellol | 8.7 | ± | 0.2 a | 9.7 | ± | 0.2 a,b | 10.2 | ± | 0.1 b | 13.8 | ± | 0.9 c,e | 16.8 | ± | 0.9 d,f | 12.7 | ± | 0.8 e | 15.7 | ± | 1.4 f |
| 2,6-dimetil-3,7-Octadiene-2,6-diol, | 29 | ± | 0.1 a | 32.8 | ± | 0.4 b,e,f,g | 38.9 | ± | 0.2 c,d,e | 41.2 | ± | 1.5 d | 36.2 | ± | 0.7 e,f | 33.3 | ± | 0.6 f,g | 32.6 | ± | 0.7 g |
| 8-Hydroxylinalool | 49.4 | ± | 2.1 a | 100.4 | ± | 0.9 b,d,g | 202.4 | ± | 0.3 c | 91.1 | ± | 7.9 d | 387.1 | ± | 8.9 e | 142.2 | ± | 11.8 f | 130.3 | ± | 9.8 f,g |
| Total | 158.8 | ± | 4.1 | 240.8 | ± | 4.3 | 366.7 | ± | 8.9 | 240.9 | ± | 12.4 | 525.2 | ± | 15.8 | 263.7 | ± | 16 | 318 | ± | 17.4 |
| % Terpenes | 0.03% | 0.04% | 0.06% | 0.04% | 0.09% | 0.04% | 0.05% | ||||||||||||||
| Esters | |||||||||||||||||||||
| Ethyl acetate | 60,290.1 | ± | 5264.2 a | 65,998.8 | ± | 4222.7 a,b | 68,955 | ± | 3516.9 a,b | 75,224.6 | ± | 6073.5 b,c | 85,400.4 | ± | 1651.2 c,d | 94,930.8 | ± | 4988.6 d | 151,421.2 | ± | 10,126 e |
| Ethyl butyrate | 0.15 | ± | 0.01 a | 27.2 | ± | 0.7 b,g | 37.5 | ± | 2.8 c | 55.3 | ± | 1.8 d | 127.1 | ± | 2.4 e | 16.7 | ± | 0.1 f | 23 | ± | 1.9 g |
| Ethyl isovalerate | 5.6 | ± | 0.3 a | 14.5 | ± | 0.2 b | 17.7 | ± | 0.1 c | 20.9 | ± | 0.8 d,g | 25.1 | ± | 0.7 e | 27.6 | ± | 0.1 f | 19.9 | ± | 2.1 g |
| Isoamyl acetate | 29.4 | ± | 0.9 a | 45.5 | ± | 1.6 b,c | 50.5 | ± | 0.3 c | 75.5 | ± | 1.9 d | 180.1 | ± | 3.8 e | 107.6 | ± | 0.1 f | 126.9 | ± | 1.4 g |
| Ethyl hexanoate | 60 | ± | 0.1 a | 107.6 | ± | 2.1 b,c,g | 105.1 | ± | 0.7 c,g | 124.9 | ± | 1.8 d,e,f | 118.9 | ± | 0.3 e,h | 133.6 | ± | 1.4 f | 115 | ± | 4.3 g,h |
| Hexyl acetate | 26.3 | ± | 0.01 a | 29.4 | ± | 0.01 a,b | 30.3 | ± | 0.01 b,c,d,e | 32.8 | ± | 0.7 c,g | 28.3 | ± | 1.4 a,e | 33.4 | ± | 0.01 d,g | 42.6 | ± | 2.1 f |
| Ethyl 2-hydroxy-3-methyl butanoate | 13.2 | ± | 1.3 a | 15.8 | ± | 0.9 a | 17.7 | ± | 0.7 a | 29.6 | ± | 0.8 b,e | 63.3 | ± | 2.7 c | 37 | ± | 1.4 d | 33.4 | ± | 3.0 e |
| Ethyl octanoate | 204.5 | ± | 6.1 a | 349.9 | ± | 32.6 b,c,g | 372 | ± | 0.6 c,g | 244.8 | ± | 22.1 d,e,f | 261.2 | ± | 26.5 e,f | 245.4 | ± | 21.8 f | 346.7 | ± | 3.1 g |
| Ethyl nonanoate | 10.9 | ± | 0.9 a | 12.2 | ± | 1.0 b,c,g | 11 | ± | 0.6 a,g | 13 | ± | 0.8 c | 16.3 | ± | 0.6 d,e,f | 15.8 | ± | 1.4 e | 17.1 | ± | 0.5 f |
| Ethyl 2-hydroxy-4-methylpentanoate | 36.8 | ± | 1.6 a | 36.2 | ± | 3.1 a | 37.4 | ± | 2.7 a | 82.6 | ± | 0.7 b | 112.9 | ± | 7.6 c | 39.3 | ± | 1.9 a | 47.8 | ± | 2.0 d |
| Isoamyl lactate | 43.3 | ± | 1.8 a | 139 | ± | 0.6 b,f | 229.3 | ± | 3.1 c | 101.2 | ± | 1.0 d | 255.5 | ± | 7.1 e | 146.6 | ± | 2.1 f | 127.8 | ± | 0.4 f |
| Ethyl decanoate | 112.1 | ± | 7.1 a | 122.4 | ± | 0.3 a | 154.8 | ± | 3.6 b | 111.8 | ± | 5.3 a | 270.2 | ± | 7.5 c,d | 127.1 | ± | 1.6 a | 273.9 | ± | 5.8 d |
| Diethyl succinate | 509.3 | ± | 12.2 a | 681.5 | ± | 2.5 b,d,e | 950.9 | ± | 48 c,f | 636.5 | ± | 51.2 d,e | 633.3 | ± | 44.2 e | 907.9 | ± | 43.2 f | 1310.2 | ± | 125.7 g |
| Ethyl 9-decenoate | 59.7 | ± | 0.8 a,c | 63.9 | ± | 2.3 a,b | 66.1 | ± | 0.8 b,d | 64.3 | ± | 1.3 a,b,d | 59.4 | ± | 0.7 a | 56.5 | ± | 1.3 c | 62.8 | ± | 0.8 a,b,d |
| Ethyl phenylacetate | 1.27 | ± | 0.01 a | 2.23 | ± | 0.01 b,c,d,f,g | 2.27 | ± | 0.03 c,d,f,g | 2.39 | ± | 0.07 d,e,f | 2.55 | ± | 0.04 e | 2.27 | ± | 0.08 f,g | 2.2 | ± | 0.08 g |
| Phenethyl acetate | 33.8 | ± | 0.1 a | 97.4 | ± | 6.8 b,e,d,f | 104.5 | ± | 4.1 c,d | 94.9 | ± | 2.6 d,f | 279.1 | ± | 5.1 e | 80.9 | ± | 2.6 f | 251 | ± | 3.1 g |
| Diethyl malate | 24.4 | ± | 0.7 a | 32.9 | ± | 0.9 b,c,d | 34.9 | ± | 0.9 c,d,e | 36 | ± | 0.9 d,e | 37 | ± | 0.6 e | 40.6 | ± | 0.7 f | 45.1 | ± | 0.9 g |
| Methyl vanillate | 242.7 | ± | 0.5 a | 287.4 | ± | 5.2 a | 610.1 | ± | 8.2 b,c | 550.3 | ± | 45.8 c,e,f | 1541.4 | ± | 18.6 d | 454 | ± | 44.7 e,f | 470.4 | ± | 62.1 f |
| Ethyl lactate | 121.4 | ± | 1.5 a | 173.8 | ± | 5.3 b,c | 155.9 | ± | 4.3 c | 204.6 | ± | 21.0 d,e | 199 | ± | 3.2 e | 271.1 | ± | 25.7 f | 256 | ± | 4.0 f |
| Butanoic acid 3-hydroxy ethyl ester | 51.8 | ± | 2.5 a | 63 | ± | 1.3 a,b | 57 | ± | 5.2 a | 76 | ± | 3.5 b,d | 231.3 | ± | 3.8 c | 82.7 | ± | 1.3 d | 105.1 | ± | 8.0 e |
| Ethyl (Z)-4-decenoate | 60.9 | ± | 1.2 a | 165.9 | ± | 3.7 b,d | 203.7 | ± | 17.7 c | 191.3 | ± | 6.1 d,c | 391.5 | ± | 6.4 e | 31.9 | ± | 1.6 a | 57.4 | ± | 0.6 a |
| Ethyl dodecanoate | 68.2 | ± | 2.6 a | 35.6 | ± | 2.0 b | 143.5 | ± | 14.1 c,f | 172 | ± | 2.8 d | 303.9 | ± | 8.1 e | 121.1 | ± | 1.0 f | 91.8 | ± | 0.3 g |
| Methyl tetradecanoate | 34.8 | ± | 1.5 a | 66.4 | ± | 7.2 b,c,d,f | 88.5 | ± | 6.6 c,d,f | 72.8 | ± | 3.8 d,f | 297.9 | ± | 18.1 e | 80.5 | ± | 0.8 f | 87.5 | ± | 1.9 f |
| Succinoic acid 2-hydroxy-3-methyl diethyl ester | 93.3 | ± | 8.1 a | 110.3 | ± | 10.4 a,b,e | 126.9 | ± | 3.4 b,c,e,f | 144.2 | ± | 4.8 c,e,f | 450.8 | ± | 36.4 d | 131.8 | ± | 1.5 e | 147.2 | ± | 2.3 f |
| Methyl hexadecanoate | 81.4 | ± | 4.6 a | 100.9 | ± | 5.2 b,e | 147.6 | ± | 0.7 c | 219.3 | ± | 10.1 d,g | 106.3 | ± | 2.1 e | 186.4 | ± | 4.3 f | 227.6 | ± | 5.2 g |
| Hexadecanoic acid, ethyl ester | 310.8 | ± | 7.4 a,f,g | 420.9 | ± | 33.5 b,d | 732.6 | ± | 20.6 c,e | 435.7 | ± | 10.9 d | 719.8 | ± | 61.2 e | 300.3 | ± | 2.5 f | 356.1 | ± | 14.5 g |
| Propanoic acid 2-methyl-propyl ester | 84.7 | ± | 0.8 a | 116.1 | ± | 11.1 b | 160 | ± | 2.4 c,d,f | 154.5 | ± | 4.5 d,f | 181.7 | ± | 0.1 e | 147.7 | ± | 8.1 f | 155.2 | ± | 9.2 f |
| Ethyl 8-nonenoato | 205.6 | ± | 3.5 a | 216.4 | ± | 3.1 a,b | 235.9 | ± | 0.8 b,f | 209.4 | ± | 2.2 a,f | 293.9 | ± | 6.5 c,d | 313 | ± | 17.6 d | 363.5 | ± | 20.5 e |
| Total | 62,816.5 | ± | 5332.3 | 69,533.2 | ± | 4366.3 | 73,838.8 | ± | 3669.9 | 79,381.1 | ± | 6282.9 | 92,587.9 | ± | 1927.1 | 99,069.5 | ± | 5177.4 | 156,584.3 | ± | 10,411.7 |
| % esters | 10.86% | 11.88% | 11.52% | 12.86% | 15.65% | 15.86% | 22.34% | ||||||||||||||
| Aldehydes | |||||||||||||||||||||
| Acetaldehyde | 4890.4 | ± | 279.7 a,b | 4810.7 | ± | 204.3 a | 5520.7 | ± | 263.1 b | 4482.1 | ± | 472.6 a | 2887.5 | ± | 294.1 c,d | 3292.9 | ± | 322.7 d | 4422.9 | ± | 417.9 a |
| Benzeneacetaldehyde | 66.6 | ± | 1.9 a | 74.3 | ± | 5.7 a,e | 131.9 | ± | 10.4 b,c,d | 145.3 | ± | 11.7 c | 119.8 | ± | 7.0 d | 80.5 | ± | 3.8 a,e | 85.1 | ± | 2.6 e |
| Nonanal | 10.5 | ± | 0.6 a,e | 13.4 | ± | 0.4 b,d | 16.2 | ± | 1.0 c | 14.2 | ± | 0.5 d | 10.7 | ± | 0.6 a | 9.9 | ± | 0.1 a,e | 8.6 | ± | 0.7 e |
| 3-methyl-butanal | 15.1 | ± | 0.5 a | 28.5 | ± | 1.5 b,c | 30.1 | ± | 0.5 c,d | 35.1 | ± | 2.6 d | 48.8 | ± | 2.6 e | 10 | ± | 0.3 a | 15.4 | ± | 0.4 a |
| Total | 4982.6 | ± | 282.7 | 4926.8 | ± | 212 | 5698.9 | ± | 275 | 4676.8 | ± | 487.4 | 3066.8 | ± | 304.4 | 3393.3 | ± | 326.9 | 4532.1 | ± | 421.6 |
| % Aldehydes | 0.86% | 0.84% | 0.89% | 0.76% | 0.52% | 0.54% | 0.65% | ||||||||||||||
| Thiols | |||||||||||||||||||||
| 3-(methylthio)-1-Propanol | 21.4 | ± | 1.8 a | 49.3 | ± | 3.8 b | 148.9 | ± | 7.5 c | 95.7 | ± | 3.7 d,f | 198.5 | ± | 3.2 e | 88.1 | ± | 8.6 f | 111.7 | ± | 2.4 g |
| Total | 21.4 | ± | 1.8 | 49.3 | ± | 3.8 | 148.9 | ± | 7.5 | 95.7 | ± | 3.7 | 198.5 | ± | 3.2 | 88.1 | ± | 8.6 | 111.7 | ± | 2.4 |
| % thiols | 0.004% | 0.01% | 0.02% | 0.02% | 0.03% | 0.01% | 0.02% | ||||||||||||||
| Acetals | |||||||||||||||||||||
| 1-(1-etoxietoxi)-pentano | 2.1 | ± | 0.1 a | 2 | ± | 0.1 a | 1.8 | ± | 0.1 a | 3.2 | ± | 0.1 b | 4.4 | ± | 0.2 c | 5.4 | ± | 0.1 d | 5.7 | ± | 0.2 e |
| Total | 2.1 | ± | 0 | 2 | ± | 0 | 1.8 | ± | 0.1 | 3.2 | ± | 0 | 4.4 | ± | 0.2 | 5.4 | ± | 0.1 | 5.7 | ± | 0.2 |
| % acetals | 0.0004% | 0.0003% | 0.0003% | 0.0010% | 0.0010% | 0.0010% | 0.0010% | ||||||||||||||
| Norisoprenoids | |||||||||||||||||||||
| 3-Oxo-α-ionol | 7.6 | ± | 0.1 a | 13.3 | ± | 0.4 b,c,d,e | 13.1 | ± | 0.1 c,d,e | 14.1 | ± | 0.2 d,e | 14.7 | ± | 0.1 e | 42.8 | ± | 0.6 f | 44.9 | ± | 1.0 f |
| Total | 7.6 | ± | 0.1 | 13.3 | ± | 0.4 | 13.1 | ± | 0.1 | 14.1 | ± | 0.2 | 14.7 | ± | 0.1 | 42.8 | ± | 0.6 | 44.9 | ± | 1 |
| % Norisoprenoids | 0.001% | 0.002% | 0.002% | 0.002% | 0.002% | 0.010% | 0.010% | ||||||||||||||
| Lactones | |||||||||||||||||||||
| Dihydro-5-pentyl-2-(3 H)-Furanona | 59.6 | ± | 0.7 a | 55.8 | ± | 4.8 a | 107.2 | ± | 2.8 b,d | 78.8 | ± | 0.5 c | 106.8 | ± | 0.3 d | 66.8 | ± | 0.2 a | 120.6 | ± | 8.4 e |
| 2,3-dihydro-benzofuran | 50.6 | ± | 1.1 a | 50 | ± | 4.0 a | 50.6 | ± | 1.1 a | 42.3 | ± | 0.8 b | 49.5 | ± | 0.8 a | 48.8 | ± | 1.5 a | 50.1 | ± | 1.6 a |
| Total | 110.2 | ± | 1.7 | 105.8 | ± | 8.8 | 157.8 | ± | 4 | 121.1 | ± | 1.4 | 156.2 | ± | 1.1 | 115.6 | ± | 1.7 | 170.7 | ± | 10 |
| % lactones | 0.02% | 0.02% | 0.02% | 0.02% | 0.03% | 0.02% | 0.02% | ||||||||||||||
| F1 | F2 | F3 | |
|---|---|---|---|
| Higher alcohols | 0.179 | 0.936 | −0.059 |
| Methanol | 0.193 | −0.862 | −0.082 |
| Acids | 0.648 | −0.533 | 0.492 |
| C-6 alcohols | 0.744 | −0.449 | 0.151 |
| Alcohols | −0.282 | 0.102 | 0.948 |
| Phenols | −0.284 | 0.831 | 0.152 |
| Terpenes and derivatives | 0.129 | −0.196 | 0.961 |
| Esters | 0.958 | 0.056 | 0.164 |
| Aldehydes | −0.355 | 0.895 | −0.242 |
| Thiols | 0.190 | −0.080 | 0.955 |
| Acetals | 0.895 | −0.420 | 0.121 |
| Norisoprenoids | 0.945 | −0.079 | −0.140 |
| Lactones | 0.513 | 0.336 | 0.769 |
| Explained variance (%) | 32.75 | 30.37 | 28.80 |
| Odorant Series | Bee Pollen Doses in Tintilla de ROTA Red Wines | ||||||
|---|---|---|---|---|---|---|---|
| Control | 0.1 g/L | 0.25 g/L | 1 g/L | 5 g/L | 10 g/L | 20 g/L | |
| Fruity | 93.03 | 142.21 | 154.61 | 134.68 | 146.87 | 129.35 | 151.40 |
| Floral | 25.25 | 31.01 | 45.26 | 43.24 | 40.41 | 26.58 | 36.55 |
| Fatty | 8.50 | 8.67 | 8.15 | 8.46 | 10.89 | 10.47 | 10.69 |
| Grassy | 1.15 | 1.15 | 1.17 | 1.45 | 1.42 | 1.26 | 1.45 |
| Dry fruit | 0.05 | 0.05 | 0.06 | 0.05 | 0.03 | 0.03 | 0.04 |
| Earthy, mushrooms | 0.60 | 0.61 | 0.52 | 0.74 | 0.78 | 0.64 | 0.33 |
| Chemical | 0.10 | 0.22 | 0.13 | 0.15 | 0.35 | 0.29 | 0.39 |
| Spicy | 0.94 | 1.52 | 2.17 | 2.09 | 1.12 | 0.75 | 1.23 |
| Phenolic | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 |
| ∑OAVT | 129.62 | 185.45 | 212.09 | 190.86 | 201.89 | 169.38 | 202.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amores-Arrocha, A.; Sancho-Galán, P.; Jiménez-Cantizano, A.; Palacios, V. Bee Pollen Role in Red Winemaking: Volatile Compounds and Sensory Characteristics of Tintilla de Rota Warm Climate Red Wines. Foods 2020, 9, 981. https://doi.org/10.3390/foods9080981
Amores-Arrocha A, Sancho-Galán P, Jiménez-Cantizano A, Palacios V. Bee Pollen Role in Red Winemaking: Volatile Compounds and Sensory Characteristics of Tintilla de Rota Warm Climate Red Wines. Foods. 2020; 9(8):981. https://doi.org/10.3390/foods9080981
Chicago/Turabian StyleAmores-Arrocha, Antonio, Pau Sancho-Galán, Ana Jiménez-Cantizano, and Víctor Palacios. 2020. "Bee Pollen Role in Red Winemaking: Volatile Compounds and Sensory Characteristics of Tintilla de Rota Warm Climate Red Wines" Foods 9, no. 8: 981. https://doi.org/10.3390/foods9080981
APA StyleAmores-Arrocha, A., Sancho-Galán, P., Jiménez-Cantizano, A., & Palacios, V. (2020). Bee Pollen Role in Red Winemaking: Volatile Compounds and Sensory Characteristics of Tintilla de Rota Warm Climate Red Wines. Foods, 9(8), 981. https://doi.org/10.3390/foods9080981








