Lactobacillus rhamnosus GG and Biochemical Agents Enrich the Shelf Life of Fresh-Cut Bell Pepper (Capsicum annuum L. var. grossum (L.) Sendt)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bell Pepper and Microbes
2.2. Preparation of Biochemical Additive Solution and Bacterial Inoculum
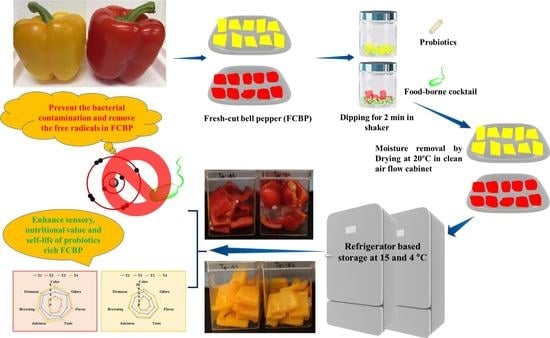
2.3. Preparation of the FCBP and Treatments
2.4. Enumeration of Microbial Colonization
2.5. DPPH Scavenging, Total Phenol, Flavonoids, Color, Texture, and Sensory Properties
3. Results and Discussion
3.1. Microbial Changes
3.2. Phytochemical Changes and Antioxidant Properties
3.3. Color and Texture Sensory Changes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, B.; Kam, D.; Min, B.; Hwa, J.; Oh, S. A vision servo system for automated harvest of sweet pepper in Korean greenhouse environment. Appl. Sci. 2019, 9, 2395. [Google Scholar] [CrossRef]
- Choi, I.-L.; Yoo, T.J.; Kang, H.-M. UV-C treatments enhance antioxidant activity, retain quality and microbial safety of fresh-cut paprika in MA storage. Hortic. Environ. Biotechnol. 2015, 56, 324–329. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Mohd Adzahan, N. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharv. Biol. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Bae, Y.-M.; Choi, N.-Y.; Heu, S.; Kang, D.-H.; Lee, S.-Y. Inhibitory effects of organic acids combined with modified atmosphere packaging on foodborne pathogens on cabbage. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 993–997. [Google Scholar] [CrossRef]
- Sivapalasingam, S.; Friedman, C.R.; Cohen, L.; Tauxe, R.V. Fresh produce: A growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 2004, 67, 2342–2353. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Shiga Toxin-Producing E. coli (STEC): Update on Outbreak in the EU, J. Update on Outbreak in the EU. ECDC, Stockholm (2011). 2011. Available online: http://ecdc.europa.eu/en/activities/sciadvice/Lists/ECDC%20Reviews/ECDC_DispForm.aspx?List=512ff74f%2D77d4%2D4ad8%2Db6d6%2Dbf0f23083f30&ID=1166&RootFolder=%2Fen%2Factivities%2Fsciadvice%2FLists%2FECDC%20Reviews (accessed on 16 January 2012).
- Zhu, Q.; Gooneratne, R.; Hussain, M.A. Listeria monocytogenes in Fresh Produce: Outbreaks, prevalence and contamination levels. Foods 2017, 6, 21. [Google Scholar] [CrossRef]
- Wu, S.; Wu, Q.; Zhang, J.; Chen, M.; Yan, Z.A.; Hu, H. Listeria monocytogenes prevalence and characteristics in retail raw foods in China. PLoS ONE 2015, 10, e0136682. [Google Scholar] [CrossRef]
- Ding, T.; Iwahori, J.I.; Kasuga, F.; Wang, J.; Forghani, F.; Park, M.-S.; Oh, D.-H. Risk assessment for Listeria monocytogenes on lettuce from farm to table in Korea. Food Control 2013, 30, 190–199. [Google Scholar] [CrossRef]
- Oliveira, M.; Usall, J.; Solsona, C.; Alegre, I.; Viñas, I.; Abadias, M. Effects of packaging type and storage temperature on the growth of foodborne pathogens on shredded ‘Romaine’ lettuce. Food Microbiol. 2010, 27, 375–380. [Google Scholar] [CrossRef]
- Luo, Y.; He, Q.; McEvoy, J.L. Effect of storage temperature and duration on the behavior of Escherichia coli O157:H7 on packaged fresh-cut salad containing romaine and iceberg lettuce. J. Food Sci. 2010, 75, M390–M397. [Google Scholar] [CrossRef]
- Alegre, I.; Abadias, M.; Anguera, M.; Oliveira, M.; Viñas, I. Factors affecting growth of foodborne pathogens on minimally processed apples. Food Microbiol. 2010, 27, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Leverentz, B.; Conway, W.S.; Alavidze, Z.; Janisiewicz, W.J.; Fuchs, Y.; Camp, M.J.; Chighladze, E.; Sulakvelidze, A. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: A model study. J. Food Prot. 2001, 64, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Alegre, I.; Abadias, M.; Anguera, M.; Usall, J.; Viñas, I. Fate of Escherichia coli O157:H7, Salmonella and Listeria innocua on minimally-processed peaches under different storage conditions. Food Microbiol. 2010, 27, 862–868. [Google Scholar] [CrossRef]
- Seo, Y.-H.; Jang, J.-H.; Moon, K.-D. Microbial evaluation of minimally processed vegetables and sprouts produced in Seoul, Korea. Food Sci. Biotechnol. 2010, 19, 1283–1288. [Google Scholar] [CrossRef]
- Lynch, M.F.; Tauxe, R.V.; Hedberg, C.W. The growing burden of foodborne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiol. Infect. 2009, 137, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Abadias, M.; Colás-Medà, P.; Usall, J.; Viñas, I. Biopreservative methods to control the growth of foodborne pathogens on fresh-cut lettuce. Int. J. Food Microbiol. 2015, 214, 4–11. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings to incorporate active ingredients to fresh-cut fruits: A review. Trends Food Sci. Technol. 2009, 20, 438–447. [Google Scholar] [CrossRef]
- Bico, S.L.S.; Raposo, M.F.J.; Morais, R.M.S.C.; Morais, A.M.M.B. Combined effects of chemical dip and/or carrageenan coating and/or controlled atmosphere on quality of fresh-cut banana. Food Control 2009, 20, 508–514. [Google Scholar] [CrossRef]
- Gorny, J.R.; Hess-Pierce, B.; Cifuentes, R.A.; Kader, A.A. Quality changes in fresh-cut pear slices as affected by controlled atmospheres and chemical preservatives. Postharv. Biol. Technol. 2002, 24, 271–278. [Google Scholar] [CrossRef]
- Peng, M.; Reichmann, G.; Biswas, D. Lactobacillus casei and its byproducts alter the virulence factors of foodborne bacterial pathogens. J. Func. Foods 2015, 15, 418–428. [Google Scholar] [CrossRef]
- Iglesias, M.B.; Echeverría, G.; Viñas, I.; López, M.L.; Abadias, M. Biopreservation of fresh-cut pear using Lactobacillus rhamnosus GG and effect on quality and volatile compounds. LWT 2018, 87, 581–588. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tappi, S.; Rocculi, P.; Gardini, F.; Lanciotti, R. Natural antimicrobials to prolong the shelf-life of minimally processed lamb’s lettuce. Postharv. Biol. Technol. 2015, 103, 35–44. [Google Scholar] [CrossRef]
- Alegre, I.; Viñas, I.; Usall, J.; Anguera, M.; Abadias, M. Microbiological and physicochemical quality of fresh-cut apple enriched with the probiotic strain Lactobacillus rhamnosus GG. Food Microbiol. 2011, 28, 59–66. [Google Scholar] [CrossRef]
- Iglesias, M.B.; Abadias, M.; Anguera, M.; Sabata, J.; Viñas, I. Antagonistic effect of probiotic bacteria against foodborne pathogens on fresh-cut pear. LWT Food Sci. Technol. 2017, 81, 243–249. [Google Scholar] [CrossRef]
- Aslam, M.; Khalid, S.; Kamran, H.; Azhar, S.; Hamid, S. Determination of sodium benzoate in selected samples of fruit juices and squashes. AJAHS 2017, 2, 26–31. [Google Scholar]
- Saravanakumar, K.; Hu, X.; Chelliah, R.; Oh, D.-H.; Kathiresan, K.; Wang, M.-H. Biogenic silver nanoparticles-polyvinylpyrrolidone based glycerosomes coating to expand the shelf life of fresh-cut bell pepper (Capsicum annuum L. var. grossum (L.) Sendt). Postharv. Biol. Technol. 2020, 160, 111039. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ardestani, A.; Yazdanparast, R. Inhibitory effects of ethyl acetate extract of Teucrium polium on in vitro protein glycoxidation. Food Chem. Toxicol. 2007, 45, 2402–2411. [Google Scholar] [CrossRef]
- Russo, P.; Peña, N.; de Chiara, M.L.V.; Amodio, M.L.; Colelli, G.; Spano, G. Probiotic lactic acid bacteria for the production of multifunctional fresh-cut cantaloupe. Food Res. Int. 2015, 77, 762–772. [Google Scholar] [CrossRef]
- Cross, M.L. Immunoregulation by probiotic lactobacilli: Pro-Th1 signals and their relevance to human health. Clin. Appl. Immunol. Rev. 2002, 3, 115–125. [Google Scholar] [CrossRef]
- McCann, M.J.; Gill, C.I.R.; O’ Brien, G.; Rao, J.R.; McRoberts, W.C.; Hughes, P.; McEntee, R.; Rowland, I.R. Anti-cancer properties of phenolics from apple waste on colon carcinogenesis in vitro. Food Chem. Toxicol. 2007, 45, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.T.; Kang, J.H.; Lee, M.S. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. 2007, 113, 358–361. [Google Scholar] [CrossRef]
- Reid, G.; Burton, J. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect. 2002, 4, 319–324. [Google Scholar] [CrossRef]
- Lee, H.Y.; Xu, H.; Lee, H.J.; Lim, T.I.; Choi, Y.B.; Ko, J.R.; Ahn, J.; Mustapha, A. Prophylactic uses of probiotics as a potential alternative to antimicrobials in food animals. Food Sci. Biotechnol. 2008, 17, 191–194. [Google Scholar]
- Karami, S.; Roayaei, M.; Zahedi, E.; Bahmani, M.; Mahmoodnia, L.; Hamzavi, H.; Rafieian-Kopaei, M. Antifungal effects of Lactobacillus species isolated from local dairy products. Int. J. Pharm. Investig. 2017, 7, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Cabo, M.L.; Braber, A.F.; Koenraad, P.M. Apparent antifungal activity of several lactic acid bacteria against Penicillium discolor is due to acetic acid in the medium. J. Food Prot. 2002, 65, 1309–1316. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Vafai, S.B.; Delaney, N.F.; Clish, C.B.; Deik, A.A.; Pierce, K.A.; Ludwig, D.S.; Mootha, V.K. Effects of sodium benzoate, a widely used food preservative, on glucose homeostasis and metabolic profiles in humans. Mol. Genet. Metab. 2015, 114, 73–79. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Sobrino-López, A.; Soledad Tapia, M.; Martín-Belloso, O. Browning inhibition in fresh-cut ‘Fuji’ apple slices by natural antibrowning agents. J. Food Sci. 2006, 71, S59–S65. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Del Real, L.A.; Gutiérrez-Cortez, E.; Cornejo-Villegas, M.A.; Quintanar-Guerrero, D. The effect of nano-coatings with α-tocopherol and xanthan gum on shelf-life and browning index of fresh-cut “red Delicious” apples. Innov. Food Sci. Emerg. Technol. 2014, 22, 188–196. [Google Scholar] [CrossRef]
- Koushesh Saba, M.; Sogvar, O.B. Combination of carboxymethyl cellulose-based coatings with calcium and ascorbic acid impacts in browning and quality of fresh-cut apples. LWT Food Sci. Technol. 2016, 66, 165–171. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Castillo, S.; Guillén, F.; Díaz-Mula, H.M.; Zapata, P.J.; Valero, D.; Serrano, M. Aloe vera gel coating maintains quality and safety of ready-to-eat pomegranate arils. Postharv. Biol. Technol. 2013, 86, 107–112. [Google Scholar] [CrossRef]
- Saxena, A.; Saxena, T.M.; Raju, P.S.; Bawa, A.S. Effect of controlled atmosphere storage and chitosan coating on quality of fresh-cut jackfruit bulbs. Food Bioproc. Technol. 2013, 6, 2182–2189. [Google Scholar] [CrossRef]
- Russo, P.; de Chiara, M.L.V.; Vernile, A.; Amodio, M.L.; Arena, M.P.; Capozzi, V.; Massa, S.; Spano, G. Fresh-Cut Pineapple as a New Carrier of Probiotic Lactic Acid Bacteria. BioMed Res. Int. 2014, 2014, 309183. [Google Scholar] [CrossRef]




| Sources | Microbial Counts (CFU g−1) | ||||
|---|---|---|---|---|---|
| TBC | TSC | TLC | TFC | TLABC | |
| Treatments | |||||
| T0 | 1959.30 ± 6.14 | 1.83 ± 0.23 | 1.57 ± 0.05 | 1000.87 ± 4.55 | 0 |
| T1 | 911.6 ± 2.56 | 0 | 0 | 753.61 ± 6.23 | 0 |
| T2 | 1031.21 ± 8.74 | 640.43 ± 2.34 | 431.36 ± 6.11 | 836.28 ± 2.56 | 0 |
| T3 | 412.2 ± 2.89 | 0 | 0 | 115.57 ± 5.79 | 86.30 ± 3.55 |
| T4 | 491.87 ± 7.25 | 245.43 ± 5.26 | 102.48 ± 2.13 | 619.59 ± 6.13 | 83.14 ± 0.84 |
| Colors | |||||
| R-FCBP | 1188.09 ± 9.25 | 225.56 ± 4.25 | 149.69 ± 1.96 | 932.94 ± 3.93 | 33.79 ± 2.84 |
| Y-FCBP | 863.96 ± 7.21 | 129.52 ± 2.15 | 64.47 ± 2.89 | 397.55 ± 3.71 | 33.98 ± 3.08 |
| Temperature (°C) | |||||
| 4 | 585.05 ± 8.25 | 115.98 ± 6.15 | 89.24 ± 2.13 | 199.06 ± 9.29 | 35.19 ± 1.85 |
| 15 | 1367.2 ± 4.25 | 239.16 ± 8.14 | 125.04 ± 1.69 | 1131.43 ± 2.30 | 32.58 ± 4.79 |
| Intervals (Days) | |||||
| 0 | 195.30 ± 4.26 | 210.83 ± 2.64 | 164.14 ± 3.73 | 24.38 ± 2.21 | 48.94 ± 1.97 |
| 3 | 672.58 ± 2.54 | 99.47 ± 6.12 | 110.58 ± 2.39 | 457.77 ± 2.14 | 32.29 ± 1.50 |
| 6 | 895.10 ± 4.27 | 125.44 ± 4.52 | 67.19 ± 1.62 | 724.84 ± 3.13 | 32.75 ± 3.65 |
| 9 | 1287.33 ± 2.21 | 234.83 ± 53.15 | 84.21 ± 5.85 | 891.25 ± 8.00 | 30.57 ± 1.07 |
| 12 | 1271.81 ± 9.25 | 153.40 ± 8.21 | 97.84 ± 1.00 | 912.70 ± 3.60 | 30.20 ± 2.51 |
| 15 | 1533.28 ± 5.26 | 192.67 ± 1.62 | 118.52 ± 2.65 | 980.75 ± 1.24 | 28.57 ± 1.35 |
| Significance | |||||
| Treatments | (F = 1115.24) ** | (F = 35,445.03) ** | (F = 23,970.39) ** | (F = 27,561.41) ** | (F = 18,963.46) ** |
| Colors | (F = 284.64) ** | (F = 5157.02) ** | (F = 6088.55) ** | (F = 85,918.17) ** | (F = 0.019) NS |
| Temperature (°C) | (F = 2489.02) ** | (F = 8512.48) ** | (F = 1054.36) ** | (F = 244,433.7) ** | (F = 66.97) ** |
| Intervals (days) | (F = 500.68) ** | (F = 1138.20) ** | (F = 666.34) ** | (F = 17,773.40) ** | (F = 421.33) ** |
| Sources | DPPH Scavenging (%) | TFaC (OD 450 nm) | TPC (OD 760 nm) |
|---|---|---|---|
| Treatments | |||
| T0 | 16.95 ± 0.31 | 0.085 ± 0.018 | 1.28 ± 0.15 |
| T1 | 17.37 ± 0.76 | 0.087 ± 0.005 | 1.81 ± 0.21 |
| T2 | 16.16 ± 1.26 | 0.081 ± 0.007 | 1.88 ± 0.18 |
| T3 | 23.27 ± 0.25 | 0.075 ± 0.010 | 1.76 ± 0.13 |
| T4 | 51.75 ± 0.62 | 0.085 ± 0.004 | 1.88 ± 0.11 |
| Color of FCBP | |||
| R-FCBP | 24.52 ± 2.61 | 0.084 ± 0.001 | 1.75 ± 0.17 |
| Y-FCBP | 25.69 ± 0.76 | 0.081 ± 0.010 | 1.70 ± 0.13 |
| Temperature | |||
| 4 | 24.16 ± 0.37 | 0.081 ± 0.004 | 1.71 ± 0.16 |
| 15 | 26.04 ± 0.24 | 0.085 ± 0.001 | 1.73 ± 0.11 |
| Intervals | |||
| 0 | 79.95 ± 0.13 | 0.117 ± 0.013 | 1.75 ± 0.01 |
| 3 | 17.11 ± 1.41 | 0.091 ± 0.015 | 1.86 ± 0.08 |
| 6 | 13.87 ± 0.76 | 0.091 ± 0.001 | 1.73 ± 0.09 |
| 9 | 15.16 ± 0.26 | 0.069 ± 0.02 | 1.71 ± 0.16 |
| 12 | 10.94 ± 0.77 | 0.072 ± 0.04 | 1.63 ± 0.08 |
| 15 | 13.58 ± 1.98 | 0.056 ± 0.011 | 1.66 ± 0.02 |
| Significance | |||
| Treatments | (F = 16,555.60) ** | (F = 12.15) ** | (F = 365.10) ** |
| Color of FCBP | (F = 20.24) ** | (F = 18.07) ** | (F = 19.48) ** |
| Temperature | (F = 52.51) ** | (F = 11.18) ** | (F = 8.6) ** |
| Intervals (days) | (F = 7175.76) ** | (F = 7.46) ** | (F = 30.63) ** |
| Sources | L | a | b | ∆E | Texture (g) |
|---|---|---|---|---|---|
| Treatments | |||||
| T0 | 22.74 ± 0.20 | 7.40 ± 1.00 | 13.76 ± 0.55 | 8.79 ± 0.20 | 597.02 ± 5.99 |
| T1 | 23.36 ± 2.04 | 9.05 ± 1.38 | 12.95 ± 1.01 | 9.32 ± 0.25 | 693.54 ± 5.49 |
| T2 | 170.06 ± 2.25 | 5.80 ± 0.73 | 18.18 ± 0.51 | 11.81 ± 0.27 | 540.99 ± 11.41 |
| T3 | 27.40 ± 1.99 | 7.99 ± 0.21 | 17.56 ± 1.99 | 8.53 ± 0.69 | 739.02 ± 2.15 |
| T4 | 27.96 ± 3.04 | 7.81 ± 0.17 | 18.55 ± 2.13 | 8.20 ± 0.41 | 449.33 ± 5.88 |
| Color of FCBP | |||||
| R | 28.92 ± 1.20 | 17.99 ± 0.18 | 12.93 ± 0.63 | 22.46 ± 0.44 | 659.12 ± 4.87 |
| Y | 79.69 ± 1.03 | 2.24 ± 0.22 | 19.46 ± 1.52 | 8.23 ± 1.77 | 548.84 ± 2.09 |
| Temperature | |||||
| 4 | 88.13 ± 2.90 | 9.01 ± 0.77 | 18.44 ± 0.55 | 8.82 ± 1.41 | 842.41 ± 9.40 |
| 15 | 20.47 ± 1.51 | 6.22 ± 0.83 | 13.96 ± 1.73 | 9.84 ± 1.81 | 365.55 ± 3.83 |
| Intervals | |||||
| 0 | 342.75 ± 0.51 | 12.46 ± 0.03 | 26.35 ± 2.69 | 5.94 ± 0.59 | 1018.74 ± 8.53 |
| 3 | 240.86 ± 0.72 | 13.18 ± 0.51 | 24.95 ± 1.53 | 6.12 ± 0.44 | 1054.24 ± 10.35 |
| 6 | 51.73 ± 0.78 | 7.86 ± 0.26 | 23.18 ± 0.61 | 10.93 ± 0.14 | 708.40 ± 13.20 |
| 9 | 31.71 ± 2.22 | 8.14 ± 0.66 | 14.90 ± 0.87 | 8.59 ± 0.65 | 524.00 ± 3.61 |
| 12 | 10.63 ± 2.05 | 3.77 ± 0.84 | 5.67 ± 1.12 | 11.79 ± 0.87 | 252.84 ± 3.34 |
| Significance | |||||
| Treatments | (F = 3.97) ** | (F = 122.09) ** | (F = 53.07) ** | (F = 5.58) ** | (F = 83.89) ** |
| Color of FCBP | (F = 3.35) ** | (F = 13.51) ** | (F = 223.21) ** | (F = 14.93) ** | (F = 8.35) ** |
| Temperature | (F = 3.35) ** | (F = 619.08) ** | (F = 120.36) ** | (F = 0.11) NS | (F = 872.56) ** |
| Intervals (days) | (F = 4.95) ** | (F = 1564.99) ** | (F = 668.91) ** | (F = 12.77) ** | (F = 831.22) ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Chelliah, R.; Hu, X.; Oh, D.H.; Wang, M.-H. Lactobacillus rhamnosus GG and Biochemical Agents Enrich the Shelf Life of Fresh-Cut Bell Pepper (Capsicum annuum L. var. grossum (L.) Sendt). Foods 2020, 9, 1252. https://doi.org/10.3390/foods9091252
Saravanakumar K, Sathiyaseelan A, Mariadoss AVA, Chelliah R, Hu X, Oh DH, Wang M-H. Lactobacillus rhamnosus GG and Biochemical Agents Enrich the Shelf Life of Fresh-Cut Bell Pepper (Capsicum annuum L. var. grossum (L.) Sendt). Foods. 2020; 9(9):1252. https://doi.org/10.3390/foods9091252
Chicago/Turabian StyleSaravanakumar, Kandasamy, Anbazhagan Sathiyaseelan, Arokia Vijaya Anand Mariadoss, Ramachandran Chelliah, Xiaowen Hu, Deog Hwan Oh, and Myeong-Hyeon Wang. 2020. "Lactobacillus rhamnosus GG and Biochemical Agents Enrich the Shelf Life of Fresh-Cut Bell Pepper (Capsicum annuum L. var. grossum (L.) Sendt)" Foods 9, no. 9: 1252. https://doi.org/10.3390/foods9091252
APA StyleSaravanakumar, K., Sathiyaseelan, A., Mariadoss, A. V. A., Chelliah, R., Hu, X., Oh, D. H., & Wang, M.-H. (2020). Lactobacillus rhamnosus GG and Biochemical Agents Enrich the Shelf Life of Fresh-Cut Bell Pepper (Capsicum annuum L. var. grossum (L.) Sendt). Foods, 9(9), 1252. https://doi.org/10.3390/foods9091252