Potential Use of Silica Nanoparticles for the Microbial Stabilisation of Wine: An In Vitro Study Using Oenococcus oeni as a Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oenococcus oeni Culture
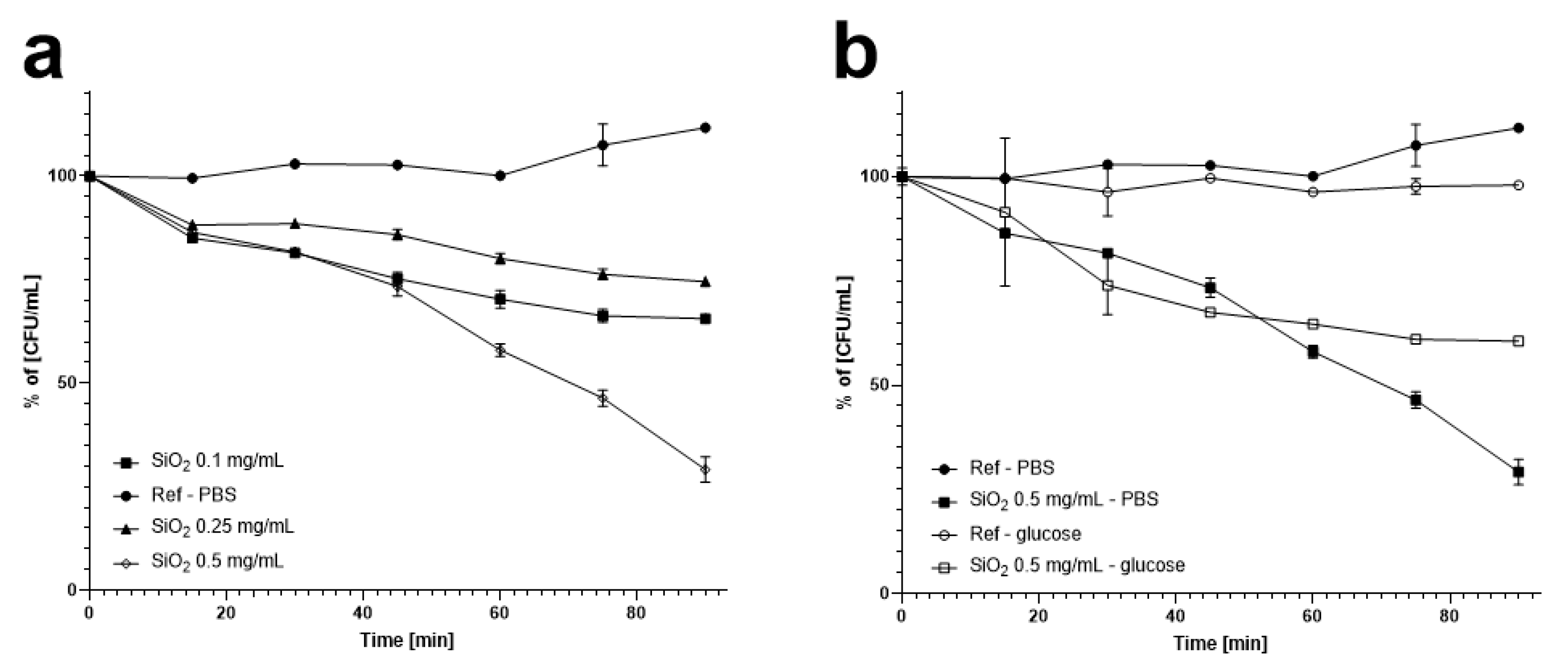
2.3. Reduction of Oenococcus oeni Numbers in PBS and Glucose by Silica Nanospheres with Stirring
2.4. Reduction of O. oeni Counts by Silica Nanospheres without Stirring
2.5. Reduction of Oenococcus oeni Numbers in Wine by Silica Nanospheres with Stirring
2.6. Silica Nanospheres Biodegradation Studies
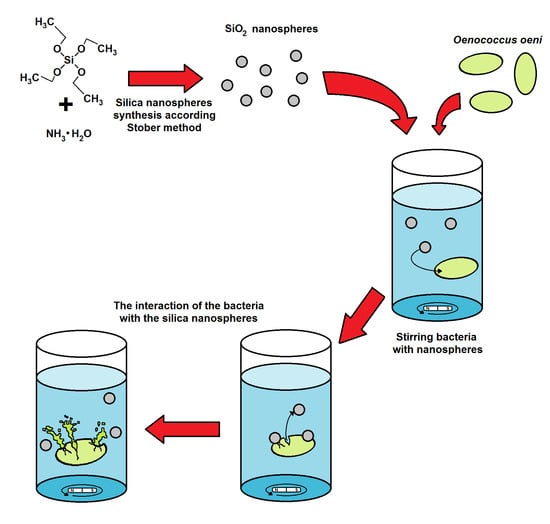
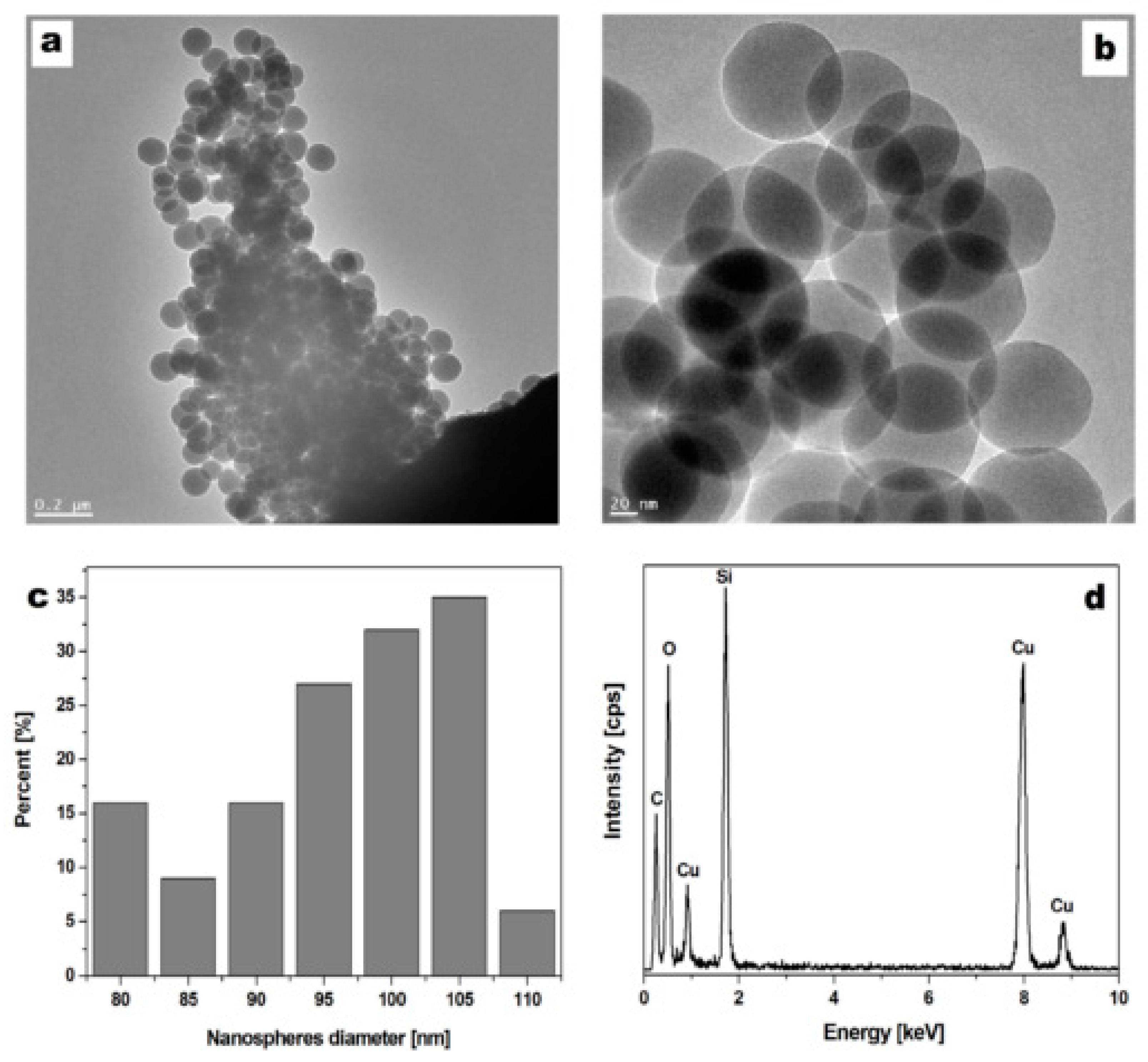
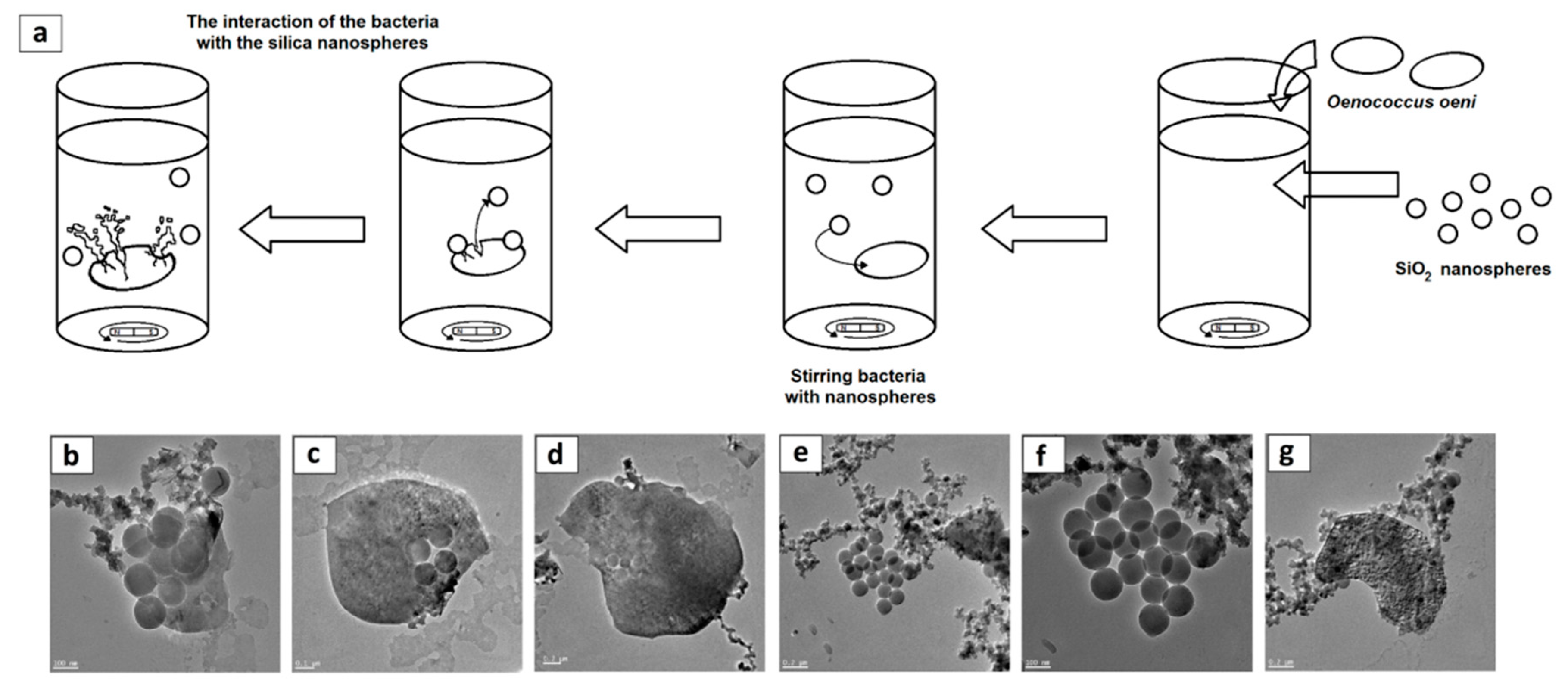
2.7. Visualization and Physiochemical Characteristics of the Silica Nanospheres
2.8. Statistical Analysis
3. Results
3.1. Silica Nanosphere Characteristics
3.2. Reduction in Oenococcus oeni Counts by Silica Nanospheres with Stirring
3.3. Reduction in O. oeni Counts by Silica Nanospheres without Stirring
3.4. Reduction of Oenococcus oeni Numbers in Wine by Silica Nanospheres with Stirring
3.5. Microscopic Visualisation
3.6. Degradation of Silica Nanospheres
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falguera, V.; Forns, M.; Ibarz, A. UV—Vis irradiation: An alternative to reduce SO2 in white wines? LWT Food Sci. Technol. 2013, 51, 59–64. [Google Scholar] [CrossRef]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative methods to SO2 for microbiological stabilization of wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Machado, R.M.D.; Toledo, M.C.F.; Vicente, E. Sulphite content in some Brazilian wines: Analytical determination and estimate of dietary exposure. Eur. Food Res. Technol. 2009, 229, 383–389. [Google Scholar] [CrossRef]
- Monro, T.M.; Moore, R.L.; Nguyen, M.C.; Ebendorff-Heidepriem, H.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Sensing free sulfur dioxide in wine. Sensors 2012, 12, 10759–10773. [Google Scholar] [CrossRef] [Green Version]
- Rizzotti, L.; Levav, N.; Fracchetti, F.; Felis, G.E.; Torriani, S. Effect of UV—C treatment on the microbial population of white and red wines, as revealed by conventional plating and PMA-qPCR methods. Food Control. 2015, 47, 407–412. [Google Scholar] [CrossRef]
- Fredericks, I.N.; Du Toit, M.; Krügel, M. Efficacy of ultraviolet radiation as an alternative technology to inactivate microorganisms in grape juices and wines. Food Microbiol. 2011, 28, 510–517. [Google Scholar] [CrossRef]
- Du Toit, M.; Pretorius, I.S. Microbial spoilage and preservation of wine: Using weapons from nature’s own arsenal: A review. S. Afr. J. Enol. Vitic. 2000, 21, 74–96. [Google Scholar] [CrossRef] [Green Version]
- Salaha, M.-I.; Kallithraka, S.; Marmaras, I.; Koussissi, E.; Tzourou, I. A natural alternative to sulphur dioxide for red wine production: Influence on colour, antioxidant activity and anthocyanin content. J. Food Compos. Anal. 2008, 21, 660–666. [Google Scholar] [CrossRef]
- Berbegal, C.; Spano, G.; Fragasso, M.; Grieco, F.; Russo, P.; Capozzi, V. Starter cultures as biocontrol strategy to prevent Brettanomyces bruxellensis proliferation in wine. Appl. Microbiol. Biotech. 2018, 102, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Grygorcewicz, B.; Chajęcka-Wierzchowska, W.; Augustyniak, A.; Wasak, A.; Stachurska, X.; Nawrotek, P.; Dołęgowska, B. In-milk inactivation of Escherichia coli O157: H7 by the environmental lytic bacteriophage ECPS-6. J. Food Saf. 2020, 40, e12747. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Portu, J.; López, N.; Santamaría, P.; Gutiérrez, A.R.; López, R.; López-Alfaro, I. Pulsed Electric Field treatment after malolactic fermentation of Tempranillo Rioja wines: Influence on microbial, physicochemical and sensorial quality. Innov. Food Sci. Emerg. Technol. 2019, 51, 57–63. [Google Scholar] [CrossRef]
- van Wyk, S.; Silva, F.V.; Farid, M.M. Pulsed electric field treatment of red wine: Inactivation of Brettanomyces and potential hazard caused by metal ion dissolution. Innov. Food Sci. Emerg. Technol. 2019, 52, 57–65. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; Bañuelos, M.A.; Sanz, P.D.; Otero, L.; Suárez-Lepe, J.A. Grape processing by high hydrostatic pressure: Effect on microbial populations, phenol extraction and wine quality. Food Bioproc. Tech. 2015, 8, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Christofi, S.; Malliaris, D.; Katsaros, G.; Panagou, E.; Kallithraka, S. Limit SO2 content of wines by applying High Hydrostatic Pressure. Innov. Food Sci. Emerg. Technol. 2020, 62, 102–342. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Bañuelos, M.A.; Puig-Pujol, A.; Guamis, B.; González, C.; Suárez-Lepe, J.A. Use of Ultra-High Pressure Homogenization processing in winemaking: Control of microbial populations in grape musts and effects in sensory quality. Innov. Food Sci. Emerg. Technol. 2018, 50, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Mijowska, K.; Cendrowski, K.; Grygorcewicz, B.; Oszmiański, J.; Nawrotek, P.; Ochmian, I.; Zielińska, B. Preliminary study on the influence of UV-C irradiation on microorganism viability and polyphenol compounds content during winemaking of ‘Regent’red grape cultivar. Pol. J. Chem. Technol. 2017, 19, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Diesler, K.; Golombek, P.; Kromm, L.; Scharfenberger-Schmeer, M.; Durner, D.; Schmarr, H.G.; Stahl, M.R.; Briviba, K.; Fischer, U. UV-C treatment of grape must: Microbial inactivation, toxicological considerations and influence on chemical and sensory properties of white wine. Innov. Food Sci. Emerg. Technol. 2019, 52, 291–304. [Google Scholar] [CrossRef]
- Błaszak, M.; Nowak, A.; Lachowicz, S.; Migdał, W.; Ochmian, I. E-Beam Irradiation and Ozonation as an Alternative to the Sulphuric Method of Wine Preservation. Molecules 2019, 24, 3406. [Google Scholar] [CrossRef] [Green Version]
- Divol, B.; Strehaiano, P.; Lonvaud-Funel, A. Effectiveness of dimethyldicarbonate to stop alcoholic fermentation in wine. Food Microbiol. 2005, 22, 169–178. [Google Scholar] [CrossRef]
- Costa, A.; Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiol. 2008, 25, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Larrea, F.; Rojo-Bezares, B.; Sáenz, Y.; Navarro, L.; Díez, L.; Portugal, C.B.; Fernández, R.; Zarazaga, M.; Torres, C. Bacteriocins for wine microbiological control and reduction of SO2 levels. Bullet. de l’OIV 2007, 80, 445. [Google Scholar]
- Ferreira, D.; Moreira, D.; Costa, E.M.; Silva, S.; Pintado, M.M.; Couto, J.A. The antimicrobial action of chitosan against the wine spoilage yeast Brettanomyces/Dekkera. J. Chitin Chitosan Sci. 2013, 1, 240–245. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, Y.; Sun, Z.; Li, X. Chitooligosaccharide as a possible replacement for sulfur dioxide in winemaking. Appl. Sci. 2020, 10, 578. [Google Scholar] [CrossRef] [Green Version]
- Gerbaux, V.; Villa, A.; Monamy, C.; Bertrand, A. Use of lysozyme to inhibit malolactic fermentation and to stabilize wine after malolactic fermentation. Am. J. Enol. Viticult. 1997, 48, 49–54. [Google Scholar]
- Chen, K.; Han, S.Y.; Li, M.; Sheng, W.J. Use of lysozyme and Oligomeric Proanthocyanidin to reduce sulfur dioxide and the evolution of volatile compounds in Italian Riesling ice wine during aging process. J. Food Process. Pres. 2017, 41, 453–465. [Google Scholar] [CrossRef]
- Sonni, F.; Cejudo Bastante, M.J.; Chinnici, F.; Natali, N.; Riponi, C. Replacement of sulfur dioxide by lysozyme and oenological tannins during fermentation: Influence on volatile composition of white wines. J. Sci. Food Agric. 2009, 89, 688–696. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Bartolomé, B.; Martínez-Rodríguez, A.J.; Pueyo, E.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Potential of phenolic compounds for controlling lactic acid bacteria growth in wine. Food Control. 2008, 19, 835–841. [Google Scholar] [CrossRef]
- Shih, M.K.; Lai, Y.H.; Lin, C.M.; Chen, Y.W.; Hou, Z.T.; Hou, C.Y. A novel application of terpene compound α-pinene for alternative use of sulfur dioxide-free white wine. Int. J. Food Prop. 2020, 23, 520–532. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zheng, X.; Xie, Y.; Ding, C.; Ruan, H.; Fan, C. Anti-bacterial and cytotoxic properties of plasma sprayed silver-containing HA coatings. J. Mater. Sci. Mater. Med. 2008, 19, 3603–3609. [Google Scholar] [CrossRef]
- Razeeb, K.M.; Podporska-Carroll, J.; Jamal, M.; Hasan, M.; Nolan, M.; McCormack, D.E.; Quilty, B.; Newcomb, S.B.; Pillai, S.C. Antimicrobial properties of vertically aligned nano-tubular copper. Mater. Lett. 2014, 128, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Cendrowski, K.; Peruzynska, M.; Markowska-Szczupak, A.; Chen, X.; Wajda, A.; Lapczuk, J.; Kurzawski, M.; Kalenczuk, R.J.; Drozdzik, M.; Mijowska, E. Mesoporous silica nanospheres functionalized by TiO2 as a photoactive antibacterial agent. J. Nanomed. Nanotechnol. 2013, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interf. Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Lim, S.H.; Phonthammachai, N.; Pramana, S.S.; White, T.J. Simple Route to Monodispersed Silica−Titania Core−Shell Photocatalysts. Langmuir 2008, 24, 6226–6231. [Google Scholar] [CrossRef]
- Cendrowski, K.; Peruzynska, M.; Markowska-Szczupak, A.; Chen, X.; Wajda, A.; Lapczuk, J.; Kurzawski, M.; Kalenczuk, R.J.; Drozdzik, M.; Mijowska, E. Antibacterial performance of nanocrystallined titania confined in mesoporous silica nanotubes. Biomed. Microdevices 2014, 16, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijowska, E.; Cendrowski, K.; Barylak, M.; Konicki, W. Sandwich-like mesoporous silica flakes for anticancer drug transport—Synthesis, characterization and kinetics release study. Colloids Surf. B Biointerfaces 2015, 136, 119–125. [Google Scholar] [CrossRef]
- Ghanbari, A.; Attar, M.M. A study on the anticorrosion performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-silica on mild steel substrate. J. Ind. Eng. Chem. 2015, 23, 145–153. [Google Scholar] [CrossRef]
- Esquena, J.; Tadros, T.F.; Kostarelos, K.; Solans, C. Preparation of narrow size distribution silica particles using microemulsions. Langmuir 1997, 13, 6400–6406. [Google Scholar] [CrossRef]
- Park, M.V.; Annema, W.; Salvati, A.; Lesniak, A.; Elsaesser, A.; Barnes, C.; McKerr, G.; Howard, C.V.; Lynch, I.; Dawson, K.A.; et al. In vitro developmental toxicity test detects inhibition of stem cell differentiation by silica nanoparticles. Toxicol. Appl. Pharmacol. 2009, 240, 108–116. [Google Scholar] [CrossRef]
- Xie, G.; Sun, J.; Zhong, G.; Shi, L.; Zhang, D. Biodistribution and toxicity of intravenously administered silica nanoparticles in mice. Arch. Toxicol. 2010, 84, 183–190. [Google Scholar] [CrossRef]
- Horszczaruk, E.; Mijowska, E.; Cendrowski, K.; Mijowska, S.; Sikora, P. Effect of incorporation route on dispersion of mesoporous silica nanospheres in cement mortar. Constr. Build. Mater. 2014, 66, 418–421. [Google Scholar] [CrossRef]
- Hbaieb, S.; Kalfat, R.; Chevalier, Y. Loading antifungal drugs onto silica particles grafted with cyclodextrins by means of inclusion complex formation at the solid surface. Int. J. Pharm. 2012, 439, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ko, Y.S.; Park, S.J.; Lee, J.S.; Cho, J.; Baek, K.Y.; Kim, I.T.; Woo, K.; Lee, J.H. Immobilization of silver nanoparticle-decorated silica particles on polyamide thin film composite membranes for antibacterial properties. J. Membr. Sci. 2016, 499, 80–91. [Google Scholar] [CrossRef]
- Sun, Z.; Cui, G.; Li, H.; Tian, Y.; Yan, S. Multifunctional dendritic mesoporous silica nanospheres loaded with silver nanoparticles as a highly active and recyclable heterogeneous catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 142–153. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Gu, H. Adsorption and desorption behaviors of DNA with magnetic mesoporous silica nanoparticles. Langmuir 2011, 27, 6099–6106. [Google Scholar] [CrossRef]
- Chen, X.; Wilgosz, K.; Cendrowski, K.; Tang, T.; Chu, P.K.; Kalenczuk, R.J.; Mijowska, E. Facile synthesis of hollow silica spheres with nanoholes. Dalton Trans. 2013, 42, 6381–6385. [Google Scholar] [CrossRef]
- Maniprasad, P.; Santra, S. Novel copper (Cu) loaded core–shell silica nanoparticles with improved Cu bioavailability: Synthesis, characterization and study of antibacterial properties. J. Biomed. Nanotechnol. 2012, 8, 558–566. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Peruzynska, M.; Szelag, S.; Trzeciak, K.; Kurzawski, M.; Cendrowski, K.; Barylak, M.; Roginska, D.; Piotrowska, K.; Mijowska, E.; Drozdzik, M. In vitro and in vivo evaluation of sandwich-like mesoporous silica nanoflakes as promising anticancer drug delivery system. Int. J. Pharm. 2016, 506, 458–468. [Google Scholar] [CrossRef]
- Berbegal, C.; Borruso, L.; Fragasso, M.; Tufariello, M.; Russo, P.; Brusetti, L.; Spano, G.; Capozzi, V.A. Metagenomic-Based approach for the characterization of bacterial diversity associated with spontaneous malolactic fermentations in wine. Int. J. Mol. Sci. 2019, 20, 3980. [Google Scholar] [CrossRef] [Green Version]
- Cimaglia, F.; Tristezza, M.; Saccomanno, A.; Rampino, P.; Perrotta, C.; Capozzi, V.; Spano, G.; Chiesa, M.; Mita, G.; Grieco, F. An innovative oligonucleotide microarray to detect spoilage microorganisms in wine. Food Control. 2018, 87, 169–179. [Google Scholar] [CrossRef]
- Russo, P.; Fragasso, M.; Berbegal, C.; Grieco, F.; Spano, G.; Capozzi, V. Chapter 2: Microorganisms Able to Produce Biogenic Amines and Factors Affecting Their Activity. In Biogenic Amines in Food; Saad, B., Tofalo, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 18–40. [Google Scholar]
- Breniaux, M.; Dutilh, L.; Petrel, M.; Gontier, E.; Campbell-Sills, H.; Deleris-Bou, M.; Krieger, S.; Teissedre, P.-L.; Jourdes, M.; Reguant, C.; et al. Adaptation of two groups of Oenococcus oeni strains to red and white wines: The role of acidity and phenolic compounds. J. Appl. Microbiol. 2018, 125, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Díez, L.; Rojo-Bezares, B.; Zarazaga, M.; Rodríguez, J.M.; Torres, C.; Ruiz-Larrea, F. Antimicrobial activity of pediocin PA-1 against Oenococcus oeni and other wine bacteria. Food Microbiol. 2012, 31, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; Cendrowski, K.; Nawrotek, P.; Barylak, M.; Mijowska, E. Investigating the interaction between Streptomyces sp. and titania/silica nanospheres. Water Air Soil Pollut. 2016, 227, 230. [Google Scholar] [CrossRef]
- Monge, M.; Moreno-Arribas, M.V. Applications of nanotechnology in wine production and quality and safety control. In Wine Safety, Consumer Preference, and Human Health; Moreno-Arribas, M.V., Sualdea, B.B., Eds.; Springer: Berlin, Germany, 2016; pp. 51–69. [Google Scholar]
- García-Ruiz, A.; Crespo, J.; López-de-Luzuriaga, J.M.; Olmos, M.E.; Monge, M.; Rodríguez-Álfaro, M.P.; Martín-Alvarez, P.J.; Bartolome, B.; Moreno-Arribas, M.V. Novel biocompatible silver nanoparticles for controlling the growth of lactic acid bacteria and acetic acid bacteria in wines. Food Control. 2015, 50, 613–619. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Monge, M.; Miralles, B.; Armentia, G.; Cueva, C.; Crespo, J.; López de Luzuriaga, J.M.; Olmos, M.E.; Bartolomé, B.; González de Llano, D.; et al. Some new findings on the potential use of biocompatible silver nanoparticles in winemaking. Innov. Food Sci. Emerg. Technol. 2019, 51, 64–72. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Escott, C.; Del Fresno, J.M.; Tesfaye, W.; Palomero, F.; Suárez-Lepe, J.A. Applications of nanotechnology in the winemaking process. Eur. Food Res. Technol. 2020, 246, 1533–1541. [Google Scholar] [CrossRef]
- Patent Number PL 232150 B1. Available online: https://api-ewyszukiwarka.pue.uprp.gov.pl/api/collection/08d0a91f424ba7e8a4c32e3d8f1dcde0 (accessed on 30 May 2019).
- Hashim, A.F.; Youssef, K.; Abd-Elsalam, K.A. Ecofriendly nanomaterials for controlling gray mold of table grapes and maintaining postharvest quality. Eur. J. Plant. Pathol. 2019, 154, 377–388. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control. 2011, 22, 408–413. [Google Scholar] [CrossRef]
- Youssef, K.; de Oliveira, A.G.; Tischer, C.A.; Hussain, I.; Roberto, S.R. Synergistic effect of a novel chitosan/silica nanocomposites-based formulation against gray mold of table grapes and its possible mode of action. Int. J. Biol. Macromol. 2019, 141, 247–258. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Agriculture and Rural Development. Expert Group for Technical Advice on Organic Production. Final Report on Food (III). 2014. Available online: https://ec.europa.eu/info/sites/info/files/food-farming-fisheries/farming/documents/egtop-final-report-food-iii_en.pdf (accessed on 6 August 2019).
- OIV, International Organisation of Vine and Wine. List of OIV Admitted Compounds and Their Status as Additives or Processing Aids and the Use Levels or Residual Limits. 2017. Available online: http://www.oiv.int/public/medias/5523/list-of-oiv-admitted-compounds.pdf (accessed on 30 June 2017).





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachnowska, K.; Cendrowski, K.; Stachurska, X.; Nawrotek, P.; Augustyniak, A.; Mijowska, E. Potential Use of Silica Nanoparticles for the Microbial Stabilisation of Wine: An In Vitro Study Using Oenococcus oeni as a Model. Foods 2020, 9, 1338. https://doi.org/10.3390/foods9091338
Pachnowska K, Cendrowski K, Stachurska X, Nawrotek P, Augustyniak A, Mijowska E. Potential Use of Silica Nanoparticles for the Microbial Stabilisation of Wine: An In Vitro Study Using Oenococcus oeni as a Model. Foods. 2020; 9(9):1338. https://doi.org/10.3390/foods9091338
Chicago/Turabian StylePachnowska, Kamila, Krzysztof Cendrowski, Xymena Stachurska, Paweł Nawrotek, Adrian Augustyniak, and Ewa Mijowska. 2020. "Potential Use of Silica Nanoparticles for the Microbial Stabilisation of Wine: An In Vitro Study Using Oenococcus oeni as a Model" Foods 9, no. 9: 1338. https://doi.org/10.3390/foods9091338