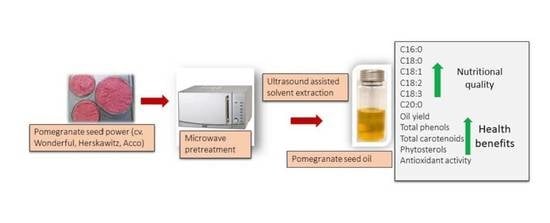
Effect of Microwave Pretreatment of Seeds on the Quality and Antioxidant Capacity of Pomegranate Seed Oil
Abstract
Share and Cite
Kaseke, T.; Opara, U.L.; Fawole, O.A. Effect of Microwave Pretreatment of Seeds on the Quality and Antioxidant Capacity of Pomegranate Seed Oil. Foods 2020, 9, 1287. https://doi.org/10.3390/foods9091287
Kaseke T, Opara UL, Fawole OA. Effect of Microwave Pretreatment of Seeds on the Quality and Antioxidant Capacity of Pomegranate Seed Oil. Foods. 2020; 9(9):1287. https://doi.org/10.3390/foods9091287
Chicago/Turabian StyleKaseke, Tafadzwa, Umezuruike Linus Opara, and Olaniyi Amos Fawole. 2020. "Effect of Microwave Pretreatment of Seeds on the Quality and Antioxidant Capacity of Pomegranate Seed Oil" Foods 9, no. 9: 1287. https://doi.org/10.3390/foods9091287
APA StyleKaseke, T., Opara, U. L., & Fawole, O. A. (2020). Effect of Microwave Pretreatment of Seeds on the Quality and Antioxidant Capacity of Pomegranate Seed Oil. Foods, 9(9), 1287. https://doi.org/10.3390/foods9091287