The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Collection and Analysis of Biological Specimens
2.3. Estimated Glomerular Filtration Rates (eGFR)
2.4. Normalization of ECd to Ecr and Ccr
2.5. Benchmark Dose Computation and Benchmark Dose–Response (BMR) Setting
2.6. Statistical Analysis
3. Results
3.1. Characterization of Cadmium Exposure by Sex and Smoking
3.2. Characterization of CKD Risk factors
3.3. Cadmium Excretion in Relation to the Risk of CKD
3.4. BMDL/BMDU Figures of ECd Associated with Reduced Glomerular Function
3.4.1. Ecr-Normalized Dataset
3.4.2. Ccr-Normalized Dataset
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Boon, P.E.; Pustjens, A.M.; Te Biesebeek, J.D.; Brust, G.M.H.; Castenmiller, J.J.M. Dietary intake and risk assessment of elements for 1- and 2-year-old children in the Netherlands. Food Chem. Toxicol. 2022, 161, 112810. [Google Scholar] [CrossRef] [PubMed]
- Fechner, C.; Hackethal, C.; Höpfner, T.; Dietrich, J.; Bloch, D.; Lindtner, O.; Sarvan, I. Results of the BfR MEAL Study: In Germany, mercury is mostly contained in fish and seafood while cadmium, lead, and nickel are present in a broad spectrum of foods. Food Chem. X 2022, 14, 100326. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, K. Epidemiology of renal tubular dysfunction in the inhabitants of a cadmium-polluted area in the Jinzu River basin in Toyama Prefecture. Tohoku J. Exp. Med. 1987, 152, 151–172. [Google Scholar] [CrossRef]
- Horiguchi, H.; Aoshima, K.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Hosoi, Y.; Katoh, T.; Kayama, F. Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. Int. Arch. Occup. Environ. Health 2010, 83, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Kurata, Y.; Katsuta, O.; Doi, T.; Kawasuso, T.; Hiratsuka, H.; Tsuchitani, M.; Umemura, T. Chronic cadmium treatment induces tubular nephropathy and osteomalacic osteopenia in ovariectomized cynomolgus monkeys. Vet. Pathol. 2014, 51, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122. [Google Scholar] [CrossRef] [PubMed]
- JECFA. Summary and Conclusions. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010; JECFA/73/SC. Food and Agriculture Organization of the United Nations/World Health Organization: Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/handle/10665/44521 (accessed on 21 September 2022).
- Satarug, S.; Vesey, D.A.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.C. The inverse association of glomerular function and urinary β2-MG excretion and its implications for cadmium health risk assessment. Environ. Res. 2019, 173, 40–47. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Dose-response analysis of the tubular and glomerular effects of chronic exposure to environmental cadmium. Int. J. Environ. Res. Public Health 2022, 19, 10572. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The source and pathophysiologic significance of excreted cadmium. Toxics 2019, 7, 55. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.C.; Phelps, K.R. The effect of cadmium on GFR is clarified by normalization of excretion rates to creatinine clearance. Int. J. Mol. Sci. 2021, 22, 1762. [Google Scholar] [CrossRef]
- Schnaper, H.W. The tubulointerstitial pathophysiology of progressive kidney disease. Adv. Chronic Kidney Dis. 2017, 24, 107–116. [Google Scholar] [CrossRef]
- Sand, S.; Victorin, K.; Filipsson, A.F. The current state of knowledge on the use of the benchmark dose concept in risk assessment. J. Appl. Toxicol. 2008, 28, 405–421. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee. Update: Use of the benchmark dose approach in risk assessment. EFSA J. 2017, 15, 4658. [Google Scholar]
- Moffett, D.B.; Mumtaz, M.M.; Sullivan, D.W., Jr.; Whittaker, M.H. Chapter 13, General Considerations of Dose-Effect and Dose-Response Relationships. In Handbook on the Toxicology of Metals, 5th ed.; Volume I: General Considerations; Nordberg, G., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 299–317. [Google Scholar]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Yimthiang, S.; Pouyfung, P.; Khamphaya, T.; Kuraeiad, S.; Wongrith, P.; Vesey, D.A.; Gobe, G.C.; Satarug, S. Effects of environmental exposure to cadmium and lead on the risks of diabetes and kidney dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 2259. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Elsherbiny, H.; Rule, A.D. In-vivo techniques for determining nephron number. Curr. Opin. Nephrol. Hypertens. 2019, 28, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef]
- Soveri, I.; Berg, U.B.; Björk, J.; Elinder, C.G.; Grubb, A.; Mejare, I.; Sterner, G.; Bäck, S.E.; SBU GFR Review Group. Measuring GFR: A systematic review. Am. J. Kidney Dis. 2014, 64, 411–424. [Google Scholar] [CrossRef]
- White, C.A.; Allen, C.M.; Akbari, A.; Collier, C.P.; Holland, D.C.; Day, A.G.; Knoll, G.A. Comparison of the new and traditional CKD-EPI GFR estimation equations with urinary inulin clearance: A study of equation performance. Clin. Chim. Acta 2019, 488, 189–195. [Google Scholar] [CrossRef]
- Phelps, K.R.; Gosmanova, E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020, 94, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Slob, W.; Moerbeek, M.; Rauniomaa, E.; Piersma, A.H. A statistical evaluation of toxicity study designs for the estimation of the benchmark dose in continuous endpoints. Toxicol. Sci. 2005, 84, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Slob, W.; Setzer, R.W. 2014. Shape and steepness of toxicological dose-response relationships of continuous endpoints. Crit. Rev. Toxicol. 2014, 44, 270–297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, T.; Jelsovsky, J.Z. Bootstrap estimation of benchmark doses and confidence limits with clustered quantal data. Risk Anal. 2007, 27, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Déruaz-Luyet, A.; Brodovicz, K.G.; Kimes, T.M.; Rosales, A.G.; Hauske, S.J. Kidney disease progression and all-cause mortality across estimated glomerular filtration rate and albuminuria categories among patients with vs. without type 2 diabetes. BMC Nephrol. 2020, 21, 167. [Google Scholar]
- George, C.; Mogueo, A.; Okpechi, I.; Echouffo-Tcheugui, J.B.; Kengne, A.P. Chronic kidney disease in low-income to middle-income countries: Case Increased Screening. BMJ Glob Health. 2017, 2, e000256. [Google Scholar] [CrossRef]
- George, C.; Echouffo-Tcheugui, J.B.; Jaar, B.G.; Okpechi, I.G.; Kengne, A.P. The need for screening, early diagnosis, and prediction of chronic kidney disease in people with diabetes in low- and middle-income countries-a review of the current literature. BMC Med. 2022, 20, 247. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Costanzi, S.; Naticchia, A.; Sturniolo, A.; Gambaro, G. Low level exposure to cadmium increases the risk of chronic kidney disease: Analysis of the NHANES 1999–2006. BMC Public Health 2010, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Tellez-Plaza, M.; Guallar, E.; Muntner, P.; Silbergeld, E.; Jaar, B.; Weaver, V. Blood cadmium and lead and chronic kidney disease in US adults: A joint analysis. Am. J. Epidemiol. 2009, 170, 1156–1164. [Google Scholar] [CrossRef]
- Lin, Y.S.; Ho, W.C.; Caffrey, J.L.; Sonawane, B. Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ. Res. 2014, 134, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.M.; Ricardo, A.C.; Persky, V.; Turyk, M. Associations between blood cadmium concentration and kidney function in the U.S. population: Impact of sex, diabetes and hypertension. Environ. Res. 2018, 169, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.F.; Hsu, P.C.; Lee, C.T.; Kung, C.T.; Chang, Y.C.; Fu, L.M.; Ou, Y.C.; Lan, K.C.; Yen, T.H.; Lee, W.C. Association between enzyme-linked immunosorbent assay-measured kidney injury markers and urinary cadmium levels in chronic kidney disease. J. Clin. Med. 2021, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Myong, J.-P.; Kim, H.-R.; Baker, D.; Choi, B. Blood cadmium and moderate-to-severe glomerular dysfunction in Korean adults: Analysis of KNHANES 2005–2008 data. Int. Arch. Occup. Environ. Health 2012, 85, 885–893. [Google Scholar] [CrossRef]
- Chung, S.; Chung, J.H.; Kim, S.J.; Koh, E.S.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Shin, S.J. Blood lead and cadmium levels and renal function in Korean adults. Clin. Exp. Nephrol. 2014, 18, 726–734. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S.J. Association of blood heavy metal levels and renal function in Korean adults. Int. J. Environ. Res. Public Health 2022, 19, 6646. [Google Scholar] [CrossRef]
- Jalili, C.; Kazemi, M.; Cheng, H.; Mohammadi, H.; Babaei, A.; Taheri, E.; Moradi, S. Associations between exposure to heavy metals and the risk of chronic kidney disease: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2021, 51, 165–182. [Google Scholar] [CrossRef]
- Byber, K.; Lison, D.; Verougstraete, V.; Dressel, H.; Hotz, P. Cadmium or cadmium compounds and chronic kidney disease in workers and the general population: A systematic review. Crit. Rev. Toxicol. 2016, 46, 191–240. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Zhu, C.; Dong, Z.; Zhang, K.; Zhao, Y.; Xu, Y. Benchmark dose for cadmium exposure and elevated N-acetyl-β-D-glucosaminidase: A meta-analysis. Environ. Sci. Pollut. Res. Int. 2016, 23, 20528–20538. [Google Scholar] [CrossRef]
- Pócsi, I.; Dockrell, M.E.; Price, R.G. Nephrotoxic biomarkers with specific indications for metallic pollutants: Implications for environmental health. Biomark. Insights 2022, 17, 11772719221111882. [Google Scholar] [CrossRef]
- Suwazono, Y.; Sand, S.; Vahter, M.; Filipsson, A.F.; Skerfving, S.; Lidfeldt, J.; Akesson, A. Benchmark dose for cadmium-induced renal effects in humans. Environ. Health Perspect. 2006, 114, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Yang, J.; Zhu, Y.; Li, Y.; Zheng, W.; Wu, M.; He, G. Dose-response evaluation of urinary cadmium and kidney injury biomarkers in Chinese residents and dietary limit standards. Environ. Health 2021, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Taylor, A.W.; Riley, M.; Byles, J.; Liu, J.; Noakes, M. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin. Nutr. 2018, 37, 276–284. [Google Scholar] [CrossRef] [PubMed]
- EFSA. European Food Safety Agency, Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar]
- Repić, A.; Bulat, P.; Antonijević, B.; Antunović, M.; Džudović, J.; Buha, A.; Bulat, Z. The influence of smoking habits on cadmium and lead blood levels in the Serbian adult people. Environ. Sci. Pollut. Res. Int. 2020, 27, 751–760. [Google Scholar] [CrossRef]
- Pappas, R.S.; Fresquez, M.R.; Watson, C.H. Cigarette smoke cadmium breakthrough from traditional filters: Implications for exposure. J. Anal. Toxicol. 2015, 39, 45–51. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Sturniolo, A.; Naticchia, A.; D’Alonzo, S.; Gambaro, G. Temporal trend of cadmium exposure in the United States population suggests gender specificities. Intern. Med. J. 2012, 42, 691–697. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Navas-Acien, A.; Caldwell, K.L.; Menke, A.; Muntner, P.; Guallar, E. Reduction in cadmium exposure in the United States population, 1988–2008: The contribution of declining smoking rates. Environ. Health Perspect. 2012, 120, 204–209. [Google Scholar] [CrossRef]
- Callan, A.; Hinwood, A.; Devine, A. Metals in commonly eaten groceries in Western Australia: A market basket survey and dietary assessment. Food Addit. Contam. A 2014, 31, 1968–1981. [Google Scholar] [CrossRef]
- Arnich, N.; Sirot, V.; Riviere, G.; Jean, J.; Noel, L.; Guerin, T.; Leblanc, J.-C. Dietary exposure to trace elements and health risk assessment in the 2nd French Total Diet Study. Food Chem. Toxicol. 2012, 50, 2432–2449. [Google Scholar] [CrossRef]
- González, N.; Calderón, J.; Rúbies, A.; Timoner, I.; Castell, V.; Domingo, J.L.; Nadal, M. Dietary intake of arsenic, cadmium, mercury and lead by the population of Catalonia, Spain: Analysis of the temporal trend. Food Chem. Toxicol. 2019, 132, 110721. [Google Scholar] [CrossRef] [PubMed]
- Butler-Dawson, J.; James, K.A.; Krisher, L.; Jaramillo, D.; Dally, M.; Neumann, N.; Pilloni, D.; Cruz, A.; Asensio, C.; Johnson, R.J.; et al. Environmental metal exposures and kidney function of Guatemalan sugarcane workers. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Win-Thu, M.; Myint-Thein, O.; Win-Shwe, T.-T.; Mar, O. Environmental cadmium exposure induces kidney tubular and glomerular dysfunction in the Myanmar adults. J. Toxicol. Sci. 2021, 46, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Skröder, H.; Hawkesworth, S.; Kippler, M.; El Arifeen, S.; Wagatsuma, Y.; Moore, S.E.; Vahter, M. Kidney function and blood pressure in preschool-aged children exposed to cadmium and arsenic-potential alleviation by selenium. Environ. Res. 2015, 140, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, E.; Tamayo-Ortiz, M.; Ariza, A.C.; Ortiz-Panozo, E.; Deierlein, A.L.; Pantic, I.; Tolentino, M.C.; Estrada-Gutiérrez, G.; Parra-Hernández, S.; Espejel-Núñez, A.; et al. Early-life dietary cadmium exposure and kidney function in 9-year-old children from the PROGRESS cohort. Toxics 2020, 8, 83. [Google Scholar] [CrossRef]
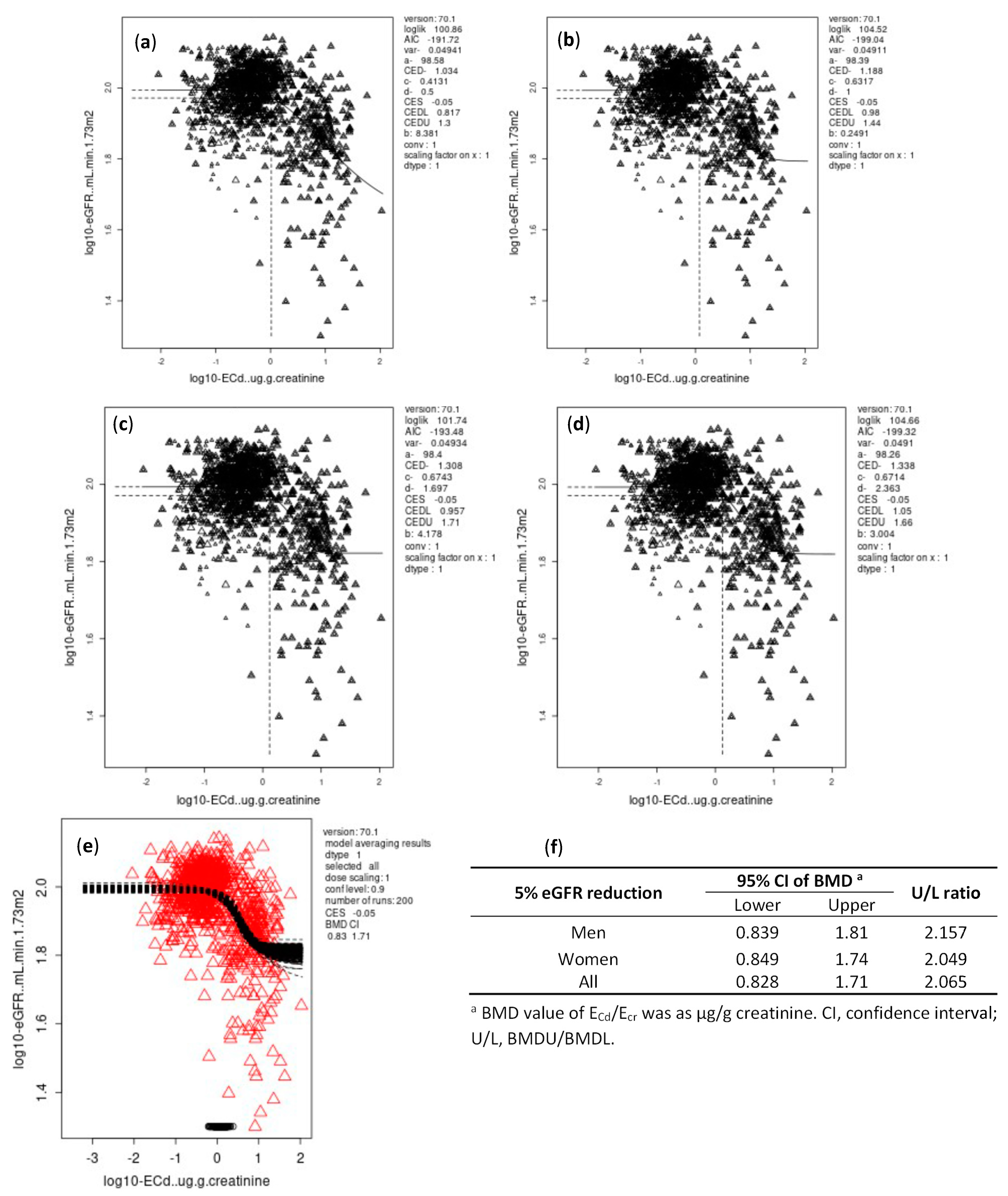
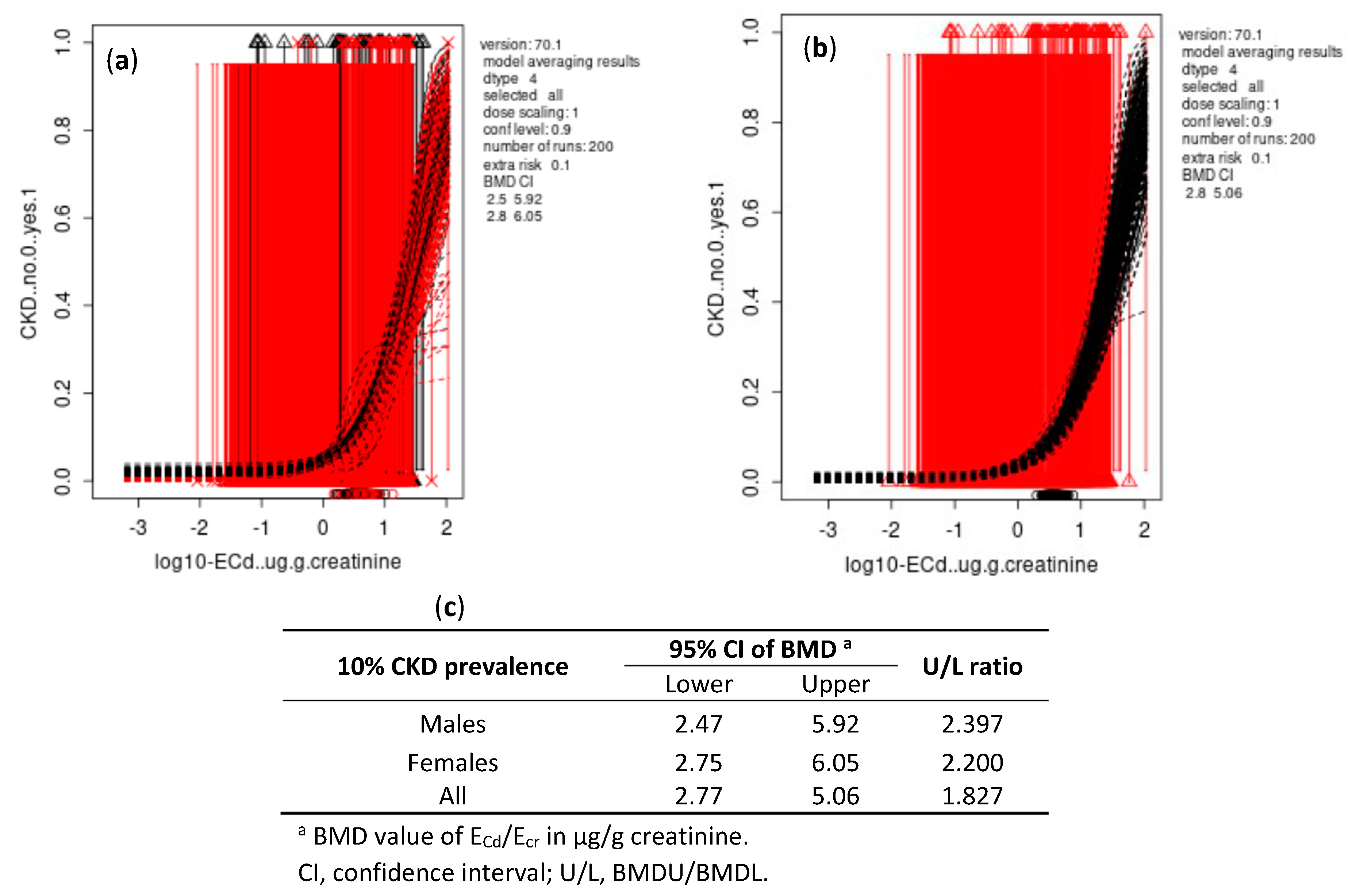
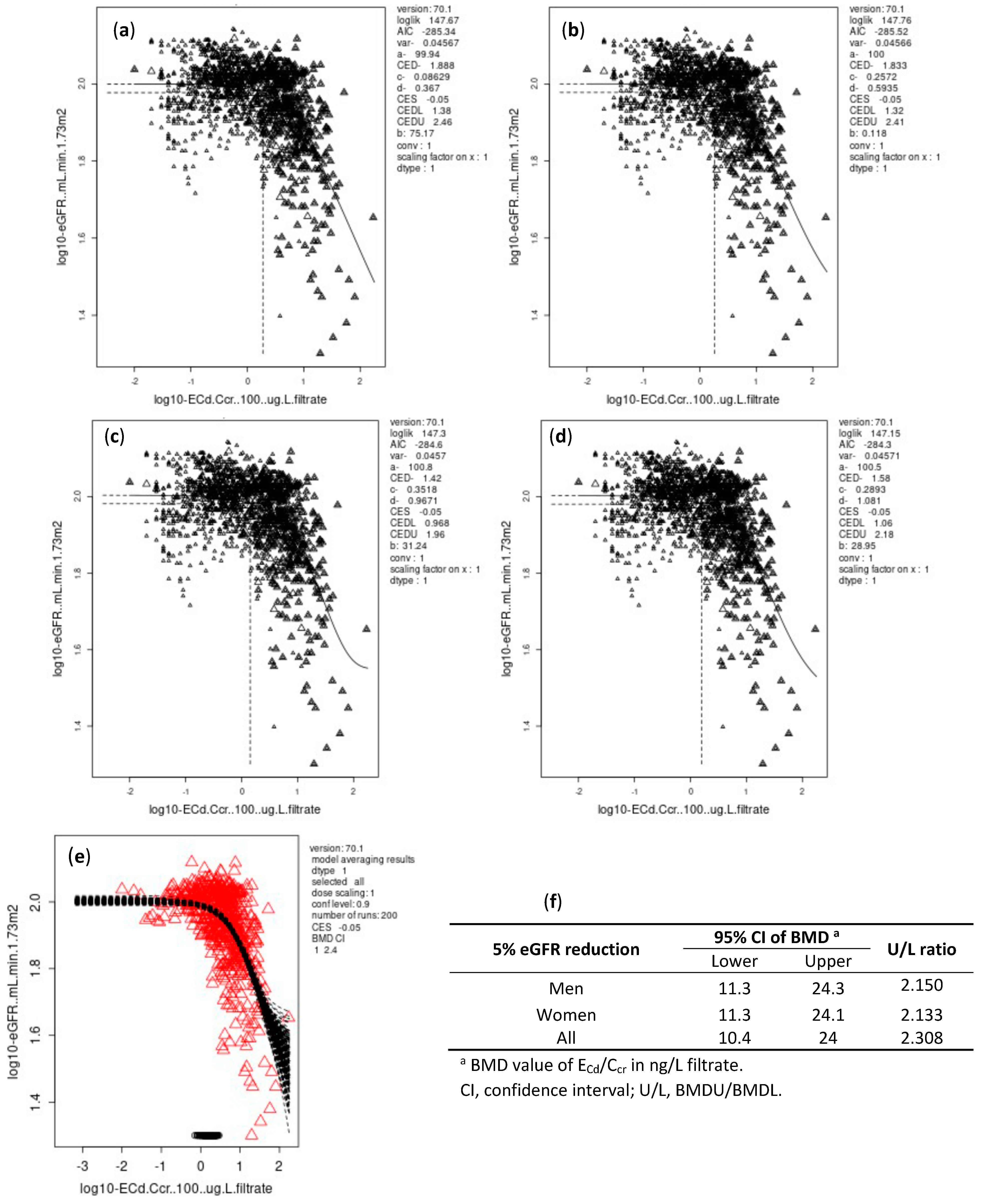
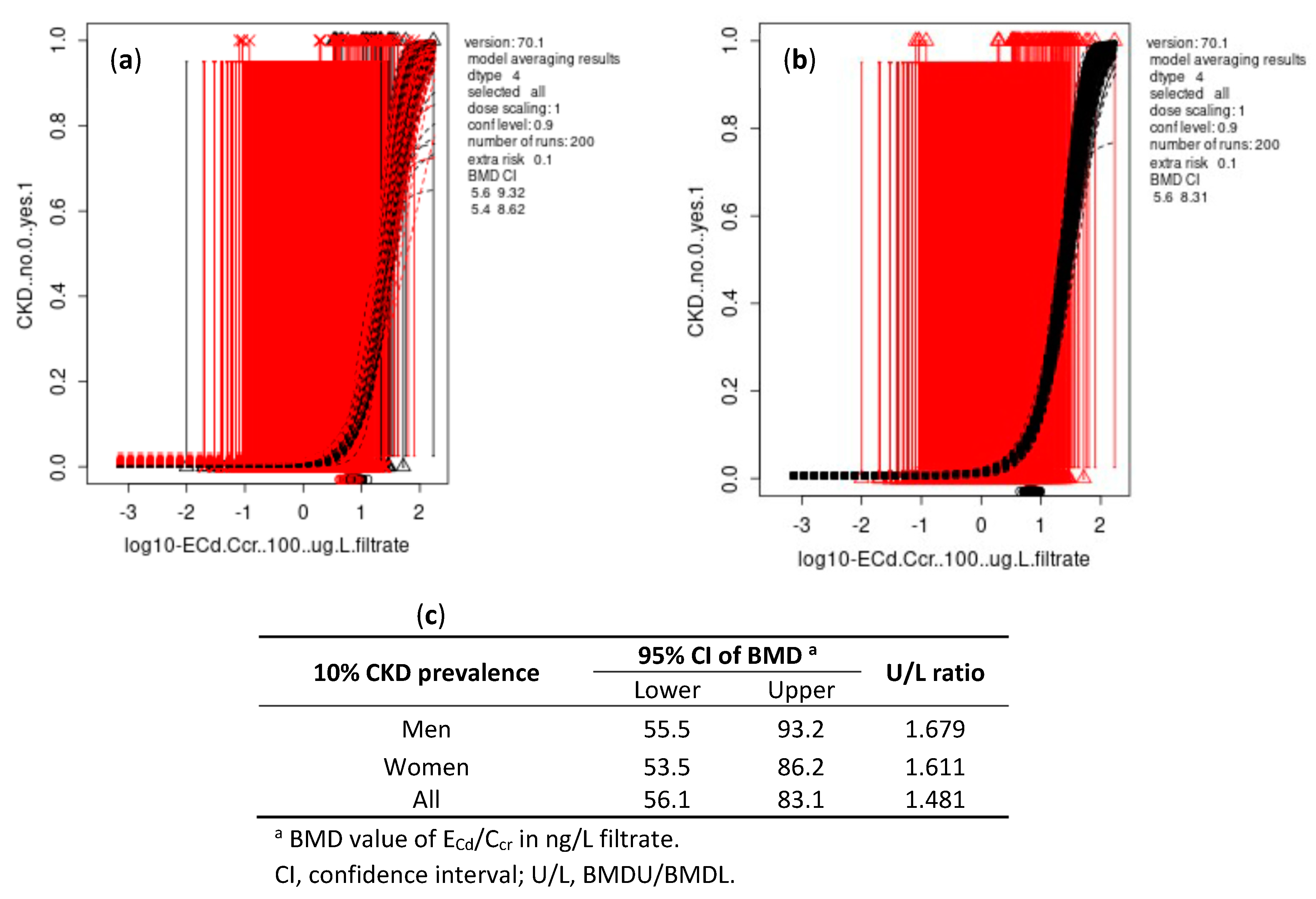
| Parameters | All subjects n 1189 (33.6% Smokers) | Males, n 493 (57.4% Smokers) | Females, n 696 (16.8% Smokers) | ||
|---|---|---|---|---|---|
| Nonsmokers n 210 | Smokers n 283 | Nonsmokers n 579 | Smokers n 117 | ||
| Age, years | 43.2 ± 14.0 | 35.9 ± 13.0 | 45.0 ± 14.8 *** | 42.6 ± 12.9 | 54.2 ± 10.1 ### |
| Hypertension (%) | 29.4 | 26.5 | 27.2 | 30.8 | 33.3 |
| BMI, kg/m2 | 23.0 ± 3.9 | 22.4 ± 3.0 | 22.3 ± 3.4 | 23.8 ± 4.0 | 22.4 ± 4.6 # |
| BMI groups (%) | |||||
| 12–18 | 10.5 | 9.2 | 12.2 * | 7.4 | 21.4 |
| 19–23 | 47.1 | 56.9 | 57.2 ** | 41.6 | 36.8 ### |
| ≥24 | 42.4 | 34.0 | 30.6 | 51.0 | 41.9 ### |
| eGFR a, mL/min/1.73 m2 | 93.7 ± 20 | 96.6 ± 17.6 | 91.9 ± 22.1 | 95.9 ± 19.3 | 81.8 ± 22.0 ### |
| eGFR ≤ 60 mL/min/1.73 m2 (%) | 6.2 | 2.4 | 8.8 ** | 4.3 | 16.2 ### |
| eGFR, mL/min/1.73 m2 (%) b | |||||
| >120 | 7.8 | 9.0 | 5.7 | 9.7 | 1.7 ### |
| 90–120 | 53.8 | 61.0 | 56.5 | 54.1 | 33.3 ### |
| 60–89 | 32.8 | 27.6 | 29.7 * | 32.8 | 49.6 ### |
| 30–59 | 5.0 | 1.9 | 7.1 ** | 3.3 | 13.7 |
| 15–29 | 0.6 | 0.5 | 1.1 | 0.2 | 1.7 |
| Plasma creatinine, mg/dL | 0.88 ± 0.24 | 1.00 ± 0.21 | 1.00 ± 0.27 | 0.76 ± 0.16 | 0.82 ± 0.27 ## |
| Urine creatinine, mg/dL | 104.4 ± 73.5 | 81.1 ± 78 | 107.0 ± 75.6 *** | 67.9 ± 68.9 | 79.8 ± 64.8 |
| Urine Cd, µg/L | 0.94 ± 9.69 | 0.32 ± 5.96 | 1.73 ± 15.9 *** | 0.75 ± 6.46 | 4.84 ± 6.38 ### |
| Normalized to Ecr as Ex/Ecr c | |||||
| ECd/Ecr, µg/g creatinine | 0.64 ± 6.12 | 0.32 ± 3.29 | 0.94 ± 8.85 *** | 0.57 ± 4.62 | 1.83 ± 7.27 ### |
| Normalized to Ccr as Ex/Ccr d | |||||
| ECd/Ccr × 100, µg/L filtrate | 1.02 ± 8.19 | 0.39 ± 4.75 | 1.61 ± 12.61 *** | 0.83 ± 5.27 | 5.00 ± 9.36 ### |
| Independent Variables/ Factors | Number of Subjects | a CKD | ||||
|---|---|---|---|---|---|---|
| β Coefficients | POR | 95% CI | p | |||
| (SE) | Lower | Upper | ||||
| Model 1 | ||||||
| Log2[(ECd/Ecr) × 103], µg/g creatinine | 917 | 0.385 (0.072) | 1.470 | 1.276 | 1.692 | <0.001 |
| Hypertension | 276 | 0.490 (0.312) | 1.632 | 0.885 | 3.008 | 0.117 |
| Gender (female) | 562 | 0.028 (0.340) | 1.029 | 0.528 | 2.002 | 0.934 |
| Smoking | 335 | 0.209 (0.337) | 1.232 | 0.637 | 2.383 | 0.536 |
| BMI, kg/m2 | ||||||
| 12–18 | 99 | Referent | ||||
| 19–23 | 431 | 0.057 (0.426) | 1.058 | 0.459 | 2.439 | 0.894 |
| ≥24 | 387 | 1.033 (0/470) | 2.810 | 1.118 | 7.064 | 0.028 |
| Age, years | ||||||
| 16–45 | 392 | Referent | ||||
| 46–55 | 348 | 2.655 (1.036) | 14.23 | 1.867 | 108.4 | 0.010 |
| 56–65 | 100 | 3.340 (1.059) | 28.21 | 3.538 | 224.9 | 0.002 |
| 66–87 | 77 | 4.950 (1.055) | 141.2 | 17.87 | 1116 | <0.001 |
| Model 2 | ||||||
| Log2[(ECd/Ccr) × 105], µg/L filtrate | 917 | 0.674 (0.107) | 1.962 | 1.589 | 2.422 | <0.001 |
| Hypertension | 276 | 0.551 (0.326) | 1.735 | 0.916 | 3.287 | 0.091 |
| Gender (female) | 562 | −0.174 (0.366) | 0.840 | 0.410 | 1.719 | 0.633 |
| Smoking | 335 | −0.058 (0.351) | 0.944 | 0.474 | 1.879 | 0.869 |
| BMI, kg/m2 | ||||||
| 12–18 | 99 | Referent | ||||
| 19–23 | 431 | 0.103 (0.457) | 1.109 | 0.452 | 2.717 | 0.822 |
| ≥24 | 387 | 1.147 (0.500) | 3.150 | 1.181 | 8.400 | 0.022 |
| Age, years | ||||||
| 16–45 | 392 | Referent | ||||
| 46–55 | 348 | 2.298 (1.036) | 9.951 | 1.305 | 75.88 | 0.027 |
| 56–65 | 100 | 3.543 (1.062) | 34.57 | 4.312 | 277.2 | 0.001 |
| 66–87 | 77 | 5.292 (1.066) | 198.6 | 24.59 | 1605 | <0.001 |
| Independent Variables/ Factors | Number of Subjects | a CKD | ||||
|---|---|---|---|---|---|---|
| β Coefficients | POR | 95% CI | p | |||
| (SE) | Lower | Upper | ||||
| Model 1 | ||||||
| Age, years | 917 | 0.126 (0.016) | 1.135 | 1.100 | 1.170 | <0.001 |
| BMI, kg/m2 | 917 | 0.082 (0.038) | 1.086 | 1.009 | 1.169 | 0.028 |
| Gender (female) | 562 | 0.124 (0.337) | 1.132 | 0.585 | 2.190 | 0.713 |
| Hypertension | 276 | 0.304 (0.310) | 1.355 | 0.738 | 2.486 | 0.327 |
| Smoking | 335 | 0.173 (0.345) | 1.189 | 0.605 | 2.338 | 0.615 |
| ECd/Ecr, µg/g creatinine | ||||||
| ≤0.37 | 358 | Referent | ||||
| 0.38–2.49 | 333 | 1.819 (0.565) | 6.164 | 2.035 | 18.67 | 0.001 |
| ≥2.5 | 226 | 2.362 (0.557) | 10.61 | 3.562 | 31.60 | <0.001 |
| Model 2 | ||||||
| Age, years | 917 | 0.141 (0.016) | 1.152 | 1.116 | 1.189 | <0.001 |
| BMI, kg/m2 | 917 | 0.099 (0.039) | 1.104 | 1.023 | 1.191 | 0.011 |
| Gender (female) | 562 | 0.191 (0.356) | 1.211 | .602 | 2.434 | 0.591 |
| Hypertension | 276 | 0.240 (0.314) | 1.271 | 0.687 | 2.353 | 0.445 |
| Smoking | 335 | −0.033 (0.359) | 0.968 | 0.479 | 1.956 | 0.927 |
| ECd/Ccr, ng/L filtrate | ||||||
| ≤9.9 | 346 | Referent | ||||
| 10–49.9 | 326 | 1.470 (0.642) | 4.350 | 1.237 | 15.30 | 0.022 |
| ≥50 | 245 | 3.036 (0.637) | 20.82 | 5.979 | 72.52 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satarug, S.; Đorđević, A.B.; Yimthiang, S.; Vesey, D.A.; Gobe, G.C. The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease. Toxics 2022, 10, 614. https://doi.org/10.3390/toxics10100614
Satarug S, Đorđević AB, Yimthiang S, Vesey DA, Gobe GC. The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease. Toxics. 2022; 10(10):614. https://doi.org/10.3390/toxics10100614
Chicago/Turabian StyleSatarug, Soisungwan, Aleksandra Buha Đorđević, Supabhorn Yimthiang, David A. Vesey, and Glenda C. Gobe. 2022. "The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease" Toxics 10, no. 10: 614. https://doi.org/10.3390/toxics10100614
APA StyleSatarug, S., Đorđević, A. B., Yimthiang, S., Vesey, D. A., & Gobe, G. C. (2022). The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease. Toxics, 10(10), 614. https://doi.org/10.3390/toxics10100614










