Headspace Gas Chromatography Coupled to Mass Spectrometry and Ion Mobility Spectrometry: Classification of Virgin Olive Oils as a Study Case
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards
2.2. Samples
2.3. Instrumentation and Software
2.4. HS-GC-IMS Analysis
2.5. HS-GC-MS Analysis
2.6. Statistical Analysis
3. Results
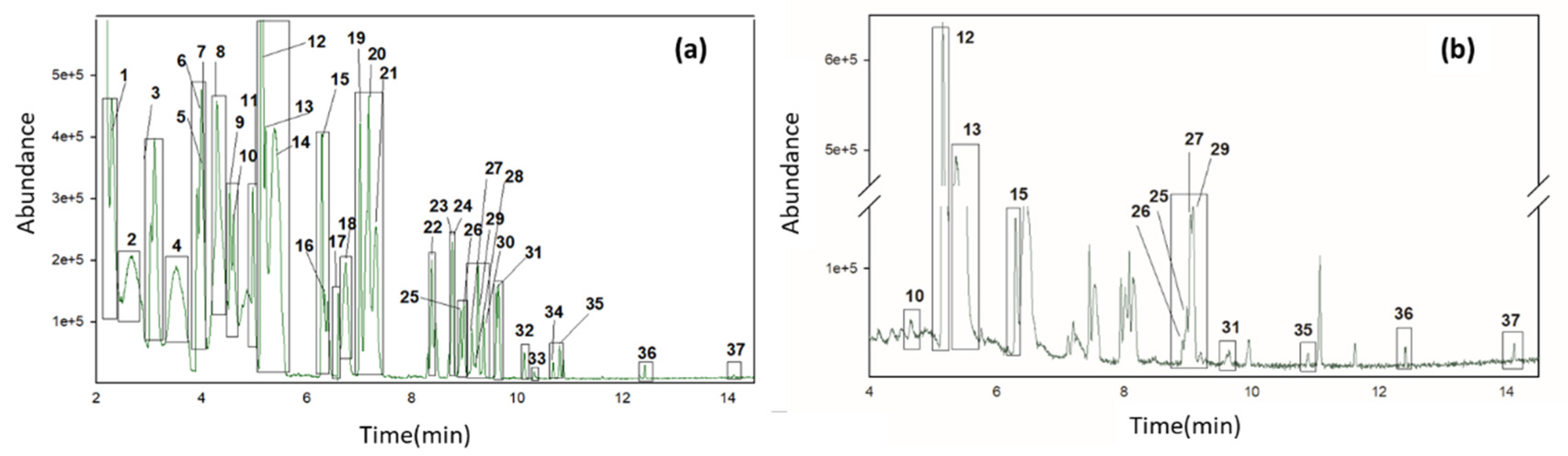
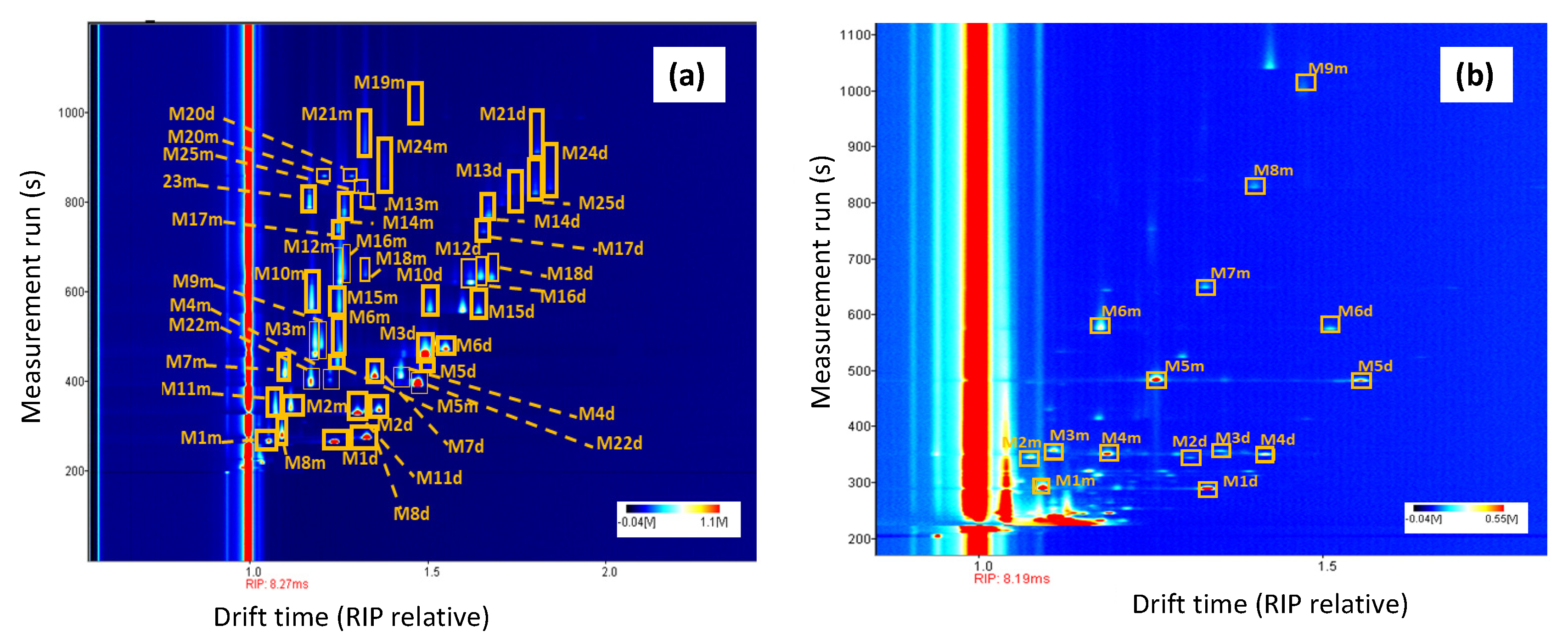
3.1. Optimization of HS-GC-MS and HS-GC-IMS Methods
3.2. Identification and Quantification of Volatile Compounds in Olive Oil Samples by HS-GC-MS and HS-GC-IMS
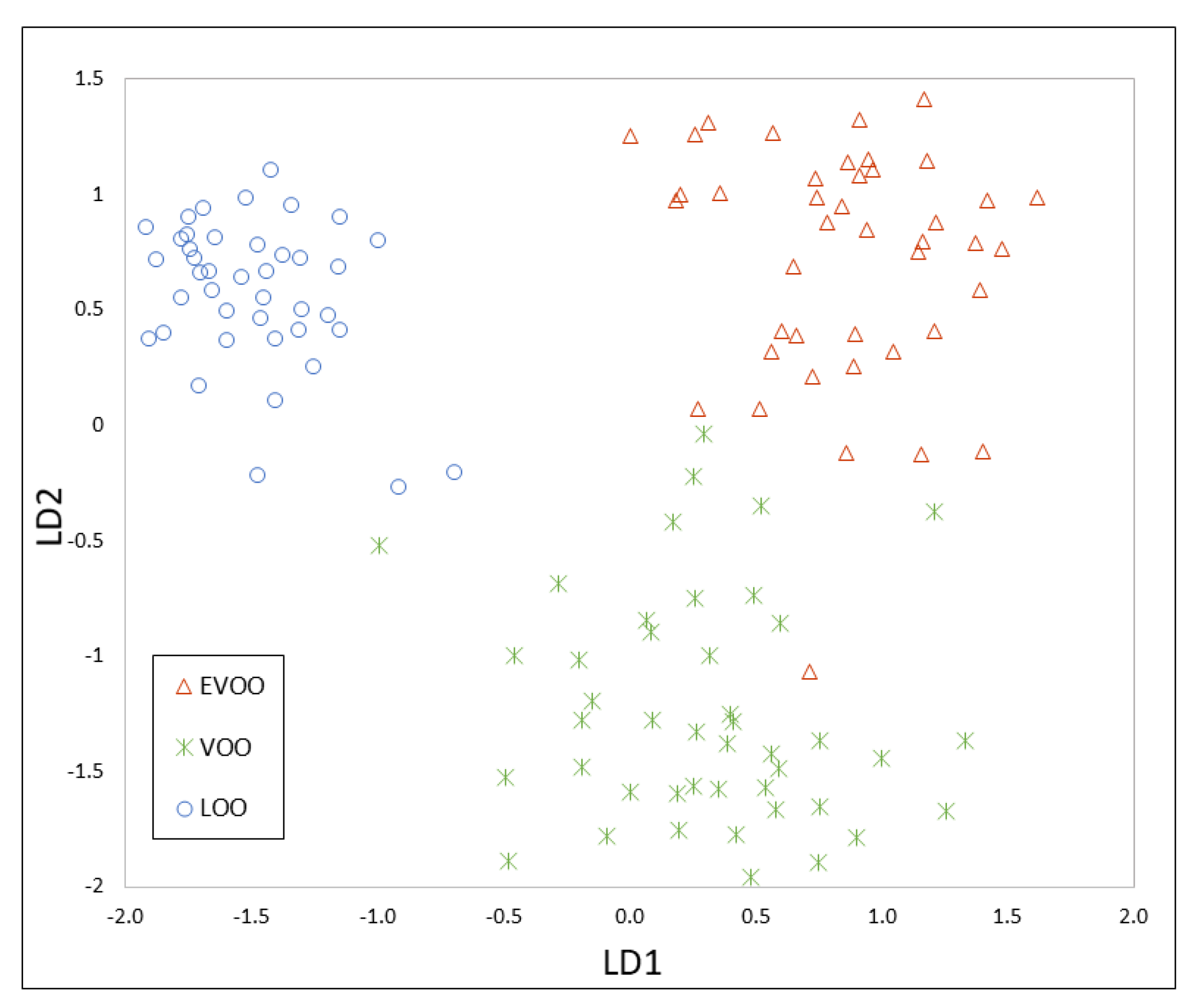
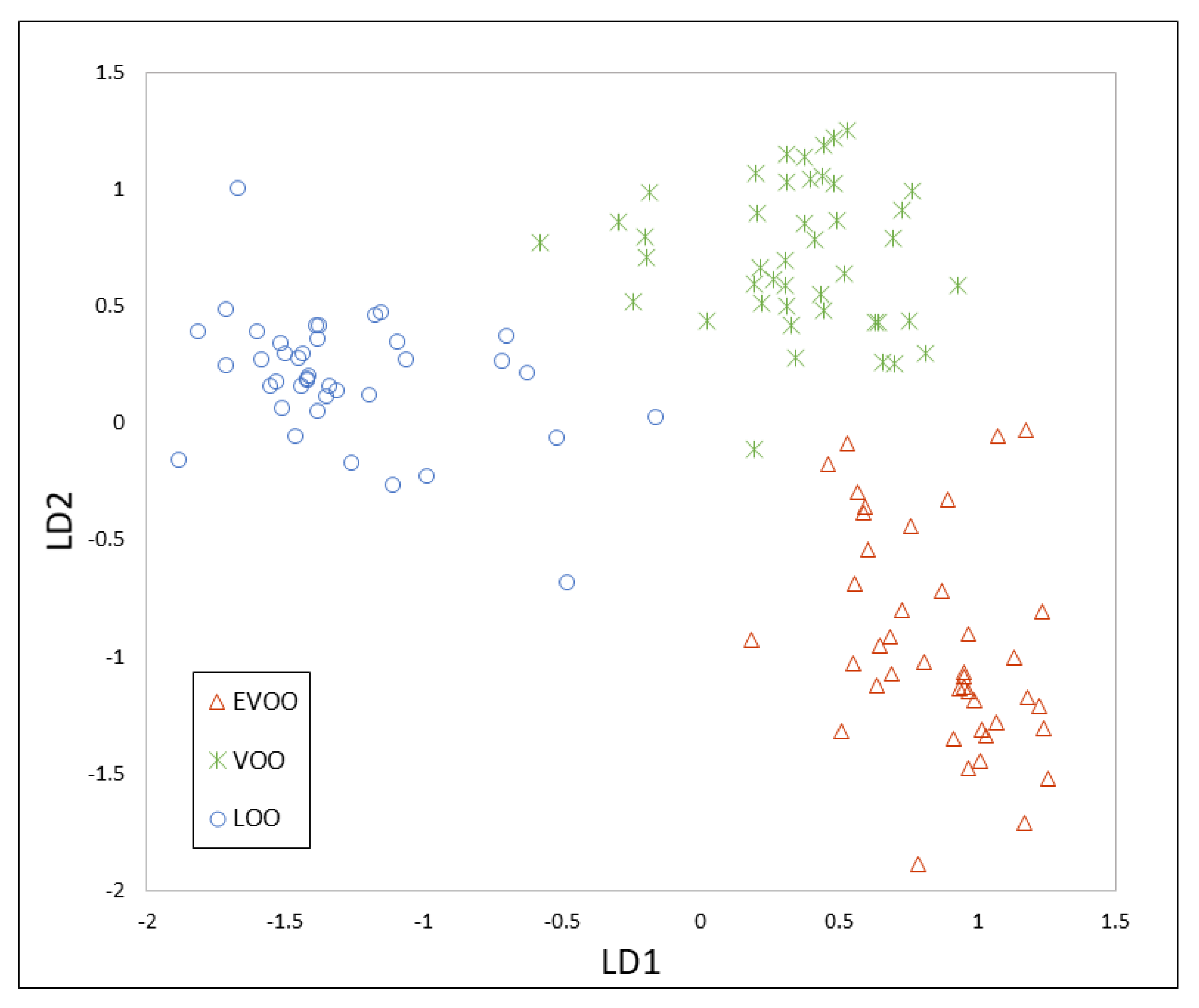
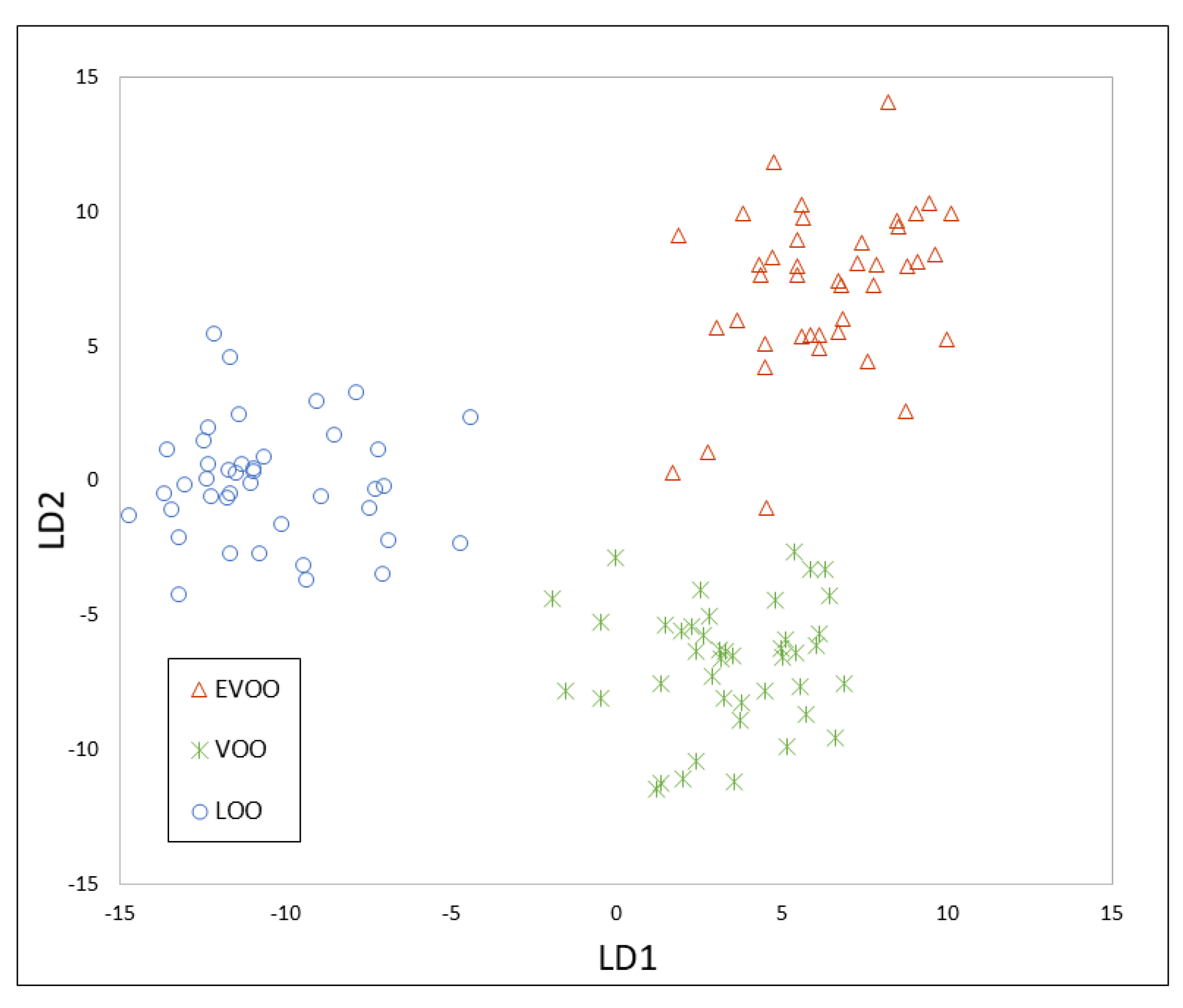
3.3. Chemometrics for Olive Oil Classification According to Its Quality
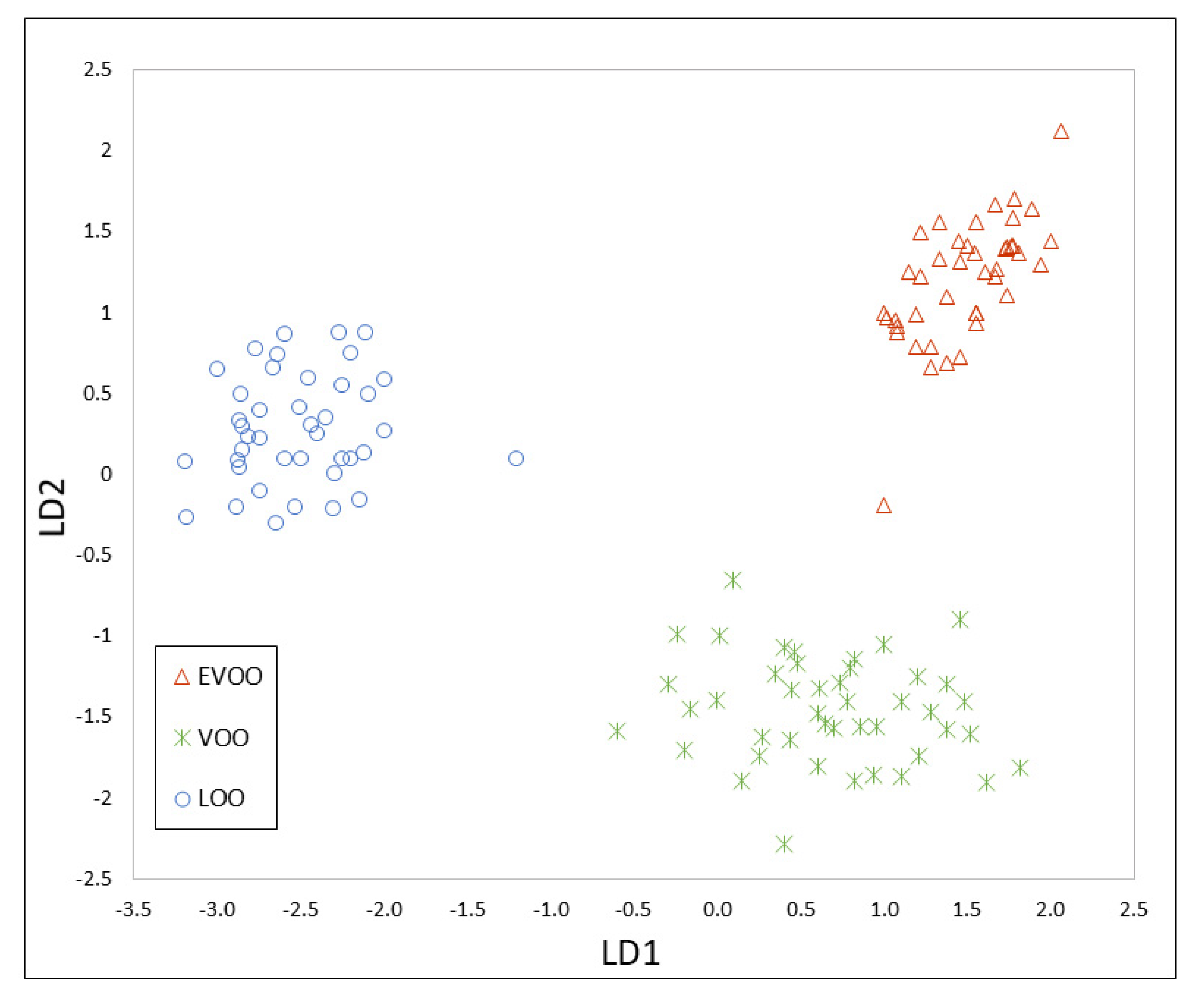
3.4. Data Fusion of MS and IMS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs. spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace—Gas chromatography–ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Nicolás, M.; Castell, A.; Campillo, N.; Viñas, P.; López-García, I.; Hernández-Córdoba, M. Untargeted headspace gas chromatography—Ion mobility spectrometry analysis for detection of adulterated honey. Talanta 2019, 205, 120123. [Google Scholar] [CrossRef]
- European Comission. Commission Regulation (EC) No 640/2008 of 4 July 2008 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 2008, 50, 11–16. [Google Scholar]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Romero, I.; García-González, D.L.; Aparicio-Ruiz, R.; Morales, M.T. Validation of SPME-GCMS method for the analysis of virgin olive oil volatiles responsible for sensory defects. Talanta 2015, 134, 394–401. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; García-González, D.L.; Morales, M.T.; Lobo-Prieto, A.; Romero, I. Comparison of two analytical methods validated for the determination of volatile compounds in virgin olive oil: GC-FID vs. GC-MS. Talanta 2018, 187, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Sales, C.; Cervera, M.I.; Gil, R.; Portolés, T.; Pitarch, E.; Beltran, J. Quality classification of Spanish olive oils by untargeted gas chromatography coupled to hybrid quadrupole-time of flight mass spectrometry with atmospheric pressure chemical ionization and metabolomics-based statistical approach. Food Chem. 2017, 216, 365–373. [Google Scholar] [CrossRef]
- Dierkes, G.; Bongartz, A.; Guth, H.; Hayen, H. Quality evaluation of olive oil by statistical analysis of multicomponent stable isotope dilution assay data of aroma active compounds. J. Agric. Food Chem. 2011, 60, 394–401 doi:101021/jf203406s. [Google Scholar] [CrossRef] [Green Version]
- Cecchi, L.; Migliorini, M.; Giambanelli, E.; Rossetti, A.; Cane, A.; Melani, F.; Mulinacci, N. Headspace solid-phase microextraction-gas chromatography-mass spectrometry quantification of the volatile profile of more than 1200 virgin olive oils for supporting the panel test in their classification: Comparison of different chemometric approaches. J. Agric. Food Chem. 2019, 67, 9112–9120. [Google Scholar] [CrossRef]
- Quintanilla-Casas, B.; Bustamante, J.; Guardiola, F.; García-González, D.L.; Barbieri, S.; Bendini, A.; Toschi, T.G.; Vichi, S.; Tres, A. Virgin olive oil volatile fingerprint and chemometrics: Towards an instrumental screening tool to grade the sensory quality. LWT 2020, 121, 108936. [Google Scholar] [CrossRef]
- Sales, C.; Portolés, T.; Johnsen, L.G.; Danielsen, M.; Beltran, J. Olive oil quality classification and measurement of its organoleptic attributes by untargeted GC–MS and multivariate statistical-based approach. Food Chem. 2019, 271, 488–496. [Google Scholar] [CrossRef]
- Berlioz, B.; Cordella, C.; Cavalli, J.F.; Lizzani-Cuvelier, L.; Loiseau, A.M.; Fernandez, X. Comparison of the amounts of volatile compounds in French protected designation of origin virgin olive oils. J. Agric. Food Chem. 2006, 54, 10092–10101. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.B.; Gargouri, B.; Flamini, G.; Bouaziz, M. Effect of agricultural sites on differentiation between Chemlali and Neb Jmel olive oils. J. Oleo Sci. 2015, 64, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vichi, S.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; López-Tamames, E. Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: Characterization of virgin olive oils from two distinct geographical areas of northern Italy. J. Agric. Food Chem. 2003, 51, 6572–6577. [Google Scholar] [CrossRef]
- Bajoub, A.; Sánchez-Ortiz, A.; Ajal, E.A.; Ouazzani, N.; Fernández-Gutiérrez, A.; Beltrán, G.; Carrasco-Pancorbo, A. First comprehensive characterization of volatile profile of north Moroccan olive oils: A geographic discriminant approach. Food Res. Int. 2015, 73, 410–417. [Google Scholar] [CrossRef]
- Purcaro, G.; Cordero, C.; Liberto, E.; Bicchi, C.; Conte, L.S. Toward a definition of blueprint of virgin olive oil by comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2014, 1334, 101–111. [Google Scholar] [CrossRef]
- Aparicio, R.; Rocha, S.M.; Delgadillo, I.; Morales, M.T. Detection of rancid defect in virgin olive oil by the electronic nose. J. Agric. Food Chem. 2000, 48, 853–860. [Google Scholar] [CrossRef]
- García-González, D.L.; Aparicio, R. Virgin olive oil quality classification combining neural network and MOS sensors. J. Agric. Food Chem. 2003, 51, 3515–3519. [Google Scholar] [CrossRef]
- Escuderos, M.E.; García, M.; Jiménez, A.; Horrillo, M.C. Edible and non-edible olive oils discrimination by the application of a sensory olfactory system based on tin dioxide sensors. Food Chem. 2013, 136, 1154–1159. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Dobao-Prieto, M.D.M.; Arce, L.; Valcárcel, M. Determination of volatile compounds by GC-IMS to assign the quality of virgin olive oil. Food Chem. 2015, 187, 572–579. [Google Scholar] [CrossRef]
- Gerhardt, N.; Birkenmeier, M.; Sanders, D.; Rohn, S.; Weller, P. Resolution-optimized headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) for non-targeted olive oil profiling. Anal. Bioanal. Chem. 2017, 409, 3933–3942. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Contreras, M.; Jurado-Campos, N.; Arce, L.; Arroyo-Manzanares, N. A robustness study of calibration models for olive oil classification: Targeted and non-targeted fingerprint approaches based on GC-IMS. Food Chem. 2019, 288, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Contreras, M.; Arroyo-Manzanares, N.; Arce, C.; Arce, L. HS-GC-IMS and chemometric data treatment for food authenticity assessment: Olive oil mapping and classification through two different devices as an example. Food Control 2019, 98, 82–93. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Arce, L.; Valcárcel, M. Multi-capillary column-ion mobility spectrometry: A potential screening system to differentiate virgin olive oils. Anal. Bioanal. Chem. 2012, 402, 489–498. [Google Scholar] [CrossRef]
- Valli, E.; Panni, F.; Casadei, E.; Barbieri, S.; Cevoli, C.; Bendini, A.; García-González, D.L.; Gallina Toschi, T. An HS-GC-IMS method for the quality classification of virgin olive oils as screening support for the panel test. Food 2020, 9, 657. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Mercader-Trejo, F.; Arce, L.; Valcárcel, M. Enhancing sensitivity and selectivity in the determination of aldehydes in olive oil by use of a Tenax TA trap coupled to a UV-ion mobility spectrometer. J. Chromatogr. A 2011, 1218, 7543–7549. [Google Scholar] [CrossRef]
- Gerhardt, N.; Schwolow, S.; Rohn, S.; Pérez-Cacho, P.R.; Galán-Soldevilla, H.; Arce, L.; Weller, P. Quality assessment of olive oils based on temperature-ramped HS-GC-IMS and sensory evaluation: Comparison of different processing approaches by LDA, kNN, and SVM. Food Chem. 2019, 278, 720–728. [Google Scholar] [CrossRef]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; García-González, D.L. Towards new analyses of aroma and volatiles to understand sensory perception of olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1114–1125. [Google Scholar] [CrossRef]
- Kiritsakis, A.K. Flavor components of olive oil—A review. J. Am. Oil Chem. Soc. 1998, 75, 673–681. [Google Scholar] [CrossRef]
- Magagna, F.; Valverde-Som, L.; Ruíz-Samblás, C.; Cuadros-Rodríguez, L.; Reichenbach, S.E.; Bicchi, C.; Cordero, C. Combined untargeted and targeted fingerprinting with comprehensive two-dimensional chromatography for volatiles and ripening indicators in olive oil. Anal. Chim. Acta 2016, 936, 245–258. [Google Scholar] [CrossRef]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 varietal virgin olive oils by their volatile compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Genovese, A.; Yang, N.; Linforth, R.; Sacchi, R.; Fisk, I. The role of phenolic compounds on olive oil aroma release. Food Res. Int. 2018, 112, 319–327. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Villani, V.; Paduano, A.; Sacchi, R. Olive oil phenolic compounds affect the release of aroma compounds. Food Chem. 2015, 181, 284–294. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Dobao-Prieto, M.M.; Arce, L.; Aguilar, J.; Cumplido, J.L.; Valcárcel, M. Ion mobility spectrometry versus classical physico-chemical analysis for assessing the shelf life of extra virgin olive oil according to container type and storage conditions. J. Agric. Food Chem. 2015, 63, 2179–2188. [Google Scholar] [CrossRef]
- Rácz, A.; Gere, A.; Bajusz, D.; Héberger, K. Is soft independent modeling of class analogies a reasonable choice for supervised pattern recognition? RSC Adv. 2018, 8, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Nikita, E.; Nikitas, P. Sex estimation: A comparison of techniques based on binary logistic, probit and cumulative probit regression, linear and quadratic discriminant analysis, neural networks, and naïve Bayes classification using ordinal variables. Int. J. Legal Med. 2020, 134, 1213–1225. [Google Scholar] [CrossRef]
| HS-GC-MS | HS-GC-IMS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | RT (min) | Linear Dynamic Range (µg g−1) | R2 | LOQ | RT (s) | Drift Time Monomer (ms) | Drift Time Dimer (ms) | Logarithmic Dynamic Range (µg g−1) | R2 | LOQ |
| Ethyl acetate | - | - | - | - | 276.2 | 9.0 | 11.0 | 0.08–20 | 0.985 | 0.08 |
| 1-penten-3-one | - | - | - | - | 328.7 | 8.8 | 10.7 | 0.08–20 | 0.989 | 0.08 |
| 2-pentanone | - | - | - | - | 334.6 | 9.2 | 11.3 | 0.15–50 | 0.993 | 0.15 |
| 4-methyl-pentan-2-one | - | - | - | - | 396.9 | 9.6 | 12.2 | 0.10–50 | 0.990 | 0.10 |
| Hexanal | 5.2 | 0.90–50 | 0.987 | 0.90 | 462.3 | 10.3 | 12.8 | 0.75–50 | 0.999 | 0.75 |
| Trans-2-pentenal | - | - | - | - | 411.8 | 9.1 | 11.2 | 0.82–50 | 0.993 | 0.82 |
| Trans-2-hexen-1-al | 6.3 | 0.38–50 | 0.995 | 0.38 | 554.4 | 9.6 | 12.4 | 0.15–50 | 0.984 | 0.15 |
| Heptanal | - | - | - | - | 631.6 | 10.8 | 13.8 | 0.42–50 | 0.976 | 0.42 |
| 6-methyl-5-hepten-2-one | 8.9 | 0.44–50 | 0.993 | 0.44 | - | - | - | - | - | - |
| 3-hexenyl acetate | 9.2 | 0.48–50 | 0.995 | 0.48 | - | - | - | - | - | - |
| Nonanal | 10.8 | 0.20–50 | 0.996 | 0.20 | 989.0 | 12.0 | ND | 0.10–50 | 0.920 | 0.10 |
| Decanal | 12.4 | 1.55–50 | 0.996 | 1.55 | - | - | - | - | - | - |
| Trans-2-decenal | 14.4 | 2.10–50 | 0.985 | 2.10 | - | - | - | - | - | - |
| Hexyl acetate | ND | ND | ND | ND | 830.6 | 11.3 | 15.5 | 0.21–20 | 0.993 | 0.21 |
| HS-GC-MS | HS-GC-IMS | Sensory Properties [20,21,22,23,24,32,33,34,35] | |||||
|---|---|---|---|---|---|---|---|
| Analyte | EVOO | VOO | LOO | EVOO | VOO | LOO | |
| Ethyl acetate | - | - | - | 0.17 ± 0.16 a | 0.46 ± 0.56 b | 0.99 ± 1.47 b | Fusty, winey-vinegary, fruity, aromatic, ethereal, sweet |
| 1-penten-3-one | - | - | - | 0.66 ± 0.09 a | 0.31 ± 0.01 b | 0.14 ± 0.01 c | Green, pungent, sweet |
| 2-pentanone | - | - | - | 0.19 ± 0.14 a | 0.17 ± 0.08 a | 0.15 ± 0.09 a | Sweet |
| 4-methyl-pentan-2-one | - | - | - | 0.70 ± 0.71 a | 0.62 ± 0.49 a | 1.52 ± 0.77 a | Fruity, sweet, ethereal |
| Hexanal | 0.70 ± 0.33 a | 0.91 ± 0.22 a | 1.10 ± 0.4 b | 0.86 ± 0.01 a | 0.97 ± 0.60 b | 0.99 ± 0.20 b | Mustiness-humidity, fusty, winey-vinegary, rancid, green-sweet, green apple, grass |
| Trans-2-pentenal | - | - | - | 0.83 ± 0.12 a | 1.06 ± 0.12 a,b | 1.30 ± 0.13 b | Winey-vinegary, pungent, green |
| Trans-2-hexen-1-al | 0.70 ± 0.28 a | 0.45 ± 0.17 b | 0.40 ± 0.19 b | 0.74 ± 0.15 a | 0.48 ± 0.19 b | 0.39 ± 0.21 b | Mustiness-humidity, fusty, winey-vinegary, rancid, bitter almond, green |
| Heptanal | - | - | - | 0.49 ± 0.10 a | 0.50 ± 0.08 a | 0.94 ± 0.12 b | Rancid, fatty, woody |
| 6-methyl-5-hepten-2-one | 0.16 ± 0.15 a | 0.18 ± 0.29 a | 0.67 ± 0.93 b | - | - | - | Mustiness-humidity, fusty, rancid, pungent, green |
| 3-hexenyl acetate | 1.01 ± 0.17 a | 0.50 ± 0.23 b | 0.47 ± 0.3 b | - | - | - | Green banana, green leaves, fruity |
| Nonanal | 0.28 ± 0.12 a | 0.31 ± 0.18 a | 0.63 ± 0.36 b | 0.35 ± 0.15 a | 0.37 ± 0.19 a | 0.76 ± 0.42 b | Rancid, fatty, waxy, pungent |
| Decanal | 1.56 ± 0.20 a | 1.63 ± 0.75 a | 1.63 ± 0.53 a | - | - | - | Rancid |
| Trans-2-decenal | 2.17 ± 1.97 a | 2.32 ± 1.39 a | 2.41 ± 1.60 b | - | - | - | Rancid |
| Hexyl acetate | - | - | - | 0.52 ± 0.01a | 0.47 ± 0.01 a | 0.22 ± 0.09 b | Fruity, green, sweet |
| Actual Classes | |||||
|---|---|---|---|---|---|
| EVOO | VOO | LOO | |||
| Predicted classes | EVOO | 6 | 1 | 0 | Success 85.71% |
| VOO | 1 | 5 | 0 | ||
| LOO | 0 | 1 | 7 | ||
| Actual Classes | |||||
|---|---|---|---|---|---|
| EVOO | VOO | LOO | |||
| Predicted classes | EVOO | 5 | 1 | 0 | Success 76.19% |
| VOO | 2 | 5 | 1 | ||
| LOO | 0 | 1 | 6 | ||
| Actual Classes | |||||
|---|---|---|---|---|---|
| EVOO | VOO | LOO | |||
| Predicted classes | EVOO | 6 | 1 | 0 | Success 85.71% |
| VOO | 1 | 5 | 0 | ||
| LOO | 0 | 1 | 7 | ||
| Actual Classes | |||||
|---|---|---|---|---|---|
| EVOO | VOO | LOO | |||
| Predicted classes | EVOO | 6 | 1 | 1 | Success 80.95% |
| VOO | 1 | 6 | 1 | ||
| LOO | 0 | 0 | 5 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Nicolás, M.; Arroyo-Manzanares, N.; Arce, L.; Hernández-Córdoba, M.; Viñas, P. Headspace Gas Chromatography Coupled to Mass Spectrometry and Ion Mobility Spectrometry: Classification of Virgin Olive Oils as a Study Case. Foods 2020, 9, 1288. https://doi.org/10.3390/foods9091288
García-Nicolás M, Arroyo-Manzanares N, Arce L, Hernández-Córdoba M, Viñas P. Headspace Gas Chromatography Coupled to Mass Spectrometry and Ion Mobility Spectrometry: Classification of Virgin Olive Oils as a Study Case. Foods. 2020; 9(9):1288. https://doi.org/10.3390/foods9091288
Chicago/Turabian StyleGarcía-Nicolás, María, Natalia Arroyo-Manzanares, Lourdes Arce, Manuel Hernández-Córdoba, and Pilar Viñas. 2020. "Headspace Gas Chromatography Coupled to Mass Spectrometry and Ion Mobility Spectrometry: Classification of Virgin Olive Oils as a Study Case" Foods 9, no. 9: 1288. https://doi.org/10.3390/foods9091288
APA StyleGarcía-Nicolás, M., Arroyo-Manzanares, N., Arce, L., Hernández-Córdoba, M., & Viñas, P. (2020). Headspace Gas Chromatography Coupled to Mass Spectrometry and Ion Mobility Spectrometry: Classification of Virgin Olive Oils as a Study Case. Foods, 9(9), 1288. https://doi.org/10.3390/foods9091288







