The Relationship between In Vitro and In Vivo Starch Digestion Kinetics of Breads Varying in Dietary Fibre
Abstract
:1. Introduction
2. Materials and Methods
2.1. Breads
2.2. Chemical Analysis
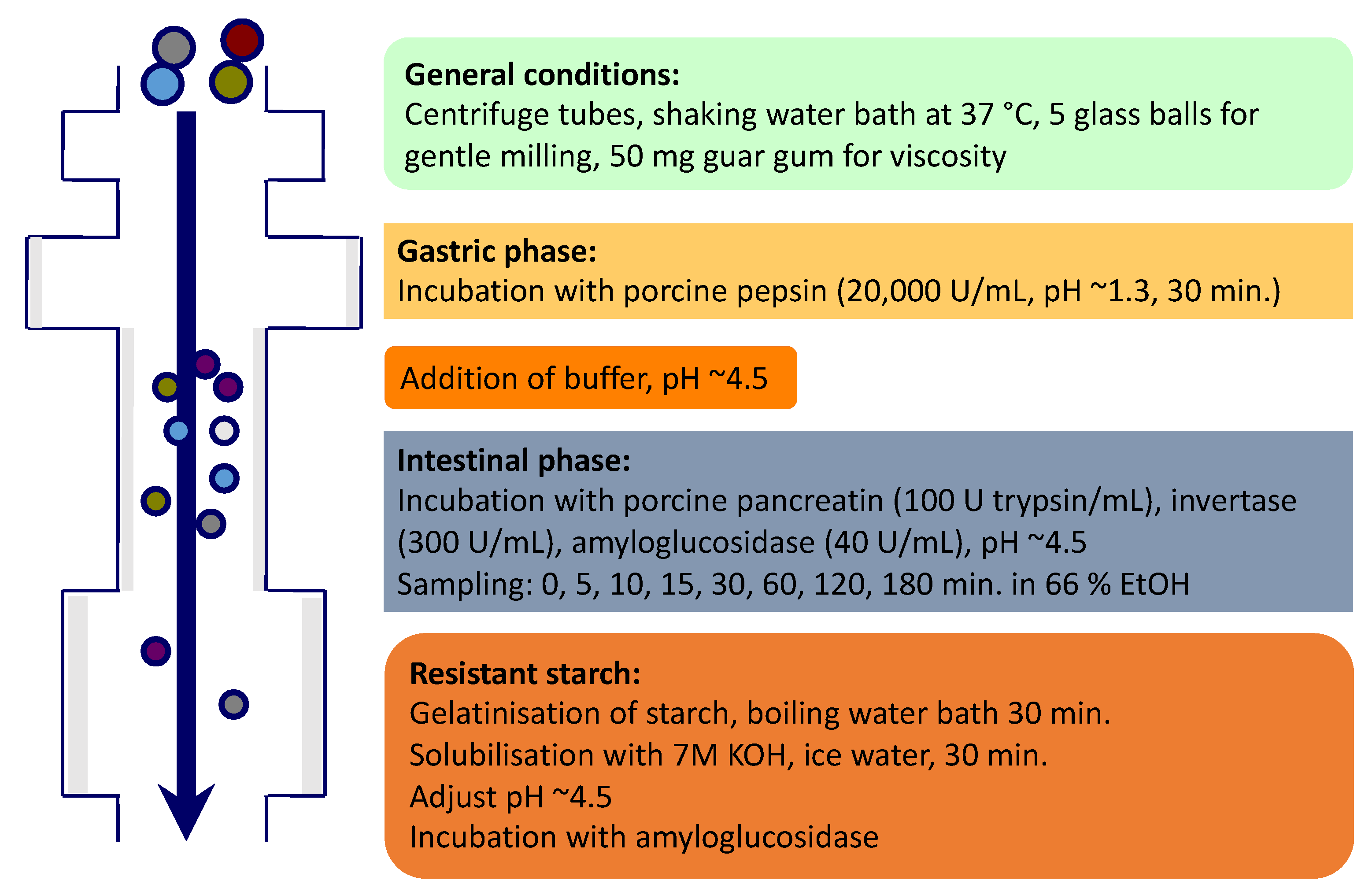
2.3. In Vitro Starch Digestion
2.4. Reference In Vivo Data
2.5. Calculations and Statistical Analysis
3. Results
3.1. Dietary Composition
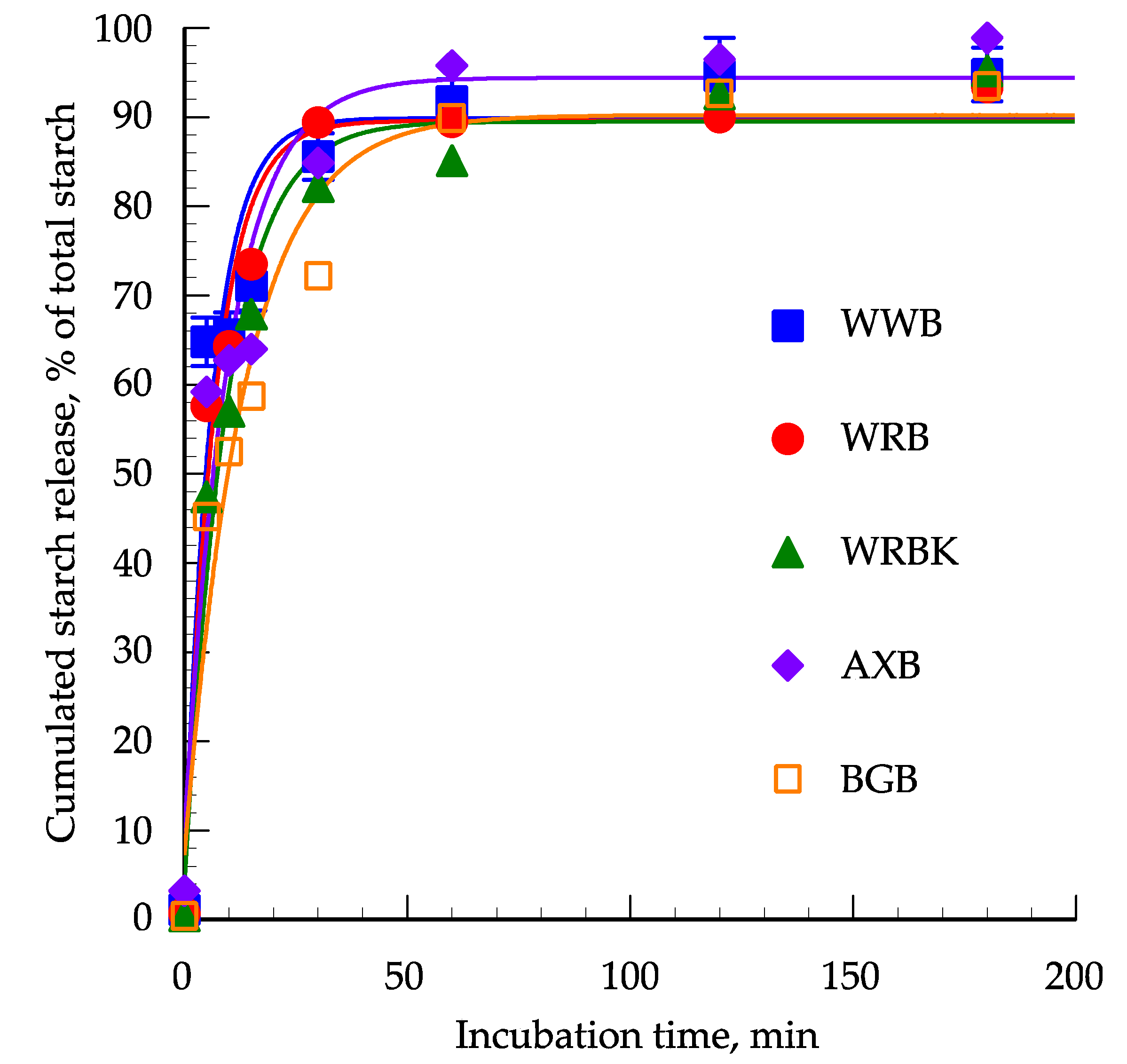
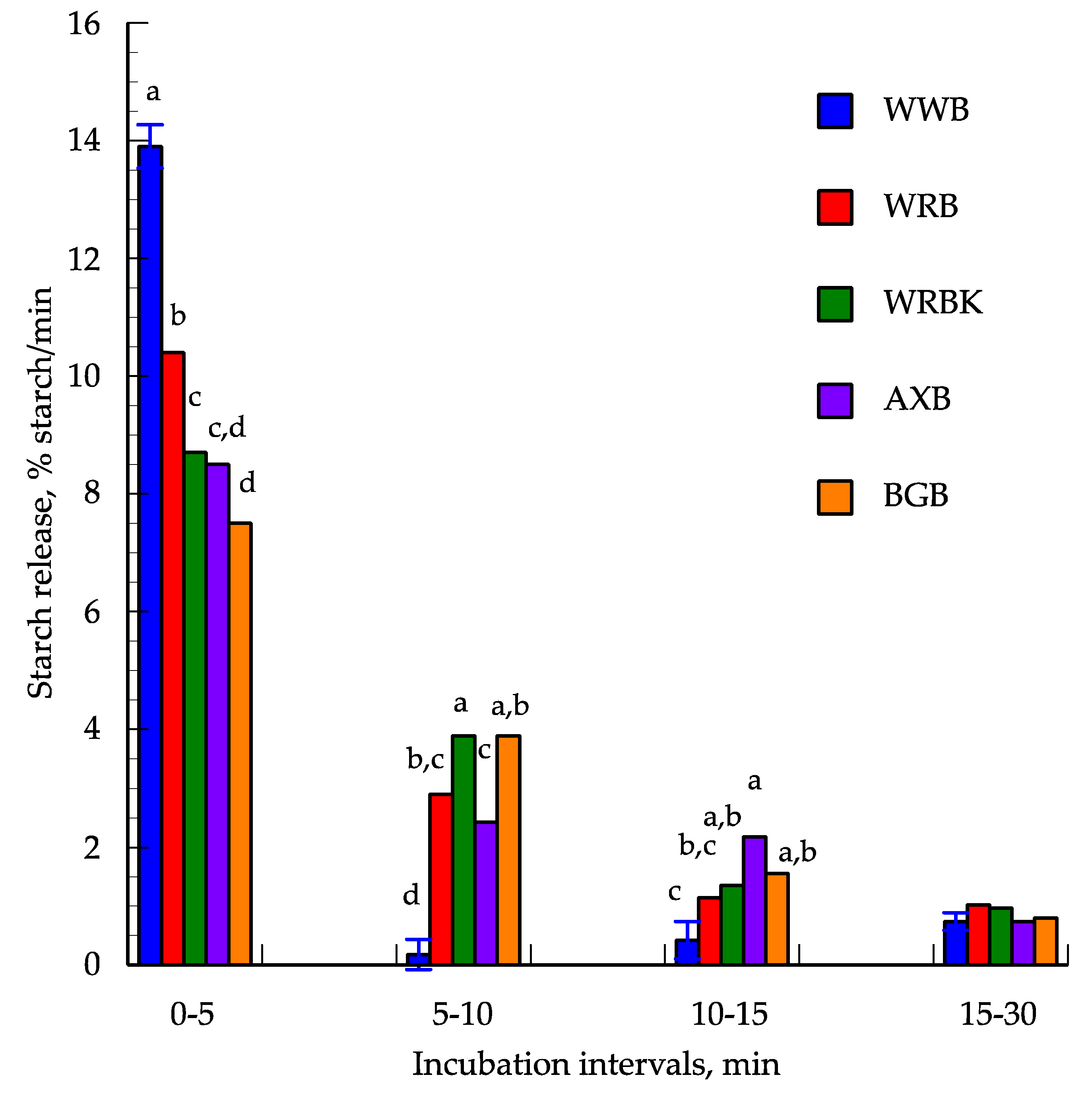
3.2. In Vitro Starch Digestion
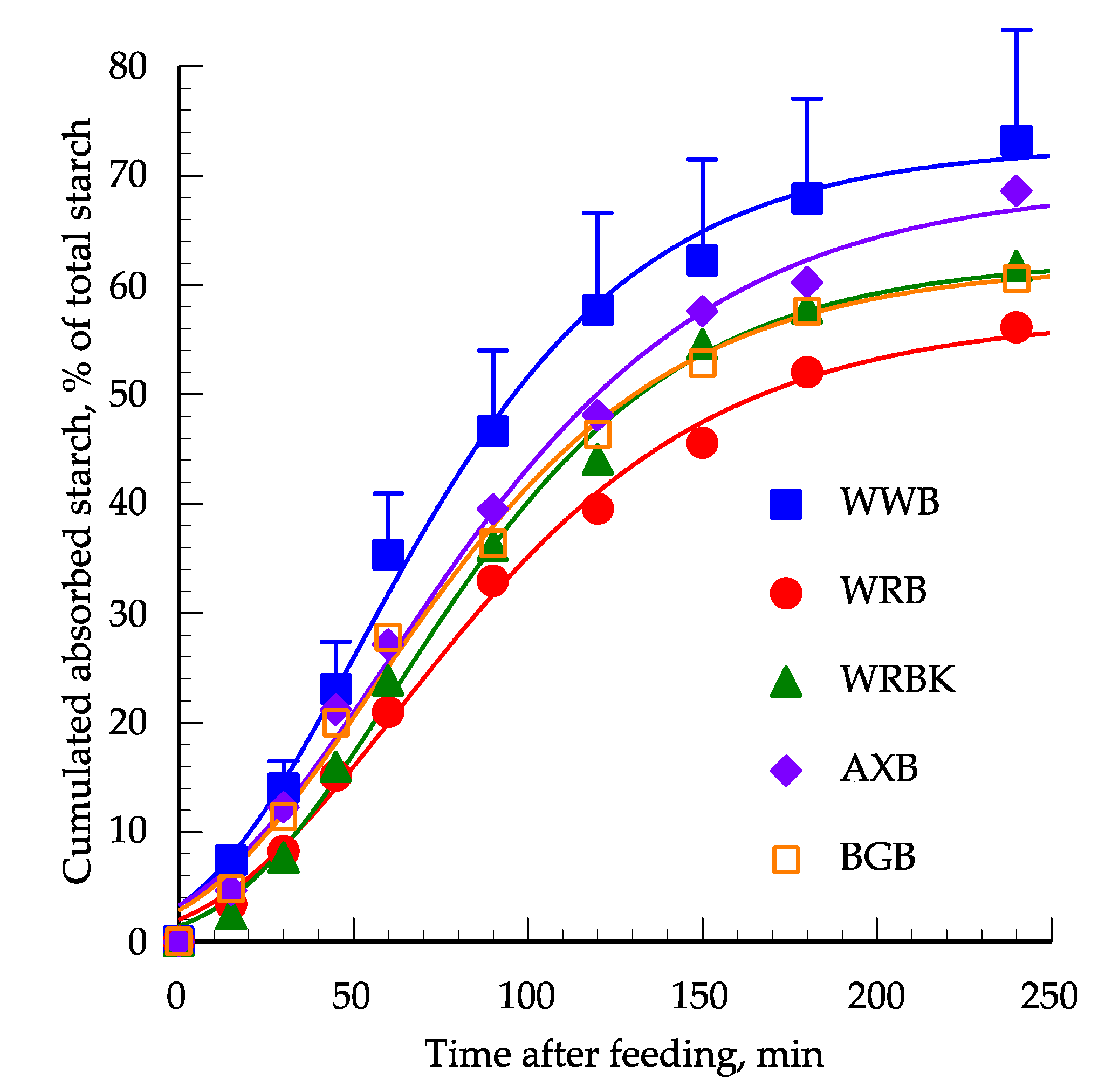
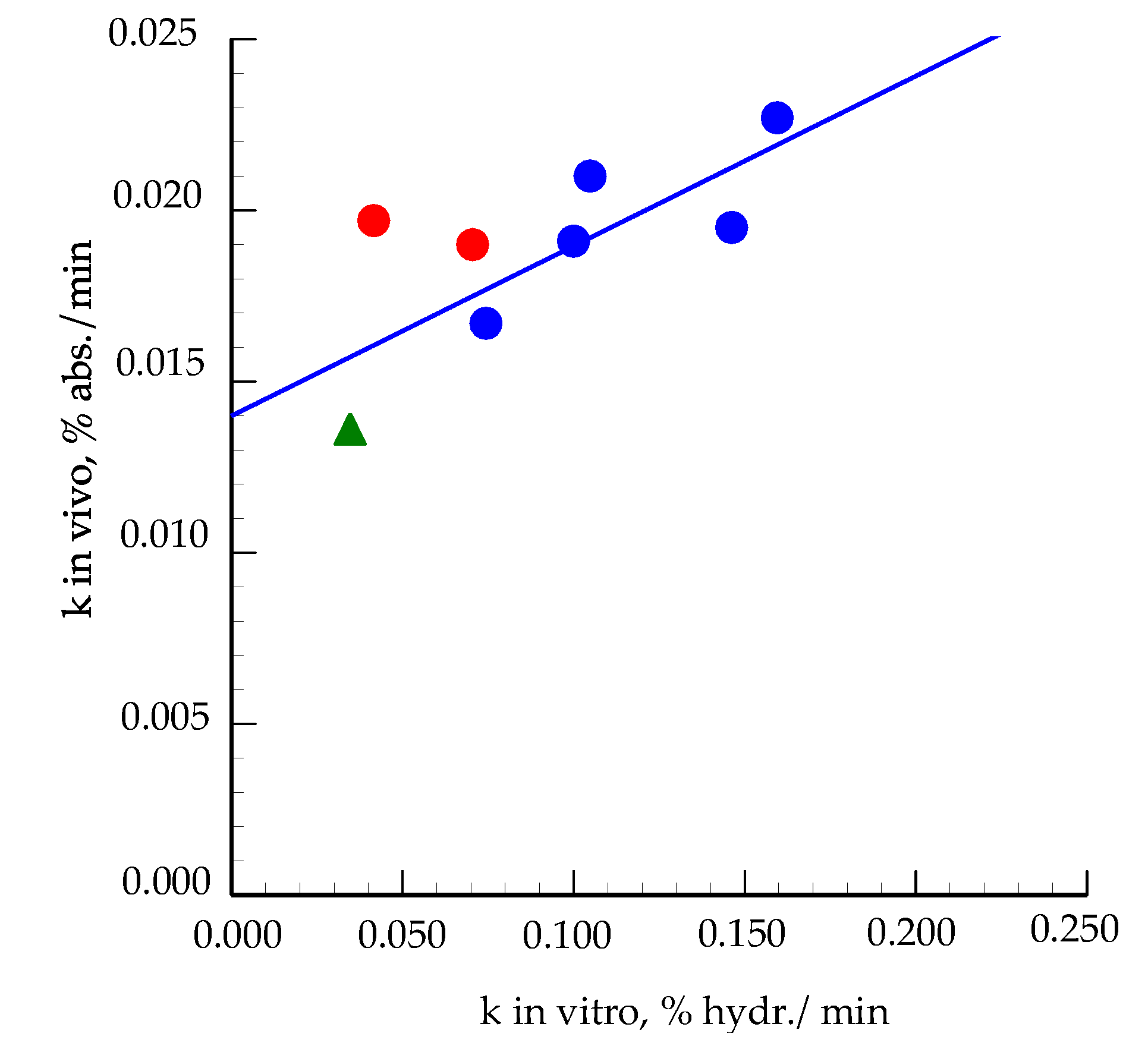
3.3. Comparison to In Vivo Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fagt, S.; Matthiessen, J.; Biltoft-Jensen, A.; Groth, M.V.; Christensen, T.; Hinsch, H.-J.; Hartkopp, H.; Trolle, E.; Lyhne, N.; Møller, A. Udviklingen i Danskernes Kost 1985-2001 Med Fokus på Sukker Alkohol Samt Motivation Og Barrierer for Sund Livsstil; Danish Institute for Food and Veterinary Research: Copenhagen, Denmark, 2004; p. 127. [Google Scholar]
- Sørensen, A.-M.; Fagt, S.; Møller, A. Dietary fibre intake in Denmark. In Dietary Fibre Intakes in Europe; Cummings, J.H., Frølich, W., Eds.; Directorate-General Science, Research and Development: Brussel, Belgium, 1993; pp. 37–39. [Google Scholar]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [Green Version]
- Fardet, A.; Leenhardt, F.; Lioger, D.; Scalbert, A.; Rémésy, C. Parameters controlling the glycaemic response to breads. Nutr. Res. Rev. 2006, 19, 18–25. [Google Scholar] [CrossRef]
- Liljeberg, H.; Granfeldt, Y.; Björck, I. Metabolic responses to starch in bread containing intact kernels versus milled flour. Eur. J. Clin. Nutr. 1992, 46, 561–575. [Google Scholar]
- Gemen, R.; De Vries, J.F.; Slavin, J.L. Relationship between molecular structure of cereal dietary fiber and health effects: Focus on glucose/insulin response and gut health. Nutr. Rev. 2011, 69, 22–33. [Google Scholar] [CrossRef]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; Mascara, T.; O’Dea, K. Arabinoxylan fiber, a byproduct of wheat flour processing, reduces the postprandial glucose response in normoglycemic subjects. Am. J. Clin. Nutr. 2000, 71, 1123–1128. [Google Scholar] [CrossRef]
- Tosh, S.M. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur. J. Clin. Nutr. 2013, 67, 310–317. [Google Scholar] [CrossRef] [Green Version]
- FDA. Guidiance for Industry: A food Labelling Guide, aa. Appendix C: Health Claims, Requirements for Health Claims Made in Labelling; U.S. Food and Drug Administration, Office of Nutritional Products, Labelling and Dietary Supplements: Washington, DC, USA, 2009.
- EFSA. Panel on Dietic Products—Nutrition and Allergies. Scientific Opinion: β-glucans from oats and barley related health claims. Appendix C. EFSA J. 2011, 9. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; O’Dea, K. Arabinoxylan fibre improves metabolic control in people with Type II diabetes. Eur. J. Clin. Nutr. 2004, 58, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.J.; Beer, M.U.; Butler, G. Evaluation of role of concentration and molecular weight of oat beta-glucan in determining effect of viscosity on plasma glucose and insulin following an oral glucose load. Br. J. Nutr. 2000, 84, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Englyst, K.; Vinoy, S.; Englyst, H.N.; Lang, V. Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br. J. Nutr. 2003, 89, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Péronnet, F.; Meynier, A.; Sauvinet, V.; Normand, S.; Bourdon, E.; Mignault, D.; St-Pierre, D.H.; Laville, M.; Rabasa-Lhoret, R.; Vinoy, S. Plasma glucose kinetics and response of insulin and GIP following a cereal breakfast in female subjects: Effect of starch digestibility. Eur. J. Clin. Nutr. 2015, 69, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Vangsøe, C.T.; Ingerslev, A.K.; Theil, P.K.; Hedemann, M.S.; Lærke, H.N.; Knudsen, K.E.B. In vitro starch digestion kinetics of diets varying in resistant starch and arabinoxylan compared with in vivo portal appearance of glucose in pigs. Food Res. Int. 2016, 88, 199–206. [Google Scholar] [CrossRef]
- Christensen, K.L.; Hedemann, M.S.; Lærke, H.N.; Jørgensen, H.; Mutt, S.J.; Herzig, K.-H.; Knudsen, K.E.B. Concentrated Arabinoxylan but Not Concentrated β-Glucan in Wheat Bread Has Similar Effects on Postprandial Insulin as Whole-Grain Rye in Porto-arterial Catheterized Pigs. J. Agric. Food Chem. 2013, 61, 7760–7768. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1990; p. 1298. [Google Scholar]
- Hansen, B. Determination of Nitrogen as Elementary N, an Alternative to Kjeldahl. Acta Agric. Scand. 1989, 39, 113–118. [Google Scholar] [CrossRef]
- Stoldt, W. Vorschlag zur Vereinheitlichung der Fettbestimmung in Lebensmitteln (Suggestion to standardise the determination of fat in foodstuffs). Fette Seifen Anstrichm. 1952, 54, 206–207. [Google Scholar] [CrossRef]
- McCleary, B.V.; Glennie-Holmes, M. Enzymic quantification of (1-3), (1-4)-ß-D-glucan in barley and malt. J. Inst. Brew. 1985, 91, 285–295. [Google Scholar] [CrossRef]
- Theander, O.; Åman, P. Studies on dietary fibre. 1. Analysis and chemical characterization of water-soluble and water-insoluble dietary fibres. Swed. J. Agric. Res. 1979, 9, 97–106. [Google Scholar]
- Kasprzak, M.M.; Lærke, H.N.; Knudsen, K.E.B. Effects of Isolated and Complex Dietary Fiber Matrices in Breads on Carbohydrate Digestibility and Physicochemical Properties of Ileal Effluent from Pigs. J. Agric. Food Chem. 2012, 60, 12469–12476. [Google Scholar] [CrossRef]
- Knudsen, K.E.B. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed. Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Lærke, H.N.; Larsen, F.H.; Knudsen, K.E.B.; Pedersen, S.; Jørgensen, A.S. Effect of Enzymatic Treatment of Different Starch Sources on the in Vitro Rate and Extent of Starch Digestion. Int. J. Mol. Sci. 2012, 13, 929–942. [Google Scholar] [CrossRef]
- Larsson, K.; Bengtsson, S. Bestämning av Lättilgängeliga Kolhydrater i Växtmaterial (Determination of Readily Available Carbohydrates in Plant Material); Methods Report no. 22; National Laboratory of Agricultural Chemistry: Uppsala, Sweden, 1983. [Google Scholar]
- Kasprzak, M.M.; Lærke, H.N.; Knudsen, K.E.B. Changes in Molecular Characteristics of Cereal Carbohydrates after Processing and Digestion. Int. J. Mol. Sci. 2012, 13, 16833–16852. [Google Scholar] [CrossRef] [Green Version]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Wood, P.J. REVIEW: Oat and Rye β-Glucan: Properties and Function. Cereal Chem. J. 2010, 87, 315–330. [Google Scholar] [CrossRef]
- Hartvigsen, M.L.; Jeppesen, P.B.; Lærke, H.N.; Njabe, E.N.; Knudsen, K.E.B.; Hermansen, K. Concentrated Arabinoxylan in Wheat Bread Has Beneficial Effects as Rye Breads on Glucose and Changes in Gene Expressions in Insulin-Sensitive Tissues of Zucker Diabetic Fatty (ZDF) Rats. J. Agric. Food Chem. 2013, 61, 5054–5063. [Google Scholar] [CrossRef]
- Hartvigsen, M.L.; Gregersen, S.; Lærke, H.N.; Holst, J.J.; Knudsen, K.E.B.; Hermansen, K. Effects of concentrated arabinoxylan and β-glucan compared with refined wheat and whole grain rye on glucose and appetite in subjects with the metabolic syndrome: A randomized study. Eur. J. Clin. Nutr. 2013, 68, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Juntunen, K.S.; E Laaksonen, D.; Autio, K.; Niskanen, L.K.; Holst, J.J.; E Savolainen, K.; Liukkonen, K.-H.; Poutanen, K.S.; Mykkänen, H.M. Structural differences between rye and wheat breads but not total fiber content may explain the lower postprandial insulin response to rye bread. Am. J. Clin. Nutr. 2003, 78, 957–964. [Google Scholar] [CrossRef]
- Liljeberg, H.; Björck, I. Bioavailability of starch in bread products. Postprandial glucose and insulin responses in healthy subjects and in vitro resistant starch content. Eur. J. Clin. Nutr. 1994, 48, 151–163. [Google Scholar] [PubMed]
- Ingerslev, A.K.; Theil, P.K.; Hedemann, M.S.; Lærke, H.N.; Knudsen, K.E.B. Resistant starch and arabinoxylan augment SCFA absorption, but affect postprandial glucose and insulin responses differently. Br. J. Nutr. 2014, 111, 1564–1576. [Google Scholar] [CrossRef] [Green Version]
- Van Kempen, T.A.T.G.; Regmi, P.R.; Matte, J.J.; Zijlstra, R.T. In Vitro Starch Digestion Kinetics, Corrected for Estimated Gastric Emptying, Predict Portal Glucose Appearance in Pigs. J. Nutr. 2010, 140, 1227–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Malagelada, J.R.; Brown, M.L.; Becker, G.; Zinsmeister, A.R. Relation between antral motility and gastric emptying of solids and liquids in humans. Am. J. Physiol. Liver Physiol. 1985, 249, G580–G585. [Google Scholar] [CrossRef] [PubMed]
- A Siegel, J.; Urbain, J.L.; Adler, L.P.; Charkes, N.D.; Maurer, A.H.; Krevsky, B.; Knight, L.C.; Fisher, R.S.; Malmud, L.S. Biphasic nature of gastric emptying. Gut 1988, 29, 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furness, J.; Rivera, L.R.; Cho, H.J.; Bravo, D.M.; Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740. [Google Scholar] [CrossRef]
- Low, A.G.; Zebrowska, T.; Heppell, L.M.J.; Smith, H.A. Influence of wheat bran, cellulose, pectin and low or high viscosity guar gum on glucose and water absorption from pig jejunum. Proc. Nutr. Soc. 1986, 45, A55. [Google Scholar]
- Lund, E.K.; Gee, J.M.; Brown, J.C.; Wood, P.J.; Johnson, I.T. Effect of oat gum on the physical properties of the gastrointestinal contents and on the uptake of D-galactose and cholesterol by rat small intestine in vitro. Br. J. Nutr. 1989, 62, 91–101. [Google Scholar] [CrossRef]
- Rérat, A.A.; Vaissade, P.; Vaugelade, P. Absorption kinetics of some carbohydrates in conscious pigs. 1. Qualitative aspects. Br. J. Nutr. 1984, 51, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Rérat, A.A.; Vaissade, P.; Vaugelade, P. Absorption kinetics of some carbohydrates in conscious pigs. 2. Quantitative aspects. Br. J. Nutr. 1984, 51, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Noah, L.; Krempf, M.; Lecannu, G.; Maugère, P.; Champ, M.M.-J. Bioavailability of starch and postprandial changes in splanchnic glucose metabolism in pigs. Am. J. Physiol. Metab. 2000, 278, E181–E188. [Google Scholar] [CrossRef] [PubMed]
| Chemical Composition | WWB | WRB | WRBK | AXB | BGB |
|---|---|---|---|---|---|
| Dry matter (g/kg as is) | 634 | 520 | 543 | 708 | 615 |
| Total starch | 711 | 588 | 608 | 514 | 612 |
| Total sugars | 47 | 32 | 23 | 39 | 58 |
| Dietary fibre | |||||
| LMW non-digestible carbohydrates | 30 | 52 | 53 | 81 | 10 |
| Resistant starch | 4 | 9 | 14 | 7 | 18 |
| Total NSP (soluble NSP) | 35 (17) | 134 (53) | 139 (50) | 116 (86) | 163 (54) |
| Cellulose | 6 | 19 | 18 | 6 | 53 |
| β-Glucan (soluble β-glucan) | 3 (2) | 21 (7) | 19 (4) | 3 (2) | 52 (40) |
| AX (soluble AX) | 17 (13) | 76 (36) | 77 (37) | 78 (66) | 32 (9) |
| Total non-digestible carbohydrates a | 69 | 195 | 206 | 204 | 190 |
| Klason lignin | 8 | 14 | 14 | 8 | 9 |
| Total dietary fibre b | 77 | 209 | 220 | 212 | 199 |
| Breads | |||||||
|---|---|---|---|---|---|---|---|
| WWB | WRB | WRBK | AXB | BGB | SEM | p-Value | |
| In vitro | |||||||
| k, % hydr./min | 0.1595 a | 0.1462 a, b | 0.1048 a, b | 0.1000 a, b | 0.0744 b | 0.02 | 0.06 |
| Asymptote,% | 89.8 | 89.6 | 89.5 | 94.4 | 90.2 | 3.1 | 0.75 |
| In vivo | |||||||
| k, % absorption/min | 0.0227 a | 0.0195 a | 0.021 | 0.0191a | 0.0167 a | 0.023 | 0.085 |
| Inflection point | 55.4 | 66.0 | 66.0 | 60.6 | 59.8 | 7.3 | 0.82 |
| Asymptote,% | 71.8 | 56.8 | 62.6 | 68.5 | 72.2 | 10.4 | 0.85 |
| kin vitro/kin vivo | 7.02 | 7.50 | 4.99 | 5.23 | 4.45 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Bonzi, P.; Vangsøe, C.T.; Nielsen, K.L.; Lærke, H.N.; Hedemann, M.S.; Knudsen, K.E.B. The Relationship between In Vitro and In Vivo Starch Digestion Kinetics of Breads Varying in Dietary Fibre. Foods 2020, 9, 1337. https://doi.org/10.3390/foods9091337
Rojas-Bonzi P, Vangsøe CT, Nielsen KL, Lærke HN, Hedemann MS, Knudsen KEB. The Relationship between In Vitro and In Vivo Starch Digestion Kinetics of Breads Varying in Dietary Fibre. Foods. 2020; 9(9):1337. https://doi.org/10.3390/foods9091337
Chicago/Turabian StyleRojas-Bonzi, Patricia, Cecilie Toft Vangsøe, Kirstine Lykke Nielsen, Helle Nygaard Lærke, Mette Skou Hedemann, and Knud Erik Bach Knudsen. 2020. "The Relationship between In Vitro and In Vivo Starch Digestion Kinetics of Breads Varying in Dietary Fibre" Foods 9, no. 9: 1337. https://doi.org/10.3390/foods9091337