Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Extraction of Phenolic Compounds
2.4. Estimation of Phenolics and Antioxidant Potential
2.5. Characterization and Quantification of Phenolics Using LC-ESI-QTOF-MS/MS and HPLC-PDA
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Estimation (TPC, TFC and TTC)
3.2. Antioxidant Potential (DPPH, ABTS, FRAP and TAC)
3.3. LC-ESI-QTOF-MS/MS Characterization
3.3.1. Phenolic Acids
3.3.2. Flavonoids
3.3.3. Other Polyphenols
3.3.4. Lignans
3.3.5. Stilbenes
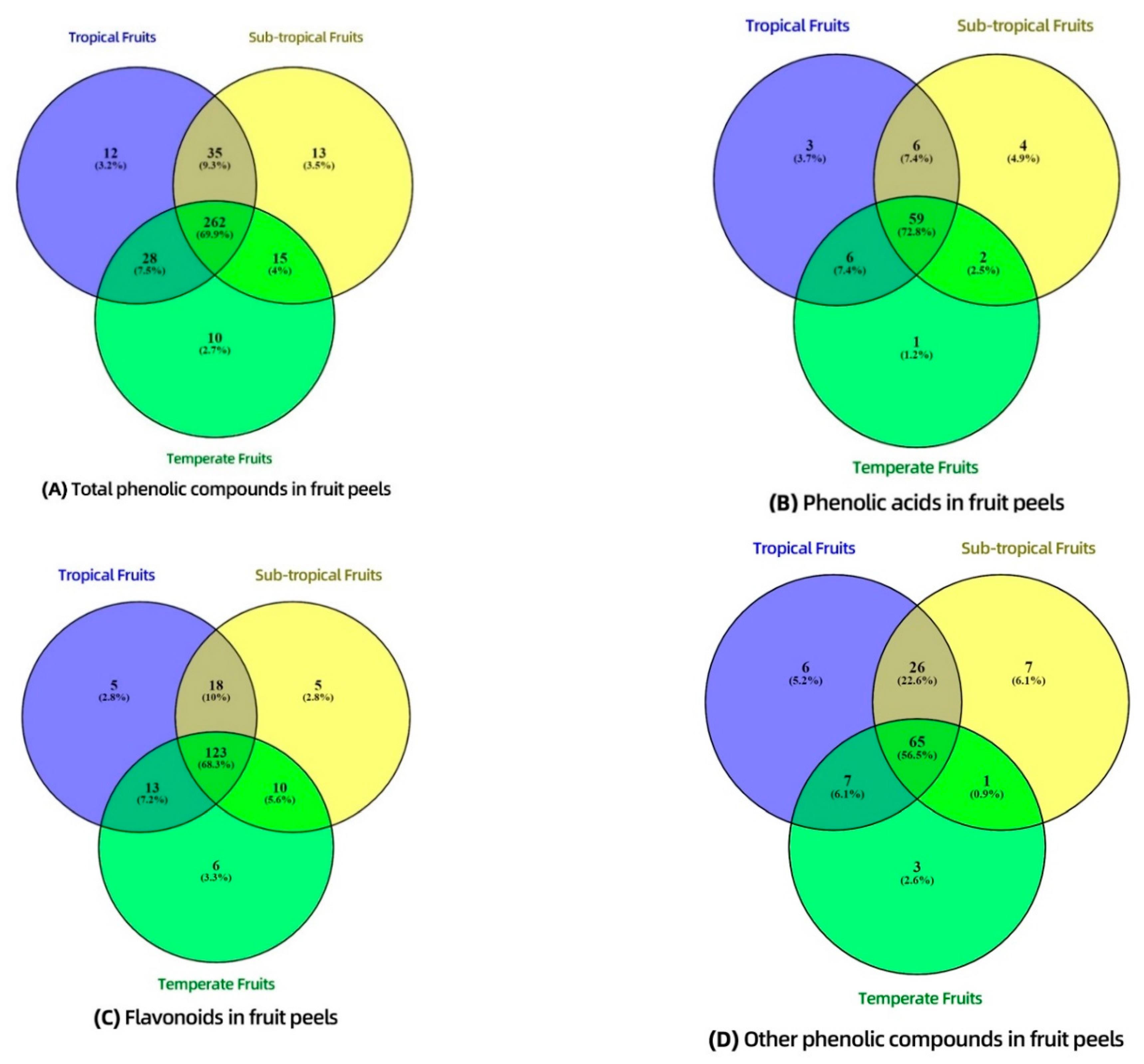
3.4. Distribution of Phenolic Compounds—Venn Diagram
3.5. HPLC-PDA Quantitative Analysis
3.5.1. Phenolic Acids
3.5.2. Flavonoids
3.6. Heat Map and Hierarchical Clustering Phenolic Compound Analysis
3.7. Correlation between Phenolic Compounds, Targeted Phenolics Quantified through HPLC-PDA and Antioxidant Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ain, H.B.U.; Saeed, F.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Food processing waste: A potential source for bioactive compounds. In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy, H.N., Bapat, V.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 625–649. [Google Scholar]
- Abu Qdais, H.; Wuensch, C.; Dornack, C.; Nassour, A. The role of solid waste composting in mitigating climate change in jordan. Waste Manag. Res. 2019, 37, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Azizan, A.; Xin, L.A.; Abdul Hamid, N.A.; Maulidiani, M.; Mediani, A.; Abdul Ghafar, S.Z.; Zulaikha Zolkeflee, N.K.; Abas, F. Potentially bioactive metabolites from pineapple waste extracts and their antioxidant and α-glucosidase inhibitory activities by (1)h nmr. Foods 2020, 9, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsuri, S.; Li, T.; Ruslan, M.S.; Amran, N.A. Antioxidant recovery from pomegranate peel waste by integrating maceration and freeze concentration technology. Int. J. Food Eng. 2020. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Borges, G.; Mullen, W.; Crozier, A. Comparison of the polyphenolic composition and antioxidant activity of european commercial fruit juices. Food Funct. 2010, 1, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Brahem, M.; Renard, C.M.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and quantification of fruit phenolic compounds of european and tunisian pear cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Fazio, A.; Iacopetta, D.; La Torre, C.; Ceramella, J.; Muià, N.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Finding solutions for agricultural wastes: Antioxidant and antitumor properties of pomegranate akko peel extracts and β-glucan recovery. Food Funct. 2018, 9, 6618–6631. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Profiling lc-dad-esi-tof ms method for the determination of phenolic metabolites from avocado (persea americana). J. Agric. Food Chem. 2011, 59, 2255–2267. [Google Scholar] [CrossRef]
- Toh, P.Y.; Leong, F.S.; Chang, S.K.; Khoo, H.E.; Yim, H.S. Optimization of extraction parameters on the antioxidant properties of banana waste. Acta Sci. Pol. Technol. Aliment. 2016, 15, 65–78. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic composition and antioxidant properties of different peach [prunus persica (l.) batsch] cultivars in china. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef] [Green Version]
- Suárez, B.; Álvarez, Á.L.; García, Y.D.; del Barrio, G.; Lobo, A.P.; Parra, F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010, 120, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, R.; Gil, D.; Miranda-Merchak, A.; Kalantzidis, G. Antihypertensive role of polyphenols. Adv. Clin. Chem. 2012, 58, 225. [Google Scholar]
- Yang, D.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of Australian herb and spices (garlic, ginger, and onion) and potential antioxidant activity. J. Food Process Preserv. 2020. [Google Scholar] [CrossRef]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in lc-ms and lc-hrms analysis and characterization of polyphenols in food. Trac. Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Laskowski, P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms profiling of australian mango peel by-product polyphenols and their potential antioxidant activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof-ms/ms characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of phenolic compounds in palm fruits (jelly and fishtail palm) and their potential antioxidant activities. Antioxidants 2019, 8, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.M.P.; Le, T.T.; Vissenaekens, H.; Gonzales, G.B.; Van Camp, J.; Smagghe, G.; Raes, K. In vitro antioxidant activity and phenolic profiles of tropical fruit by-products. Int. J. Food Sci. Technol. 2019, 54, 1169–1178. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M.C. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal. Chim. Acta 2001, 427, 119–127. [Google Scholar] [CrossRef]
- Shrestha, N.; Shrestha, S.; Bhattarai, A. Determination of Ascorbic Acid in Different Citrus Fruits of Kathmandu Valley. J. Med. Biol. Sci. Res. 2016, 2, 9–14. [Google Scholar]
- Xi, W.; Zhang, G.; Jiang, D.; Zhou, Z. Phenolic compositions and antioxidant activities of grapefruit (citrus paradisi macfadyen) varieties cultivated in china. Int. J. Food Sci. Nutr. 2015, 66, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Irkin, R.; Dogan, S.; Degirmenioglu, N.; Diken, M.E.; Guldas, M. Phenolic content, antioxidant activities and stimulatory roles of citrus fruits on some lactic acid bacteria. Arch. Biol. Sci. 2015, 67, 1313–1321. [Google Scholar] [CrossRef]
- Nurliyana, R.d.; Syed Zahir, I.; Mustapha Suleiman, K.; Aisyah, M.R.; Kamarul Rahim, K. Antioxidant study of pulps and peels of dragon fruits: A comparative study. Int. Food Res. J. 2010, 17, 367–375. [Google Scholar]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of caesalpinia bonduc (l.) roxb. Polym. J. 2017, 10, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Marina, Z.; Noriham, A. Quantification of total phenolic compound and in vitro antioxidant potential of fruit peel extracts. Int. Food Res. J. 2014, 21. [Google Scholar]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.H.F.; Visentainer, J.V. Antioxidant activity, phenolics and uplc–esi(–)–ms of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; YANO, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, S.C.; Azlan, A.; Chan, S.H.; Khoo, H.E. Extracts of peel and different parts of md2 pineapple as potent nutraceuticals. TJPS 2017, 41, 49. [Google Scholar]
- Wong, Y.S.; Sia, C.M.; Eng, H.; Ang, Y.K.; Chang, S.K.; Yim, H.S. Influence of extraction conditions on antioxidant properties of passion fruit (passiflora edulis) peel. Acta Sci. Pol. Technol. Aliment. 2014, 13, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Montañez, G.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; Velázquez-de la Cruz, G.; de León, J.A.; Navarro-Ocaña, A. Evaluation of extraction methods for preparative scale obtention of mangiferin and lupeol from mango peels (mangifera indica l.). Food Chem. 2014, 159, 267–272. [Google Scholar]
- Masibo, M.; He, Q. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability; Phenolic Compounds Biological Activity, Ed.; InTech: Rijeka, Croatia, 2017; pp. 1–24. [Google Scholar]
- Chang, C.-H.; Lin, H.-Y.; Chang, C.-Y.; Liu, Y.-C. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Ajila, C.M.; Naidu, K.A.; Bhat, S.G.; Rao, U.J.S.P. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Castro-Vazquez, L.; Alañón, M.E.; Rodríguez-Robledo, V.; Pérez-Coello, M.S.; Hermosín-Gutierrez, I.; Díaz-Maroto, M.C.; Jordán, J.; Galindo, M.F.; Arroyo-Jiménez, M.d.M. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (citrus paradisi macf.). Oxid. Med. Cell. Longev. 2016, 2016, 8915729. [Google Scholar] [CrossRef] [Green Version]
- Pal, R.S.; Kumar, V.A.; Arora, S.; Sharma, A.K.; Kumar, V.; Agrawal, S. Physicochemical and antioxidant properties of kiwifruit as a function of cultivar and fruit harvested month. Braz. Arch. Biol. Technol. 2015, 58, 262–271. [Google Scholar] [CrossRef]
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, É.R.; Paschoal, J.A.R.; Melo, P.S.; Alencar, S.M.d. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE 2018, 13, e0192577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Arellano, H.F.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Neuroprotective effects of methanolic extract of avocado persea americana (var. Colinred) peel on paraquat-induced locomotor impairment, lipid peroxidation and shortage of life span in transgenic knockdown parkin drosophila melanogaster. Neurochem. Res. 2019, 44, 1986–1998. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A. Characterization of the antioxidant properties of phenolic extracts from some citrus peels. J. Food Sci. Technol. 2012, 49, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by frap assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Mehmood, T.; Khan, M.R.; Shabbir, M.A.; Zia, M.A. Phytochemical profiling and hplc quantification of citrus peel from different varieties. Prog. Nutr. 2018, 20, 279–288. [Google Scholar]
- Yang, P.; Xu, F.; Li, H.F.; Wang, Y.; Li, F.C.; Shang, M.Y.; Liu, G.X.; Wang, X.; Cai, S.Q. Detection of 191 taxifolin metabolites and their distribution in rats using hplc-esi-it-tof-msn. Molecules 2016, 21, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar-Avello, D.; Lozano-Castellon, J.; Mardones, C.; Perez, A.J.; Saez, V.; Riquelme, S.; von Baer, D.; Vallverdu-Queralt, A. Phenolic profile of grape canes: Novel compounds identified by lc-esi-ltq-orbitrap-ms. Molecules 2019, 24, 3763. [Google Scholar]
- Wang, X.Y.; Yan, K.J.; Ma, X.H.; Li, W.; Chu, Y.; Guo, J.H.; Li, S.M.; Zhou, S.P.; Zhu, Y.H.; Liu, C.X. Simultaneous determination and pharmacokinetic study of protocatechuic aldehyde and its major active metabolite protocatechuic acid in rat plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. Sci. 2016, 54, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Choi, H.K.; Moon, J.Y.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Comparative antioxidant and antiproliferative activities of red and white pitayas and their correlation with flavonoid and polyphenol content. J Food Sci 2011, 76, C38–C45. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhang, A.; Sun, H.; Zhang, Y. Chapter 23—systematic characterization of the absorbed components of acanthopanax senticosus stem. In Serum Pharmacochemistry of Traditional Chinese Medicine; Wang, X., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 313–336. [Google Scholar]
- Wang, J.; Jia, Z.; Zhang, Z.; Wang, Y.; Liu, X.; Wang, L.; Lin, R. Analysis of chemical constituents of melastoma dodecandrum lour. By uplc-esi-q-exactive focus-ms/ms. Molecules 2017, 22, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasot, G.; Martinez-Huelamo, M.; Vallverdu-Queralt, A.; Mercader-Marti, M.; Estruch, R.; Lamuela-Raventos, R.M. Identification of phenolic metabolites in human urine after the intake of a functional food made from grape extract by a high resolution ltq-orbitrap-ms approach. Food Res. Int. 2017, 100, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.B.; Luz, L.R.; Guedes, J.A.C.; Porto, D.D.; Silva, M.F.S.; Silva, G.S.; Riceli, P.R.V.; Canuto, K.M.; Brito, E.S.; Zampieri, D.S.; et al. Metabolomics-based discovery of biomarkers with cytotoxic potential in extracts of myracrodruon urundeuva. J. Braz. Chem. Soc. 2020, 31, 775–787. [Google Scholar] [CrossRef]
- Wang, Y.F.; Vorsa, N.; Harrington, P.D.; Chen, P. Nontargeted metabolomic study on variation of phenolics in different cranberry cultivars using uplc-im—Hrms. J. Agric. Food Chem. 2018, 66, 12206–12216. [Google Scholar] [CrossRef]
- Zeng, Y.; Lu, Y.; Chen, Z.; Tan, J.; Bai, J.; Li, P.; Wang, Z.; Du, S. Rapid characterization of components in bolbostemma paniculatum by uplc/ltq-orbitrap msn analysis and multivariate statistical analysis for herb discrimination. Molecules 2018, 23, 1155. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, M. Quantification of free coumarin and its liberation from glucosylated precursors by stable isotope dilution assays based on liquid chromatography-tandem mass spectrometric detection. J. Agric. Food Chem. 2008, 56, 796–801. [Google Scholar] [CrossRef]
- Geng, C.A.; Chen, H.; Chen, X.L.; Zhang, X.M.; Lei, L.G.; Chen, J.J. Rapid characterization of chemical constituents in saniculiphyllum guangxiense by ultra fast liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. Int. J. Mass Spectrom. 2014, 361, 9–22. [Google Scholar] [CrossRef]
- Yang, S.; Shan, L.; Luo, H.; Sheng, X.; Du, J.; Li, Y. Rapid classification and identification of chemical components of schisandra chinensis by uplc-q-tof/ms combined with data post-processing. Molecules 2017, 22, 1778. [Google Scholar] [CrossRef] [Green Version]
- Stella, L.; De Rosso, M.; Panighel, A.; Vedova, A.D.; Flamini, R.; Traldi, P. Collisionally induced fragmentation of m-h (-) species of resveratrol and piceatannol investigated by deuterium labelling and accurate mass measurements. Rapid Commun. Mass Spectrom. 2008, 22, 3867–3872. [Google Scholar] [CrossRef]
- Reed, K.A. Identification of Phenolic Compounds from Peanut Skin Using hplc-msn; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2009. [Google Scholar]
- Pereira-Netto, A.B. Tropical fruits as natural, exceptionally rich, sources of bioactive compounds. Int. J. Fruit Sci. 2018, 18, 231–242. [Google Scholar] [CrossRef]
- He, S.; Wu, B.; Pan, Y.; Jiang, L. Stilbene oligomers from parthenocissus laetevirens: Isolation, biomimetic synthesis, absolute configuration, and implication of antioxidative defense system in the plant. J. Org. Chem. 2008, 73, 5233–5241. [Google Scholar] [CrossRef]
- Cammell, M.E.; Knight, J.D. Effects of climatic change on the population dynamics of crop pests. In Advances in Ecological Research; Begon, M., Fitter, A.H., Macfadyen, A., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 22, pp. 117–162. [Google Scholar]
- Gang, D.R.; Dinkova-Kostova, A.T.; Davin, L.B.; Lewis, N.G. Phylogenetic links in plant defense systems: Lignans, isoflavonoids, and their reductases. In Phytochemicals for Pest Control; Hedin, P.A., Hollingworth, R.M., Eds.; American Chemical Society: Washington, DC, USA, 1997; Volume 658, pp. 58–89. [Google Scholar]
- Palafox-Carlos, H.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of major phenolic compounds from mango (mangifera indica, cv. Ataulfo) fruit by hplc–dad–ms/ms-esi and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Kim, Y.; Brecht, J.K.; Talcott, S.T. Antioxidant phytochemical and fruit quality changes in mango (mangifera indica l.) following hot water immersion and controlled atmosphere storage. Food Chem. 2007, 1055, 1327–1334. [Google Scholar] [CrossRef]
- Hu, K.; Dars, A.G.; Liu, Q.; Xie, B.; Sun, Z. Phytochemical profiling of the ripening of chinese mango (mangifera indica l.) cultivars by real-time monitoring using uplc-esi-qtof-ms and its potential benefits as prebiotic ingredients. Food Chem. 2018, 256, 171–180. [Google Scholar] [CrossRef]
- Gorinstein, S.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Vearasilp, S.; Haruenkit, R.; Ruamsuke, P.; Katrich, E.; Tashma, Z. Antioxidant properties and bioactive constituents of some rare exotic thai fruits and comparison with conventional fruits: In vitro and in vivo studies. Food Res. Int. 2011, 44, 2222–2232. [Google Scholar] [CrossRef]
- Rebello, L.P.G.; Ramos, A.M.; Pertuzatti, P.B.; Barcia, M.T.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Flour of banana (musa aaa) peel as a source of antioxidant phenolic compounds. Food Res. Int. 2014, 55, 397–403. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Pal, J.; Prakash, A.; Pandey, G.; Raju, C. Comparatives study of antioxidant activity and total phenolic contents of pomegranate and orange peels extracts. J. Pharmacogn. Phytochem. 2017, 6, 1359–1362. [Google Scholar]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin c and antioxidant activity in wasted parts of sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Labat, A.; Scobbie, L.; Duncan, G.J.; Duthie, G.G. Phenolic acid content of fruits commonly consumed and locally produced in scotland. Food Chem. 2009, 115, 100–104. [Google Scholar] [CrossRef]
- Mihailović, N.; Mihailovic, V.; Kreft, S.; Ciric, A.; Joksović, L.; Djurdjevic, P. Analysis of phenolics in the peel and pulp of wild apples ( malus sylvestris (l.) mill.). J. Food Compos. Anal. 2017, 67. [Google Scholar]
- Veberic, R.; Trobec, M.; Herbinger, K.; Hofer, M.; Grill, D.; Stampar, F. Phenolic compounds in some apple (malus domestica borkh) cultivars of organic and integrated production. J. Sci. Food Agric. 2005, 85, 1687–1694. [Google Scholar] [CrossRef]
- López-Cobo, A.; Verardo, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. Use of hplc-and gc-qtof to determine hydrophilic and lipophilic phenols in mango fruit (mangifera indica l.) and its by-products. Food Res. Int. 2017, 100, 423–434. [Google Scholar]
- Zain, N.M.; Nazeri, M.A.; Azman, N.A. Assessment on bioactive compounds and the effect of microwave on pitaya peel. J. Teknol. 2019, 81, 11–19. [Google Scholar]
- Silva, L.M.R.d.; Figueiredo, E.A.T.d.; Ricardo, N.M.P.S.; Vieira, I.G.P.; Figueiredo, R.W.d.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Immanuel, G. Extraction of antioxidants from fruit peels and its utilization in paneer. J. Food Process. Technol. 2014, 5, 1. [Google Scholar]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of abts/dpph assays to measure antioxidant capacity in popular antioxidant-rich us foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]




| Sample | TPC (mg GAE/g) | TFC (mg QE/g) | TTC (mg CE/g) | DPPH (mg AAE/g) | ABTS (mg AAE/g) | FRAP (mg AAE/g) | TAC (mg AAE/g) |
|---|---|---|---|---|---|---|---|
| Apple peel | 10.82 ± 0.51 e | 1.22 ± 0.10 b–e | 2.25 ± 0.12 e | 5.20 ± 0.25 b | 4.96 ± 0.17 d | 3.20 ± 0.04 d | 2.97 ± 0.16 d |
| Apricot peel | 5.60 ± 0.27 f,g | 1.22 ± 0.09 b–e | 1.07 ± 0.05 f | 3.73 ± 0.55 c | 3.21 ± 0.04 e | 2.27 ± 0.11 e | 2.28 ± 0.04 e,f |
| Avocado peel | 18.79 ± 1.46 c | 1.24 ± 0.11 b–d | 9.01 ± 0.20 a | 8.67 ± 0.44 a | 7.19 ± 0.72 c | 3.65 ± 0.07 c | 4.50 ± 0.16 c |
| Banana peel | 6.13 ± 0.25 f,g | 1.32 ± 0.12 b,c | 1.22 ± 0.08 f | 1.20 ± 0.12 e | 1.31 ± 0.03 g,h | 0.81 ± 0.03 i,j | 2.36 ± 0.22 e,f |
| Custard apple peel | 15.72 ± 0.74 d | 1.21 ± 0.08 b–e | 8.32 ± 0.56 a–c | 2.52 ± 0.52 d | 4.00 ± 0.44 e | 1.51 ± 0.02 f | 2.58 ± 0.04 d,e |
| Dragon fruit peel | 0.45 ± 0.12 k | 0.03 ± 0.01 h | 0.03 ± 0.01 h | 1.03 ± 0.16 e | 0.56 ± 0.08 h | 0.06 ± 0.01 l | 0.19 ± 0.02 i |
| Grapefruit peel | 27.22 ± 1.00 a | 0.82 ± 0.14 f | 7.60 ± 0.35 c | 9.17 ± 0.19 a | 10.79 ± 0.56 a | 9.22 ± 0.25 a | 8.77 ± 0.34 a |
| kiwi fruit peel | 5.30 ± 0.40 g,h | 0.45 ± 0.06 g | 3.51 ± 0.33 d | 5.03 ± 0.39 b | 8.95 ± 0.18 b | 1.13 ± 0.10 g–i | 0.79 ± 0.05 h |
| Lime peel | 23.32 ± 2.07 b | 1.14 ± 0.17 c–e | 8.42 ± 0.63 a,b | 2.73 ± 0.34 d | 1.46 ± 0.14 g | 0.92 ± 0.07 h–j | 2.27 ± 0.08 e,f |
| Mango peel | 27.51 ± 0.63 a | 1.75 ± 0.08 a | 8.99 ± 0.13 a | 8.67 ± 0.49 a | 9.32 ± 0.24 b | 6.19 ± 0.26 b | 6.19 ± 0.23 b |
| Melon peel | 2.39 ± 0.02 i–k | 0.03 ± 0.01 h | 0.02 ± 0.01 h | 0.48 ± 0.28 e | 1.16 ± 0.20 g,h | 0.08 ± 0.01 l | 0.93 ± 0.23 g–h |
| Nectarine peel | 1.53 ± 0.04 j,k | 0.09 ± 0.01 h | 0.23 ± 0.18 h | 1.29 ± 0.09 e | 1.25 ± 0.13 g,h | 0.91 ± 0.07 h–j | 0.97 ± 0.05 g,h |
| Orange peel | 21.31 ± 1.37 b | 1.08 ± 0.06 c–f | 8.12 ± 0.26 b,c | 4.79 ± 0.31 b | 3.36 ± 0.16 e | 2.44 ± 0.12 e | 2.55 ± 0.08 d,e |
| Papaya peel | 3.13 ± 0.15 h–j | 1.06 ± 0.07 c–f | 1.09 ± 0.04 f | 1.13 ± 0.11 e | 3.30 ± 0.17 e | 0.91 ± 0.07 i,j | 1.12 ± 0.13 g,h |
| Passion fruit peel | 1.55 ± 0.21 j,k | 0.04 ± 0.01 h | 0.19 ± 0.02 h | 0.72 ± 0.13 e | 1.04 ± 0.07 g,h | 0.42 ± 0.04 k | 1.32 ± 0.05 g |
| Peach peel | 5.84 ± 0.33 f,g | 1.02 ± 0.08 d–f | 0.16 ± 0.05 h | 1.33 ± 0.11 e | 1.03 ± 0.06 g,h | 0.89 ± 0.07 i,j | 1.13 ± 0.07 g,h |
| Pear peel | 4.30 ± 0.29 g–i | 1.07 ± 0.12 c–f | 0.10 ± 0.03 h | 0.84 ± 0.12 e | 1.21 ± 0.06 g,h | 0.65 ± 0.08 j,k | 1.18 ± 0.03 g,h |
| Pineapple peel | 7.83 ± 0.35 f | 1.47 ± 0.07 b | 1.23 ± 0.05 f | 1.30 ± 0.07 e | 2.36 ± 0.06 f | 1.30 ± 0.16 f,g | 2.00 ± 0.14 f |
| Plum peel | 4.81 ± 0.30 g,h | 0.96 ± 0.08 e,f | 0.29 ± 0.05 g,h | 1.01 ± 0.10 e | 1.19 ± 0.08 g,h | 0.71 ± 0.04 j,k | 0.87 ± 0.04 h |
| Pomegranate peel | 3.89 ± 0.21 g–i | 0.97 ± 0.10 e,f | 0.99 ± 0.02 f–g | 4.60 ± 0.08 b,c | 3.34 ± 0.09 e | 1.25 ± 0.13 f–h | 2.40 ± 0.18 e,f |
| Fruit Peels | Gallic Acid | Protocatechuic Acid | Caftaric Acid | Chlorogenic Acid | p-hydroxybenzoic Acid | Caffeic Acid | Syringic Acid | Coumaric Acid | Ferulic Acid | Sinapinic Acid | Sum of Phenolic Acids |
|---|---|---|---|---|---|---|---|---|---|---|---|
| APL-P | 4.2 ± 0.9 d | 7.4 ± 0.4 a | - | 11.2 ± 0.1 b | 6.5 ± 0.8 c | 2.1 ± 0.9 c | 1.1 ± 0.7 g | - | 1.2 ± 0.3 h | - | 33.7 ± 1.5 C |
| APR-P | 2.1 ± 0.6 g | - | 2.4 ± 0.4 e | 5.9 ± 0.2 e | 3.1 ± 0.3 f | 3.5 ± 0.1 b | - | - | 4.5 ± 0.1 c | 1.3 ± 0.1 e | 22.8 ± 1.7 D |
| AVO-P | 3.2 ± 0.5 e | 4.2 ± 0.2 b | 1.2 ± 0.1 g | 9.5 ± 0.1 c | 4.5 ± 0.6 d | - | 3.5 ± 0.3 d | 4.1 ± 0.1 b | - | 2.7 ± 0.6 c | 32.9 ± 2.5 C |
| BNA-P | 1.2 ± 0.2 i | - | - | 4.5 ± 0.5 f | 2.8 ± 0.1 g | - | 4.1 ± 0.5 c | - | 3.7 ± 0.2 d | - | 16.3 ± 1.9 G |
| CTA-P | 1.4 ± 0.2 h | - | 4.7 ± 0.9 c | 7.5 ± 0.3 d | 4.5 ± 0.9 d | - | - | 1.8 ± 0.4 f | 1.8 ± 0.3 g | 3.9 ± 0.8 b | 25.6 ± 1.6 D |
| DGF-P | 1.1 ± 0.5 i | - | 3.5 ± 0.5 d | 4.1 ± 0.9 g | 1.2 ± 0.7 i | - | 3.1 ± 0.9 d | 2.8 ± 0.1 d | 2.7 ± 0.8 e | - | 18.5 ± 2.1 F |
| GRF-P | 5.4 ± 0.9 c | 3.4 ± 0.4 c | 7.8 ± 0.5 a | - | 3.5 ± 0.9 e | 4.2 ± 0.5 a | - | 3.1 ± 0.8 c | 2.1 ± 0.4 f | 1.7 ± 0.7 d | 31.2 ± 1.9 C |
| KWF-P | 1.1 ± 0.9 i | - | 4.2 ± 0.4 c | 3.2 ± 0.5 i | 2.8 ± 0.1 g | - | 2.1 ± 0.1 e | 4.1 ± 0.8 b | 1.2 ± 0.7 h | - | 18.7 ± 1.7 F |
| LMN-P | - | 1.2 ± 0.8 e | 2.4 ± 0.5 e | - | 4.2 ± 0.4 d | 1.2 ± 0.6 e | - | 2.1 ± 0.4 e | 4.5 ± 0.9 c | 4.9 ± 0.7 a | 20.5 ± 2.1 E |
| MNG-P | 14.5 ± 0.4 a | - | 2.1 ± 0.1 f | 13.8 ± 0.9 a | 10.5 ± 0.4 a | 4.5 ± 0.4 a | 11.5 ± 0.7 a | 5.1 ± 0.2 a | 6.3 ± 0.4 a | 3.9 ± 0.9 b | 72.2 ± 4.5 A |
| MEL-P | - | 1.1 ± 0.7 e | - | 1.6 ± 0.3 j | - | - | 2.3 ± 0.1 e | - | 1.2 ± 0.2 h | - | 6.2 ± 1.2 M |
| NEC-P | 1.5 ± 0.7 h | 1.2 ± 0.2 e | - | 4.5 ± 0.4 f | 2.8 ± 0.1 g | 1.7 ± 0.9 d | - | - | 1.1 ± 0.1 h | - | 12.8 ± 1.9 I |
| ORN-P | 5.4 ± 0.9 c | - | 3.1 ± 0.4 d | 5.6 ± 0.3 e | 3.6 ± 0.1 e | - | 1.8 ± 0.2 f | - | 2.1 ± 0.7 f | 1.8 ± 0.2 d | 23.4 ± 2.3 D |
| PAP-P | 2.4 ± 0.7 f | - | 5.6 ± 0.1 b | - | 2.9 ± 0.2 g | - | 2.4 ± 0.9 e | - | 1.8 ± 0.4 g | - | 15.1 ± 1.1 H |
| PSN-P | - | - | 5.2 ± 0.8 b | - | 2.1 ± 0.4 h | - | 3.5 ± 0.3 d | - | 1.1 ± 0.1 h | - | 11.9 ± 1.9 J |
| PEC-P | 1.5 ± 0.4 h | 1.2 ± 0.1 e | - | 3.7 ± 0.9 h | - | 1.8 ± 0.2 d | - | - | 2.7 ± 0.1 e | 1.4 ± 0.9 e | 12.3 ± 1.3 I |
| PER-P | 1.1 ± 0.7 i | - | 2.1 ± 0.8 f | - | 3.2 ± 0.3 f | - | 1.5 ± 0.4 f | - | 1.2 ± 0.1 h | - | 9.1 ± 1.7 L |
| PIN-P | 1.5 ± 0.9 h | 2.1 ± 0.2 d | - | - | 1.2 ± 0.1 i | 1.1 ± 0.5 e | - | 2.8 ± 0.3 d | - | 1.9 ± 0.7 d | 10.6 ± 1.9 K |
| PLM-P | 1.4 ± 0.3 h | - | - | - | 3.8 ± 0.1 e | - | 1.7 ± 0.7 f | - | 4.2 ± 0.4 c | - | 11.1 ± 2.1 J |
| POM-P | 6.7 ± 0.1 b | 7.4 ± 0.6 a | - | 11.8 ± 0.7 b | 9.8 ± 0.1 b | 4.5 ± 0.7 a | 6.7 ± 0.9 b | - | 5.8 ± 0.2 b | - | 52.7 ± 3.9 B |
| Fruit Peels | Catechin | Epicatechin | Epicatechin Gallate | Quercetin-3-Galactoside | Quercetin-3-Glucuronide | Quercetin-3-Glucoside | Kaempferol-3-Glucoside | Diosmin | Quercetin | Kaempferol | Sum of Flavonoids |
|---|---|---|---|---|---|---|---|---|---|---|---|
| APL-P | 3.2 ± 0.8 b | - | 1.5 ± 0.1 d | 5.7 ± 0.6 b | - | 4.5 ± 0.9 a | - | 2.1 ± 0.4 b | 9.6 ± 0.9 b | - | 26.6 ± 1.9 C |
| APR-P | - | 2.1 ± 0.8 b | 2.3 ± 0.1 b | - | 3.8 ± 0.7 d | 3.5 ± 0.2 b | 3.2 ± 0.4 b | - | 6.9 ± 0.7 c | 4.5 ± 0.1 d | 26.3 ± 2.1 C |
| AVO-P | 2.1 ± 0.9 d | 1.8 ± 0.4 c | - | 4.9 ± 0.7 c | 1.7 ± 0.8 f | 2.8 ± 0.1 c | 1.9 ± 0.5 e | 1.7 ± 0.1 c | 3.9 ± 0.9 g | 2.9 ± 0.4 f | 23.7 ± 2.7 D |
| BNA-P | 1.5 ± 0.7 e | - | - | 3.8 ± 0.9 d | - | 1.7 ± 0.1 e | - | - | 4.8 ± 0.8 e | - | 11.8 ± 2.1 H |
| CTA-P | 2.1 ± 0.4 d | - | 1.9 ± 0.7 c | - | 1.4 ± 0.1 f | - | 1.7 ± 0.5 e | - | 3.2 ± 0.9 h | 3.9 ± 0.4 d | 14.2 ± 1.9 G |
| DGF-P | 7.5 ± 0.9 a | - | 1.5 ± 0.1 d | 4.5 ± 0.7 c | 1.7 ± 0.4 f | - | 2.4 ± 0.7 d | - | 4.9 ± 0.3 e | 3.5 ± 0.7 e | 26.0 ± 1.1 C |
| GRF-P | - | - | 2.1 ± 0.7 b | 2.9 ± 0.1 e | - | 1.7 ± 0.7 e | 1.9 ± 0.3 e | 1.1 ± 0.7 d | 5.9 ± 0.1 d | 4.2 ± 0.9 d | 19.8 ± 1.9 E |
| KWF-P | 1.5 ± 0.2 e | - | 1.1 ± 0.9 e | - | 4.5 ± 0.3 c | - | 1.2 ± 0.8 f | 1.7 ± 0.1 c | 5.4 ± 0.2 d | 7.1 ± 0.7 b | 22.5 ± 2.7 D |
| LMN-P | 2.7 ± 0.1 c | - | - | 4.8 ± 0.7 c | 8.7 ± 0.2 b | - | 3.7 ± 0.4 a | 1.9 ± 0.6 b | - | 1.2 ± 0.1 h | 23.0 ± 1.3 D |
| MNG-P | 7.1 ± 0.3 a | - | 3.2 ± 0.9 a | 10.9 ± 0.1 a | 11.5 ± 0.7 a | - | 2.7 ± 0.4 c | - | 11.9 ± 0.4 a | 9.8 ± 0.7 a | 57.1 ± 2.4 A |
| MEL-P | - | 1.9 ± 0.3 c | - | 2.8 ± 0.9 e | - | 1.7 ± 0.1 e | - | - | 4.5 ± 0.3 f | - | 10.9 ± 1.2 I |
| NEC-P | 2.1 ± 0.8 d | - | 1.7 ± 0.1 d | 1.8 ± 0.7 g | - | - | 2.9 ± 0.7 c | - | 2.9 ± 0.9 i | 3.1 ± 0.1 e | 14.5 ± 2.1 G |
| ORN-P | 3.5 ± 0.1 b | 2.4 ± 0.4 b | - | 3.8 ± 0.2 d | - | - | 3.9 ± 0.1 a | - | - | 1.9 ± 0.8 g | 15.5 ± 1.7 F |
| PAP-P | - | 1.7 ± 0.3 c | 1.9 ± 0.9 c | - | 4.7 ± 0.1 c | - | 1.7 ± 0.9 e | - | 4.9 ± 0.4 e | 2.9 ± 0.2 f | 17.8 ± 2.1 F |
| PSN-P | 1.8 ± 0.7 e | - | - | 1.7 ± 0.1 f | - | 2.1 ± 0.9 d | - | 1.7 ± 0.1 c | - | 3.1 ± 0.8 e | 10.4 ± 1.4 I |
| PEC-P | 1.2 ± 0.5 f | - | 1.9 ± 0.3 c | 2.1 ± 0.4 f | - | 1.8 ± 0.2 e | - | 1.1 ± 0.1 d | - | 4.1 ± 0.7 d | 12.2 ± 1.7 H |
| PER-P | 1.8 ± 0.7 e | 1.9 ± 0.9 c | - | - | 1.7 ± 0.4 f | - | 1.1 ± 0.1 f | - | - | 7.4 ± 0.7 b | 13.9 ± 2.1 G |
| PIN-P | - | - | 1.5 ± 0.1 d | - | - | 3.7 ± 0.1 b | - | 3.1 ± 0.6 a | - | 6.5 ± 0.7 c | 14.8 ± 1.9 G |
| PLM-P | 3.1 ± 0.1 b | - | 1.4 ± 0.9 d | 2.7 ± 0.3 e | - | 3.1 ± 0.5 c | - | 1.9 ± 0.7 b | - | 4.1 ± 0.2 d | 16.3 ± 2.1 F |
| POM-P | 2.1 ± 0.1 d | 4.1 ± 0.3 a | - | 5.7 ± 0.1 b | 2.1 ± 0.7 e | - | 3.6 ± 0.2 a | 1.1 ± 0.3 d | 9.4 ± 0.9 b | 7.6 ± 0.7 b | 35.7 ± 4.7 B |
| Variables | TPC | TFC | TTC | DPPH | ABTS | FRAP | TAC | Phenolic Acids |
|---|---|---|---|---|---|---|---|---|
| TFC | 0.488 * | |||||||
| TTC | 0.932 ** | 0.457 * | ||||||
| DPPH | 0.718 ** | 0.396 | 0.720 ** | |||||
| ABTS | 0.591 ** | 0.270 | 0.622 ** | 0.904 ** | ||||
| FRAP | 0.722 ** | 0.314 | 0.603 ** | 0.868 ** | 0.835 ** | |||
| TAC | 0.780 ** | 0.397 | 0.668 ** | 0.850 ** | 0.779 ** | 0.967 ** | ||
| Phenolic acids | 0.496 * | 0.343 | 0.515 * | 0.761 ** | 0.628 * | 0.614 ** | 0.640 ** | |
| Flavonoids | 0.349 | 0.232 | 0.355 | 0.633 * | 0.535 * | 0.473 * | 0.452 * | 0.911 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. https://doi.org/10.3390/foods9091206
Suleria HAR, Barrow CJ, Dunshea FR. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods. 2020; 9(9):1206. https://doi.org/10.3390/foods9091206
Chicago/Turabian StyleSuleria, Hafiz A. R., Colin J. Barrow, and Frank R. Dunshea. 2020. "Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels" Foods 9, no. 9: 1206. https://doi.org/10.3390/foods9091206
APA StyleSuleria, H. A. R., Barrow, C. J., & Dunshea, F. R. (2020). Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods, 9(9), 1206. https://doi.org/10.3390/foods9091206








