Comparative Proteomics of Milk Fat Globule Membrane (MFGM) Proteome across Species and Lactation Stages and the Potentials of MFGM Fractions in Infant Formula Preparation
Abstract
:1. Introduction
2. Bovine MFGs and the MFGM
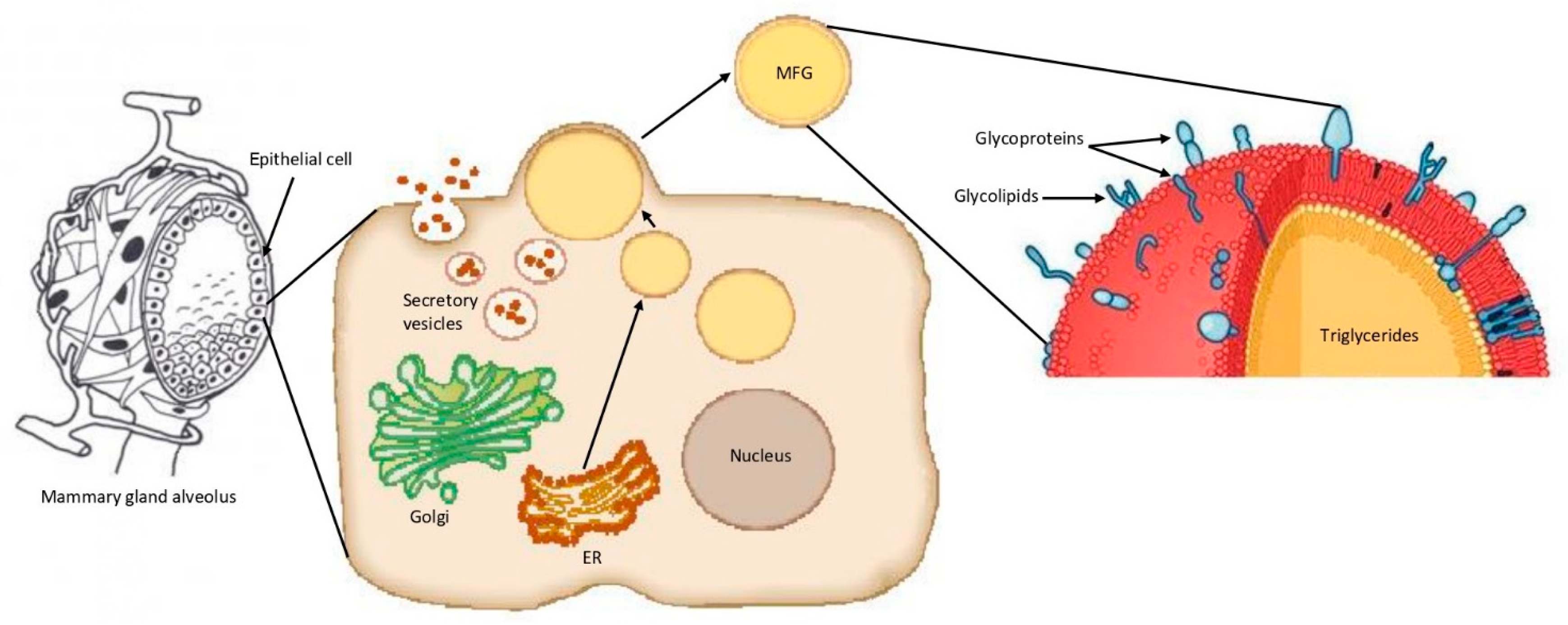
2.1. Formation of Bovine MFGs and MFGM
2.2. Lipids in MFG and MFGM, and the Role of Choline
2.3. Major MFGM Proteins
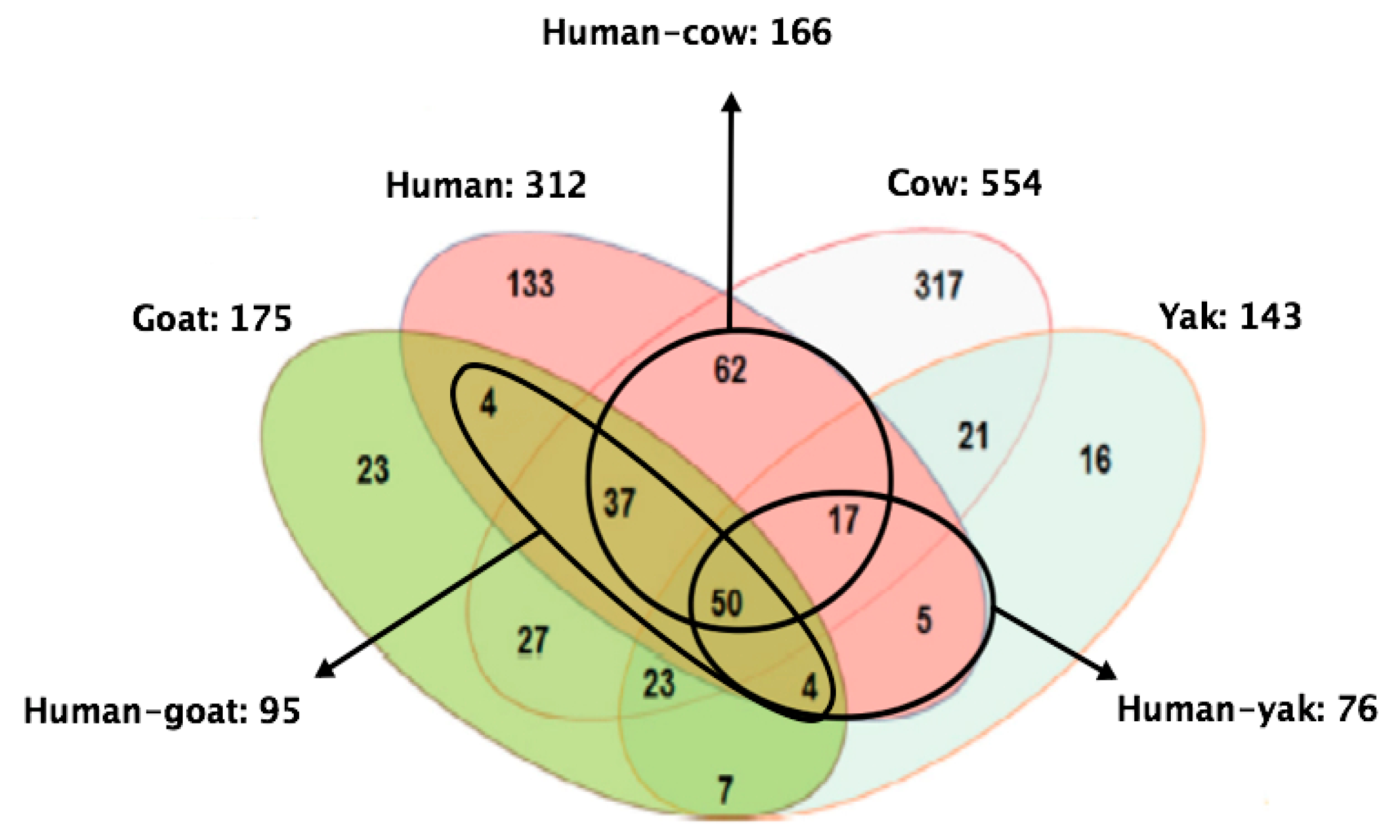
3. Comparison of MFGM Proteome between Different Species and Lactation Stages
4. MFGM: Potentials in Infant Formula Preparation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MFG | Milk fat globule |
| MFGM | Milk fat globule membrane |
| TG | Triglyceride |
| LDL | Low density lipoprotein |
| FA | Fatty acid |
| SCFA | Short-chain fatty acid |
| MCFA | Medium-chain fatty acid |
| LCFA | Long-chain fatty acid |
| PE | Phosphatidylethanolamine |
| PC | Phosphatidylcholine |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
| SM | Sphingomyelin |
| GI | Gastrointestinal |
| ADPH | Long-chain fatty acid |
| BTN | Butyrophilin |
| MUC1 | Mucin 1 |
| XDH/XO | Xanthine dehydrogenase/oxidase |
| PAS III | Periodic acid Schiff III |
| FABP | Fatty acid-binding protein |
References
- Bernard, L.; Bonnet, M.; Delavaud, C.; Delosiere, M.; Ferlay, A.; Fougere, H.; Graulet, B. Milk Fat Globule in Ruminant: Major and Minor Compounds, Nutritional Regulation and Differences Among Species. Eur. J. Lipid Sci. Tech. 2018, 120, 1700039. [Google Scholar] [CrossRef]
- Mulder, H.; Walstra, P. The Milk Fat Globule. Emulsion Science as Applied to Milk Products and Comparable Foods. In Commonwealth Agricultural Bureau; Farnham Royal and Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1974. [Google Scholar]
- Arranz, E.; Corredig, M. Invited review: Milk phospholipid vesicles, their colloidal properties, and potential as delivery vehicles for bioactive molecules. J. Dairy Sci. 2017, 100, 4213–4222. [Google Scholar] [CrossRef] [Green Version]
- Evers, J.M. The milkfat globule membrane—Compositional and structural changes post secretion by the mammary secretory cell. Int. Dairy J. 2004, 14, 661–674. [Google Scholar] [CrossRef]
- Evers, J.M. The milkfat globule membrane—Methodologies for measuring milkfat globule (membrane) damage. Int. Dairy J. 2004, 14, 747–760. [Google Scholar] [CrossRef]
- Spitsberg, V.L. Invited review: Bovine milk fat globule membrane as a potential nutraceutical. J. Dairy Sci. 2005, 88, 2289–2294. [Google Scholar] [CrossRef]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Conway, V.; Gauthier, S.; Pouilot, Y. Buttermilk: Much more than a source of milk phospholipids. Anim. Front. 2014, 4, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Singh, H. The milk fat globule membrane-A biophysical system for food applications. Curr. Opin. Colloid Interface Sci. 2008, 11, 154–163. [Google Scholar] [CrossRef]
- Danthine, S.; Blecker, C.; Paquot, M.; Innocente, N.; Deroanne, C. Évolution des connaissances sur la membrane du globule gras du lait: Synthèse bibliographique. Lait 2000, 80, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Pinotti, L.; Baldi, A.; Dell’Orto, V. Comparative mammalian choline metabolism with emphasis on the high-yielding dairy cow. Nutr. Res. Rev. 2002, 15, 315–331. [Google Scholar] [CrossRef] [Green Version]
- Pinotti, L.; Baldi, A.; Politis, I.; Rebucci, R.; Sangalli, L.; Dell’Orto, V. Rumen-protected choline administration to transition cows: Effects on milk production and vitamin E status. J. Vet. Med. A 2003, 50, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Michalski, M.C.; Briard, V.; Michel, F.; Tasson, F.; Poulain, P. Size distribution of fat globules in human colostrum, breast milk, and infant formula. J. Dairy Sci. 2005, 88, 1927–1940. [Google Scholar] [CrossRef] [Green Version]
- Zanabria, R.; Tellez, A.M.; Griffiths, M.; Corredig, M. Milk fat globule membrane isolate induces apoptosis in HT-29 human colon cancer cells. Food Funct. 2013, 4, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Zanabria, R.; Griffiths, M.W.; Corredig, M. Does structure affect biological function? Modifications to the protein and phospholipids fraction of the milk fat globule membrane after extraction affect the antiproliferative activity of colon cancer cells. J. Food Biochem. 2020, 44, 13104. [Google Scholar] [CrossRef]
- Ji, X.; Xu, W.; Cui, J.; Ma, Y.; Zhou, S. Goat and buffalo milk fat globule membranes exhibit better effects at inducing apoptosis and reduction the viability of HT-29 cells. Sci. Rep. 2019, 9, 2577. [Google Scholar] [CrossRef] [Green Version]
- Fuller, K.L.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Jiménez-Flores, R.; Donovan, S.M. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. J. Dairy Sci. 2013, 96, 3488–3497. [Google Scholar] [CrossRef] [Green Version]
- Struijs, K.; Van de Wiele, T.; Le, T.T.; Debyser, G.; Dewettinck, K.; Devreese, B.; Van Camp, J. Milk fat globule membrane glycoproteins prevent adhesion of the colonic microbiota and result in increased bacterial butyrate production. Int. Dairy J. 2013, 32, 99–109. [Google Scholar] [CrossRef]
- Huang, S.; Wu, Z.; Liu, C.; Han, D.; Feng, C.; Wang, S.; Wang, J. Milk Fat Globule Membrane Supplementation Promotes Neonatal Growth and Alleviates Inflammation in Low-Birth-Weight Mice Treated with Lipopolysaccharide. Biomed. Res. Int. 2019, 2019, 4876078. [Google Scholar] [CrossRef] [Green Version]
- Demmer, E.; Van Loan, M.D.; Rivera, N.; Rogers, T.S.; Gertz, E.R.; German, J.B.; Smilowitz, J.T.; Zivkovic, A.M. Addition of a dairy fraction rich in milk fat globule membrane to a high-saturated fat meal reduces the postprandial insulinaemic and inflammatory response in overweight and obese adults. J. Nutr. Sci. 2016, 5, e14. [Google Scholar] [CrossRef] [Green Version]
- Hernell, O.; Timby, N.; Domellöf, M.; Lönnerdal, B. Clinical benefits of milk fat globule membranes for infants and children. J. Pediatr. 2016, 173, S60–S65. [Google Scholar] [CrossRef] [Green Version]
- Hanuš, O.; Samková, E.; Křížová, L.; Hasoňová, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability-A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markiewicz-Keszycka, M.; Grażyna-Runowska, G.; Lipińska, P.; Wójtowski, J. Fatty Acid Profile of Milk—A Review. Bull. Vet. Inst. Pulawy 2013, 57, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Bernard, L.; Leroux, C.; Chilliard, Y. Nutritional regulation of mammary lipogenesis and milk fat in ruminant: Contribution to sustainable milk production. Rev. Colomb. Cienc. Pecu. 2013, 26, 292–302. [Google Scholar]
- Neville, M.C.; Picciano, M.F. Regulation of milk lipid secretion and composition. Annu. Rev. Nutr. 1997, 17, 159–183. [Google Scholar] [CrossRef]
- Månsson, H.L. Fatty Acids in Bovine Milk Fat. Food Nutr. Res. 2008, 52, 1–3. [Google Scholar]
- Hintze, K.J.; Snow, D.; Burtenshaw, I.; Ward, R.E. Nutraceutical properties of milk fat globular membrane. In Biotechnol. Biopolymers; Elnashar, M., Ed.; Springer: Paris, France, 2011; pp. 321–342. [Google Scholar]
- Lopez, C. Milk fat globules enveloped by their biological membrane: Unique colloidal assemblies with a specific composition and structure. Curr. Opin. Colloid Interface Sci. 2011, 16, 391–404. [Google Scholar] [CrossRef]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef] [Green Version]
- Argov-Argaman, N. Symposium review: Milk fat globule size—Practical implications and metabolic regulation. J. Dairy Sci. 2019, 102, 2783–2795. [Google Scholar] [CrossRef] [Green Version]
- Keenan, T.W.; Morré, D.J.; Olson, D.E.; Yunghans, W.N.; Patton, S. Biochemical and morphological comparison of plasma membrane and milk fat globule membrane from bovine mammary gland. J. Cell Biol. 1970, 44, 80–93. [Google Scholar] [CrossRef]
- Reece, W.O. Dukes’ Physiology of Domestic Animals, 12th ed.; Melvin, J., Swenson, W., Reece, O., Eds.; Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- Horseman, N.D.; Collier, R.J. Serotonin: A local regulator in the mammary gland epithelium. Annu. Rev. Anim. Biosci. 2014, 2, 353–374. [Google Scholar] [CrossRef]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Milk_fat_globule_membrane (accessed on 19 August 2020).
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders-A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Zhang, M.; Xing, Z.; Coleman, D.N.; Liang, Y.; Loor, J.J.; Yang, G. Knockout of butyrophilin subfamily 1 member A1 (BTN1A1) alters lipid droplet formation and phospholipid composition in bovine mammary epithelial cells. J. Anim. Sci. Biotechnol. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.V.; Kitchen, B.J. Reviews of the progress of dairy science: The bovine milk fat globule membrane-its formation, composition, structure and behaviour in milk and dairy products. J. Dairy Res. 1983, 50, 107–133. [Google Scholar] [CrossRef]
- Rohlfs, E.M.; Garner, S.C.; Mar, M.H.; Zeisel, S.H. Glycerophosphocholine and phosphocholine are the major choline metabolites in rat milk. J. Nutr. 1993, 123, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.; Hagemeister, H. Composition of milk. In World Animal Science-C Production-System Approach, Dairy Cattle Production; Gravert, H.O., Ed.; Elsevier Applied Science Publishers: Amsterdam, The Netherlands, 1987; pp. 107–171. [Google Scholar]
- Noble, R.C. Digestion, absorption and transport of lipids in ruminant animals. In Lipid Metabolism in Ruminant Animals; Christie., W.W., Ed.; Pergamon Press: Oxford, UK, 1981; pp. 57–93. [Google Scholar]
- Bitman, J.; Wood, D.L. Changes in milk fat phospholipids during lactation. J. Dairy Sci. 1990, 73, 1208–1216. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Mar, M.; Zhou, Z.; Da Costa, K.A. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J. Nutr. 1995, 125, 3049–3054. [Google Scholar]
- Kinsella, J.E. Preferential labelling of phosphatidylcholine during phospholipid synthesis by bovine mammary tissue. Lipids 1973, 8, 393–400. [Google Scholar] [CrossRef]
- Piepenbrink, M.S.; Overton, T.R. Liver metabolism and production of periparturient dairy cattle fed rumen-protected choline. J. Dairy Sci. 2000, 83, 257. [Google Scholar]
- Pinotti, L.; Campagnoli, A.; Sangalli, L.; Rebucci, R.; Dell’Orto, V.; Baldi, A.. Metabolism of periparturient dairy cows fed rumen-protected choline. J. Anim. Feed Sci. 2004, 13, 551–554. [Google Scholar] [CrossRef]
- Baldi, A.; Pinotti, L. Choline metabolism in high-producing dairy cows: Metabolic and nutritional basis. Can. J. Sci. 2006, 86, 207–212. [Google Scholar] [CrossRef]
- Cooke, R.F.; Silva Del Río, N.; Caraviello, D.Z.; Bertics, S.J.; Ramos, M.H.; Grummer, R.R. Supplemental choline for prevention and alleviation of fatty liver in dairy cattle. J. Dairy Sci. 2007, 90, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Bruckmaier, R.; D’ambrosio, F.; Campagnoli, A.; Pecorini, C.; Rebucci, R.; Pinotti, L. Rumen-protected choline supplementation in periparturient dairy goats: Effects on liver and mammary gland. J. Agric. Sci. 2011, 150, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, B.; Lv, J.; Dong, L.; Zhang, L.; Wang, T. Choline supplementation improves the lipid metabolism of intrauterine-growth-restricted pigs. Asian Australas. J. Anim. Sci. 2018, 31, 686–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinotti, L.; Campagnoli, A.; Dell’Orto, V.; Baldi, A. Choline: Is there a need in lactating dairy cow? Livest. Prod. Sci. 2005, 98, 149–152. [Google Scholar] [CrossRef]
- Pinotti, L.; Campagnoli, A.; D’Ambrosio, F.; Susca, F.; Innocenti, M.; Rebucci, R.; Fusi, E.; Cheli, F.; Savoini, G.; Dell’Orto, V.; et al. Rumen-Protected Choline and Vitamin E Supplementation in Periparturient Dairy Goats: Effects on Milk Production and Folate, Vitamin B12 and Vitamin E Status. Animals 2008, 2, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Polidori, C.; Campagnoli, A.; Dell’Orto, V.; Baldi, A. A meta-analysis of the effects of rumen protected choline supplementation on milk production in dairy cows. In Energy and Protein Metabolism and Nutrition; EAAP Publication No. 127; Crovetto, G.M., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2010; pp. 321–322. [Google Scholar]
- Pinotti, L. Vitamin-Like Supplementation in Dairy Ruminants: The Case of Choline. In Milk Production-An. Up-to-Date Overview of Animal Nutrition, Management and Health; Chaiyabutr, N., Ed.; IntechOpen: London, UK, 2012; pp. 65–86. [Google Scholar]
- Pinotti, L.; Manoni, M.; Fumagalli, F.; Rovere, N.; Tretola, M.; Baldi, A. The role of micronutrients in high-yielding dairy ruminants: Choline and vitamin E. Ank. Univ. Vet. Fak. Derg. 2020, 67, 209–214. [Google Scholar] [CrossRef]
- Arshad, U.; Zenobi, M.G.; Staples, C.R.; Santos, J.E.P. Meta-analysis of the effects of supplemental rumen-protected choline during the transition period on performance and health of parous dairy cow. J. Dairy Sci. 2020, 103, 282–300. [Google Scholar] [CrossRef]
- Mather, I.H. A review and proposed nomenclature for major proteins of the milk-fat globule membrane. J. Dairy Sci. 2000, 83, 203–247. [Google Scholar] [CrossRef]
- Brink, L.R.; Herren, A.W.; McMillen, S.; Fraser, K.; Agnew, M.; Roy, N.; Lönnerdal, B. Omics analysis reveals variations among commercial sources of bovine milk fat globule membrane. J. Dairy Sci. 2020, 103, 3002–3016. [Google Scholar] [CrossRef] [Green Version]
- Vissac, C.; Lemery, D.; Le Corre, L.; Fustier, P.; Dechelotte, P.; Maurizis, J.C.; Bignon, Y.J.; Bernard-Gallon, D.J. Presence of BRCA1 and BRCA2 proteins in human milk fat globules after delivery. Biochim. Biophys. Acta 2002, 1586, 50–56. [Google Scholar] [CrossRef] [Green Version]
- OECD/FAO. Agricultural Outlook 2019–2028; OECD Publishing: Paris, France; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Gallier, S.; Vocking, K.; Post, J.A.; Van De Heijning, B.; Acton, D.; van der Beek, E.M.; Van Baalen, T. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloid Surf. B 2015, 136, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Wang, X.; Zhang, W.; Liu, L.; Pang, X.; Zhang, S.; Lv, J. Comparative proteomics of milk fat globule membrane in different species reveals variations in lactation and nutrition. Food Chem. 2016, 196, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Sun, X.; Guo, M. Comparative Proteomics of Whey and Milk Fat Globule Membrane Proteins of Guanzhong Goat and Holstein Cow Mature Milk. J. Food Sci. 2019, 84, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Cavaletto, M.; Giuffrida, M.G.; Conti, A. The proteomic approach to analysis of human milk fat globule membrane. Clin. Chim. Acta. 2004, 347, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, D.; Giuffrida, M.G.; Cavaletto, M.; Garoffo, L.P.; Dellavalle, G.; Napolitano, L.; Giunta, C.; Fabris, C.; Bertino, E.; Coscia, A.; et al. Structural proteome of human colostral fat globule membrane proteins. Proteomics 2003, 3, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, T.A.; Lippolis, J.D. Bovine milk fat globule membrane proteome. J. Dairy Res. 2006, 73, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Hettinga, K.; van Valenberg, H.; de Vries, S.; Boeren, S.; van Hooijdonk, T.; van Arendonk, J.; Vervoort, J. The host defense proteome of human and bovine milk. PLoS ONE 2011, 6, e19433. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, N.; Wang, W.; Zhao, X.; Zhang, Y.; Han, R.; Ma, L.; Zhao, S.; Li, S.; Guo, T.; et al. N-glycosylation proteomic characterization and cross-species comparison of milk fat globule membrane proteins from mammals. Proteomics 2016, 16, 2792–2800. [Google Scholar] [CrossRef]
- Cao, X.; Zheng, Y.; Wu, S.; Yang, N.; Yue, X. Characterization and comparison of milk fat globule membrane N-glycoproteomes from human and bovine colostrum and mature milk. Food Funct. 2019, 10, 5046–5058. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Cao, X.; Yang, M.; Han, H.; Kong, F.; Yue, X. Quantitative proteomic analysis of milk fat globule membrane (MFGM) proteins from donkey colostrum and mature milk. Food Funct. 2019, 10, 4256–4268. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Sun, X.; Jiang, S.; Guo, M. Characterization of the milk fat globule membrane proteome in colostrum and mature milk of Xinong Saanen goats. J. Dairy Sci. 2020, 103, 3017–3024. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, J.W.; Chu, C.S.; Tang, N.; Waddell, K.; Grimm, R.; Lebrilla, C.B. Label-free liquid chromatography-tandem mass spectrometry analysis with automated phosphopeptide enrichment reveals dynamic human milk protein phosphorylation during lactation. Anal. Biochem. 2011, 408, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Deng, W.; Cao, X.; Wang, L.; Yu, N.; Zheng, Y.; Wu, J.; Wu, R.; Yue, X. Quantitative Phosphoproteomics of Milk Fat Globule Membrane in Human Colostrum and Mature Milk: New Insights into Changes in Protein Phosphorylation during Lactation. J. Agric. Food Chem. 2020, 68, 4546–4556. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D. Developmental changes in the milk fat globule membrane proteome during the transition from colostrum to milk. J. Dairy Sci. 2008, 91, 2307–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zheng, N.; Zhao, X.W.; Zhang, Y.D.; Han, R.W.; Ma, L.; Zhao, S.; Li, S.; Guo, T.; Wang, J. Proteomic characterization and comparison of mammalian milk fat globule proteomes by iTRAQ analysis. J. Proteom. 2015, 116, 34–43. [Google Scholar] [CrossRef]
- Liao, Y.; Alvarado, R.; Phinney, B.; Lönnerdal, B. Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J. Proteome Res. 2011, 10, 3530–3541. [Google Scholar] [CrossRef]
- Spertino, S.; Cipriani, V.; De Angelis, C.; Giuffrida, M.G.; Marsano, F.; Cavaletto, M. Proteome profile and biological activity of caprine, bovine and human milk fat globules. Mol. Biosyst. 2012, 8, 967–974. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, E.; Hernell, O.; Lönnerdal, B.; Domellöf, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Demmelmair, H.; Prell, C.; Timby, N.; Lönnerdal, B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients 2017, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (IOM) of the National Academics. Infant Formula Evaluating the Safety of New Ingredients; The National Academics Press: Washington, DC, USA, 2004. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, R.G.; Ferris, A.M.; Lammi-Keefe, C.J.; Henderson, R.A. Lipids of bovine and human milks: A comparison. J. Dairy Sci. 1990, 73, 223–240. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Jasińska-Melon, E.; Mojska, H.; Olędzka, G.; Wesołowska, A.; Szostak-Węgierek, D. The Concentration of Omega-3 Fatty Acids in Human Milk Is Related to Their Habitual but Not Current Intake. Nutrients 2019, 11, 1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corkins, K.G.; Shurley, T. What’s in the Bottle? A Review of Infant Formulas. Nutr. Clin. Pr. 2016, 31, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 2012, 88, 595–601. [Google Scholar] [CrossRef]
- Lönnerdal, B. Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulas. Am. J. Clin. Nutr. 2014, 99, 712S–717S. [Google Scholar] [CrossRef] [Green Version]
- Billeaud, C.; Puccio, G.; Saliba, E.; Guillois, B.; Vaysse, C.; Pecquet, S.; Steenhout, P. Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: A randomized controlled multicenter non-inferiority trial in healthy term infants. Clin. Med. Insights Pediatr. 2014, 8, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Lönnerdal, B. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 561–568. [Google Scholar] [CrossRef]
- Givens, I. Milk and Dairy Foods, 1st ed.; Academic Press: London UK, 2020; pp. 1–417. [Google Scholar]
- Ye, A.; Cui, J.; Singh, H. Effect of the fat globule membrane on in vitro digestion of milk fat globules with pancreatic lipase. Int. Dairy J. 2010, 20, 822–829. [Google Scholar] [CrossRef]
- Ramírez, M.; Amate, L.; Gil, A. Absorption and distribution of dietary fatty acids from different sources. Early Hum. Dev. 2001, 65, 95–101. [Google Scholar] [CrossRef]
- Agnew, J.E.; Holdsworth, C.D. The effect of fat on calcium absorption from a mixed meal in normal subjects, patients with malabsorptive disease, and patients with a partial gastrectomy. Gut 1971, 12, 973–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, V.; Sandoz, L.; Garcia-Rodenas, C.L. Importance of the regiospecific distribution of long-chain saturated fatty acids on gut comfort, fat and calcium absorption in infants. Prostaglandins Leukot. Essent. Fat. Acids. 2017, 121, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Bribiesca, E.; Turgeon, S.L.; Britten, M. Effect of calcium on fatty acid bioaccessibility during in vitro digestion of Cheddar-type cheeses prepared with different milk fat fractions. J. Dairy Sci. 2017, 100, 2454–2470. [Google Scholar] [CrossRef] [PubMed]
- Tomarelli, R.M.; Meyer, B.J.; Weaber, J.R.; Bernhart, F.W. Effect of Positional Distribution on the Absorption of the Fatty Acids of Human Milk and Infant Formulas. Nutr. J. 1968, 95, 583–590. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Dillard, C.J. Composition, structure and absorption of milk lipids: A source of energy, fat-soluble nutrients and bioactive molecules. Crit. Rev. Food Sci. Nutr. 2006, 46, 57–92. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, R.; Holland, J.W.; Deeth, H.C.; Alewood, P.F. Milk proteomics. Int. Dairy J. 2004, 14, 1013–1023. [Google Scholar] [CrossRef]




| Product | MFGM (mg/100 g) |
|---|---|
| Cheese (25% fat) | 150 |
| Milk (skimmed, 0.5% fat) | 15 |
| Milk (whole, 3.5% fat) | 35 |
| Yogurt (1.5% fat) | 15 |
| Cream (38% fat) | 200 |
| Components | Abbreviation | Functions |
|---|---|---|
| Polar Lipids | ||
| Phosphatidylcholine | PC | Structural maintenance of MFGM; cholesterol regulation and lipoproteins metabolism |
| Phosphatidylethanolamine | PE | Structural membrane regulation |
| Phosphatidylinositol | PI | Cell signaling; PI3K-Akt pathway regulation |
| Phosphatidylserine | PS | Apoptosis regulation |
| Sphingomyelin | SM | Myelinization; metabolized to ceramide and sphingosine (second messengers that regulate cell growth and cell cycle) |
| Cholesterol | - | Structural maintenance of MFGM (lipid rafts complexes with SM) |
| Proteins | ||
| Adipophilin | ADPH | Lipolysis regulation |
| Butyrophilin | BTN | MFG synthesis regulation |
| Mucin 1 | MUC 1 | Decoy receptor for pathogens; inhibition of in vitro rotavirus infectivity |
| Xanthine dehydrogenase/oxidase | XDH/XO | Structural maintenance of MFGM; antimicrobial activity (ROS/RNS production) |
| Fatty acid-binding protein | FABP | Fatty acid transport; MFG lipid synthesis |
| Breast related cancer antigens 1/2 | BRCA 1/2 | Onco-suppressor activity |
| Choline | - | Precursor of phospholipids and SM; hepatic lipid metabolism |
| Gangliosides | - | Cognitive development |
| Item | Human Breast Milk | Cow-Based IF |
|---|---|---|
| Energy (kcal/100 mL) | 65 | 60–70 |
| Digestible carbohydrates (g/100 kcal) | 8.2–10.4 | 9–14 |
| Lipids (g/100 kcal) | 3.7–9.1 | 4.4–6 |
| Proteins (g/100 kcal) | 1.3 (0.8–2.1) a | 1.8–2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoni, M.; Di Lorenzo, C.; Ottoboni, M.; Tretola, M.; Pinotti, L. Comparative Proteomics of Milk Fat Globule Membrane (MFGM) Proteome across Species and Lactation Stages and the Potentials of MFGM Fractions in Infant Formula Preparation. Foods 2020, 9, 1251. https://doi.org/10.3390/foods9091251
Manoni M, Di Lorenzo C, Ottoboni M, Tretola M, Pinotti L. Comparative Proteomics of Milk Fat Globule Membrane (MFGM) Proteome across Species and Lactation Stages and the Potentials of MFGM Fractions in Infant Formula Preparation. Foods. 2020; 9(9):1251. https://doi.org/10.3390/foods9091251
Chicago/Turabian StyleManoni, Michele, Chiara Di Lorenzo, Matteo Ottoboni, Marco Tretola, and Luciano Pinotti. 2020. "Comparative Proteomics of Milk Fat Globule Membrane (MFGM) Proteome across Species and Lactation Stages and the Potentials of MFGM Fractions in Infant Formula Preparation" Foods 9, no. 9: 1251. https://doi.org/10.3390/foods9091251
APA StyleManoni, M., Di Lorenzo, C., Ottoboni, M., Tretola, M., & Pinotti, L. (2020). Comparative Proteomics of Milk Fat Globule Membrane (MFGM) Proteome across Species and Lactation Stages and the Potentials of MFGM Fractions in Infant Formula Preparation. Foods, 9(9), 1251. https://doi.org/10.3390/foods9091251